Abstract
Diffusion tensor imaging (DTI) is a powerful method to visualize white matter, but its use in patients with acute stroke remains limited because of the lack of corresponding histologic information. In this study, we addressed this issue using a hypoxia–ischemia (HI)-induced thrombotic model of stroke in adult mice. At 6, 15, and 24 hours after injury, animals were divided into three groups for (1) in vivo T2- and diffusion-weighted magnetic resonance imaging, followed by histochemistry, (2) ex vivo DTI and electron microscopy, and (3) additional biochemical or immunochemical assays. The temporal changes of diffusion anisotropy and histopathology were compared in the fimbria, internal capsule, and external capsule. We found that HI caused a rapid reduction of axial and radial diffusivities in all three axonal bundles. A large decrease in fractional anisotropy, but not in axial diffusivity per se, was associated with structural breakdown of axons. Furthermore, the decrease in radial diffusivity correlated with swelling of myelin sheaths and compression of the axoplasma. The gray matter of the hippocampus also exhibited a high level of diffusion anisotropy, and its reduction signified dendritic degeneration. Taken together, these results suggest that cross-evaluation of multiple DTI parameters may provide a fuller picture of axonal and dendritic injury in acute ischemic stroke.
Keywords: axial diffusivity, fractional anisotropy, leukoaraiosis, oligodendrocyte, radial diffusivity, white matter
Introduction
White matter (WM), occupying 50% of total brain volume in humans, has a metabolic rate similar to that of gray matter (GM) (Zhang and Sejnowski, 2000; Goldberg and Ransom, 2003). Yet, WM receives a disproportionately small blood supply and little collateral circulation, making it particularly susceptible to hypoxic-ischemic insults (Dewar et al, 1999; Arai and Lo, 2009). However, although the importance of WM injury in cerebrovascular diseases is recognized, it is difficult to distinguish it from GM damage and determine the point of irreversible WM injury in acute stroke using traditional imaging methods.
Diffusion tensor imaging (DTI) is a relatively new magnetic resonance imaging (MRI) technique to visualize axonal organization and to measure directional water diffusion in the nervous system (Basser et al, 1994). In DTI, measurements of water diffusion along multiple axes are fitted into a three-dimensional (3D) model to determine three eigenvalues in the principal (λ1) and two shorter, perpendicular axes (λ2 and λ3) of the ‘diffusion ellipsoid'. The three eigenvalues are used to calculate several DTI indexes, including fractional anisotropy (FA), axial/longitudinal diffusivity (λII), and radial/transverse diffusivity (λ⊥) (Mori and Zhang, 2006). Fractional anisotropy, scaled from 0 (isotropic) to 1 (anisotropic), is a measurement of the relative difference of the three eigenvalues. Axial diffusivity, equivalent to λ1, represents water motion along the length of axons, which can be reduced by structural breakdown or focal enlargement–constriction of the neurites according to theoretical modeling (Beaulieu, 2002; Budde and Frank, 2010). Radial diffusivity, the average of λ2 and λ3, indicates perpendicular water diffusion across the axon, which is hindered by barriers such as myelin sheaths (Song et al, 2002).
In trauma- or axotomy-induced Wallerian degeneration, axial diffusivity steadily declines and radial diffusivity gradually increases, resulting in a progressive reduction of FA (MacDonald et al, 2007; Zhang et al, 2009). In contrast, patients with acute ischemic stroke often exhibit a rapid reduction of both axial and radial diffusivities, accompanied by little to no change of initial FA (Yang et al, 1999; Bhagat et al, 2008; Sakai et al, 2009). A sizable reduction of FA only occurred ∼24 hours after stroke onset, which was postulated to indicate irreversible axonal injury (Bhagat et al, 2008). However, this hypothesis of important clinical application is yet to be validated, because to date, there are no DTI–neuropathology correlation data in patients with acute ischemic stroke (<24 hours).
In this study, we describe DTI measurements and corresponding histologic changes in a rodent model of thrombotic stroke induced by cerebral hypoxia–ischemia (HI) (Adhami et al, 2006). We show that a combined hypoxic-ischemic insult triggers a rapid reduction of both axial and radial diffusivities in multiple axonal tracts, similar to the pattern observed in acute stroke patients. In contrast, a large reduction of FA only occurs slowly and is restricted to the nerve tracts with severe axonal destruction. Furthermore, the rapid reduction of radial diffusivity correlates with oligodendrocyte swelling and compression of the axoplasma. Finally, the reduction of anisotropy contrast inside the GM of the hippocampus signifies dendritic degeneration. Taken together, these results suggest that DTI can be used to detect and distinguish multiple aspects of axonal and dendritic injuries in acute ischemic stroke.
Materials and methods
Animal Surgery
Male CD-1 mice (Charles River, Wilmington, MA, USA) and Thy1-YFP mice (Jackson Laboratories, Bar Harbor, ME, USA; stock number 003782; Feng et al (2000)), aged 8 to 12 weeks, were used in this study. Details of the HI-induced thrombotic model of stroke have been described previously (Adhami et al, 2006). In brief, animals were anesthetized using 1% to 2% isoflurane while maintaining respiration at 80 to 120 breaths per minute. Partial cerebral ischemia was established by permanent unilateral (right) common carotid artery occlusion. After carotid ligation, hypoxia was initiated by administering 7.5% O2 balanced by nitrogen through a gas mask for 50 minutes under anesthesia. The body temperature of mice was maintained at 37°C±0.5°C using a thermo-controller connected to a rectal probe and heating light. The animal procedures were approved by the Institutional Animal Care and Use Committee and conform to the NIH Guide for Care and Use of Laboratory Animals.
At 2 hours after hypoxia, mice were evaluated for neurologic symptoms, and those walking in circles towards the ipsilateral hemisphere were used for analysis in this study. Approximately 80% of HI-challenged animals fulfilled this criterion, and the mortality rate was <10% in CD-1 and Thy1-YFP mice by 24 hours recovery. In total, the following six groups of CD-1 mice were used for sequential MRI histology examinations.
Six hours after HI: in vivo T2-MRI and apparent diffusion coefficient (ADC) scan, followed by Nissl and anti-myelin basic protein (anti-MBP) stain (n=4).
Fifteen hours after HI: in vivo T2-MRI and ADC scan, followed by Nissl and anti-MBP stain (n=4).
Twenty-four hours after HI: in vivo T2-MRI and ADC scan, followed by Nissl and anti-MBP stain (n=4).
Six hours after HI: in vivo T2-MRI, ex vivo DTI, followed by electron microscopy (EM) study (n=3).
Fifteen hours after HI: in vivo T2-MRI, ex vivo DTI, followed by EM study (n=3).
Twenty-four hours after HI: in vivo T2-MRI, ex vivo DTI, followed by EM study (n=3).
Additional CD-1 and Thy1-YFP mice were killed at 1, 6, 15, or 24 hours after HI for various biochemical, histochemical, and immunocytochemical evaluations without MRI or EM (the number of animals used for each assay is indicated in text).
In Vivo Magnetic Resonance Imaging
All data were collected on a Bruker BioSpec 7-T system (Bruker BioSpec 70/30, Karlsruhe, Germany) equipped with 400 mT/m actively shielded gradients. Animals were anesthetized by 1% to 2% isoflurane delivered by oxygen, and the respiration rate was maintained at 60 to 100 breaths per minute. The core temperature was maintained at 37°C by warm air circulated through the magnet bore. All animals were scanned with a custom-built radio frequency coil. Anatomic data were acquired with a 3D, fast spin-echo sequence and the following parameters: echo train length=16, TEeff (echo timeeff)/TR (repetition time)=70.56/1,000 msec, FOV (field of view)=32 × 19.2 × 19.2 mm3, matrix size=256 × 96 × 96, and 1 average, resulting in a resolution of 125 × 200 × 200 μm3, with total acquisition time under 10 minutes. The anatomic image was used as a reference for the subsequent diffusion-weighted images. Two-dimensional diffusion-weighted images were collected using a conventional Stejskal–Tanner single spin-echo sequence with a b-value of 800 seconds/mm2, FOV=51.2 × 19.2 mm2, TE/TR=22/1,000 msec, 2 averages, matrix=256 × 96, resolution=200 × 200 μm2 in plane, and 600-μm slice thickness. The acquisition time was ∼20 minutes. Analysis was performed using the ParaVision 4.0 software package (Bruker BioSpec 70/30, Karlsruhe, Germany). Regions of interest were prescribed in the striatum, hippocampus, and rostral and caudal areas of the cortex. Apparent diffusion coefficients were averaged separately for the ipsilateral and contralateral sides at each time point.
Ex Vivo Magnetic Resonance Imaging
Ex vivo DTI was performed to assess the damage in the WM structures. Animals were killed immediately after the in vivo MRI scan by transcardial perfusion of fixatives. The brains were excised, stored in phosphate-buffered saline at 4°C for <2 weeks, and equilibrated to the ambient bore temperature for ∼30 minutes. All ex vivo images were acquired using a custom-built solenoid transmit/receive coil. Whole-brain data were collected using a 3D, conventional spin-echo DTI sequence. The DTI parameters were: b=800 seconds/mm2 gradient separation/duration (D/δ)=12/4 msec, FOV=32 × 12 × 12 mm3, TE/TR=22/1,000 msec, 1 average, matrix=256 × 96 × 96, resolution=125 μm isotropic. The total scan time was ∼18 hours. Quantification of DTI measures including FA, λII, and λ⊥ was performed using DTIstudio software (free Sharewave reported in Jiang et al, 2006).
Biochemistry
Brain samples for lipid peroxidation were taken from the cerebral cortex on either side of the brain, and homogenized in the phosphate buffer. The amount of malondialdehyde was quantified using a commercial kit following the manufacturer's instructions (Oxis Research, Portland, OR, USA).
Histology
All immunohistochemistry procedures were performed on frozen brain sections following transcardial perfusion of 4% paraformaldehyde and sucrose cryoprotection. The following antibodies were used: a rabbit polyclonal antibody against fibrinogen (a gift from Dr J Degen), MBP (Chemicon, Temecula, CA, USA), Olig2 (Chemicon), MAP2 (Sigma, St Louis, MO, USA), and NeuN (Chemicon). Biotinylated secondary antibodies and streptavidin conjugated to Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Carlsbad, CA, USA) were used to amplify immunosignals. The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) was performed as described previously (Yang et al, 2009). Cerebral perfusion analysis was performed by injecting fluorescein isothiocyanate-dextran (5 μg in 100 μL phosphate-buffered saline of 2 × 106 molecular weight; Sigma) into the left ventricle of animals at 4 hours after HI. For detection of superoxide, the unfixed mouse brains were removed and snap frozen on dry ice. Brain sections of 10-μm thickness were then incubated with DHE (dihydroethidium) (5 μmol/L; Invitrogen, Carlsbad, CA, USA) in phosphate buffer for 30 minutes at 37°C in a light-protected humidified chamber. The in vivo Turnbull's reaction histochemistry for detection of ferrous ion [Fe(II)] was performed as described previously (Yu et al, 2001). A published mouse atlas was used to determine brain locations and anatomic terminology (Franklin and Paxinos, 2008).
Electron Microscopy
At indicated times after HI, animals were perfused transcardially with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 mol/L phosphate buffer. The brains were removed and horizontal 100-μm-thick sections were cut using a vibratome. Areas of the fimbria, external capsule, and the hippocampus from the HI-challenged and contralateral hemispheres were dissected out and postfixed with 1% OsO4. The tissues were embedded in epoxy resin (Ted Pella, Redding, CA, USA). Thin sections of 70-nm thickness were cut using a Leica UC6 ultramicrotome (Leica, Deerfield, IL, USA) and stained with uranyl acetate and lead citrate. The sections were analyzed in a JEM 1200 EXII electron microscope (JEOL, Peabody, MA, USA).
Statistical Analyses
All data were analyzed using SPSS for Windows version 12.0 (SPSS, Chicago, IL, USA). Data were first analyzed using a general linear model analysis with time, side (contralateral or ipsilateral), and location as factors. Side and time were significant factors for all locations, but there was no significant side × location effect. There was a significant time × side effect for axial diffusivity and a significant location × time effect for FA. To determine the specific interactions, all data were analyzed for each side using one-way ANOVA (analysis of variance) with time as the factor. Contrasts were defined in this analysis comparing the 6- and 15-hour time points and the 6- and 24-hour time points. After that analysis, each time point was analyzed comparing the contralateral and ipsilateral sides by Student's t-test. Quantitative indices for the hippocampal subfields were not calculated because the contrast between these structures disappeared on the anisotropy maps at 15 and 24 hours.
Results
Cerebral Hypoxia–Ischemia Produces Progressive Gray Matter Damage and Occasional Axonal T2-Hyperintensity
To induce stroke, mice were treated with unilateral ligation of the common carotid artery, followed by 50 minutes systemic hypoxia (7.5% O2) with the core temperature kept at 37.5°C±0.5°C. This model produced thrombosis and infarction on the HI-challenged side of the brain, while causing no discernible damage to the contralateral side of the brain (Adhami et al, 2006). The undamaged contralateral side of the brain thus served as the internal control for MRI and additional analyses. In Supplementary Figure 1, we showed that at 4 hours after injury, there were still many punctate fibrin clots and pockets of perfusion deficits on the HI-challenged side of the brain but not on the contralateral hemisphere, suggesting an extended period of thrombosis in small arteries.
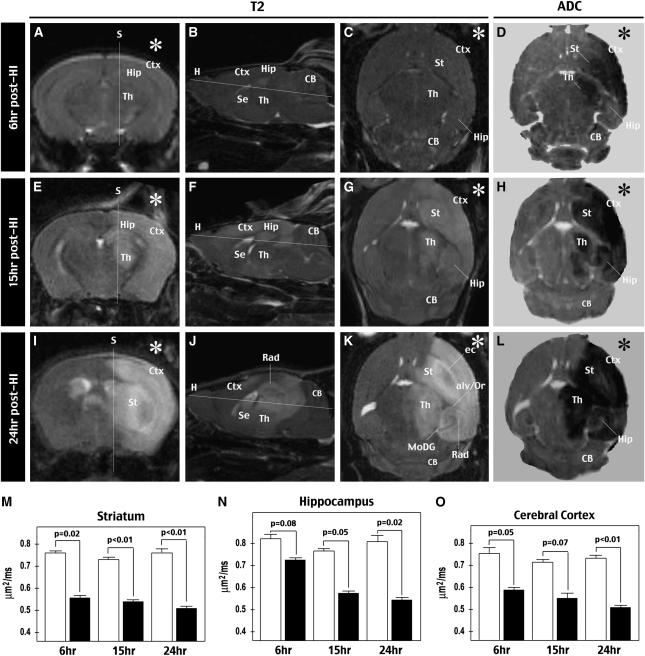
For in vivo 3D T2-weighted MRI correlated with ADC maps, different groups of mice were imaged at 6 hours (Figures 1A to 1D), 15 hours (Figures 1E to 1H), or 24 hours (Figures 1I to 1L) after the HI insult (n=4 for each time point). This analysis showed a gradual increase of T2 signals (an indication of water accumulation) and expansion of affected areas on the HI-injured hemisphere from 6 to 24 hours recovery. Consistent with the carotid artery-supplying territories, the damaged regions included the striatum, cerebral cortex, hippocampus, and thalamus, but spared the cerebellum and the brainstem that receive blood supply from the vertebral arteries. Interestingly, T2-weighted MRI occasionally showed hyperintensity along the nerve tracts, suggesting axonal edema, on the HI-challenged hemisphere at 15 to 24 hours recovery. For example, Figure 1K shows increased T2 signals in the external capsule (ec), the molecular layer of the dentate gyrus (MoDG), and the alveus and stratum oriens (alv/Or) in the ipsilateral hippocampus.
Figure 1.
Evaluation of HI-induced brain damage by in vivo T2- and diffusion-weighted MRI. (A to C, E to G, and I to K) T2-weighted MRI of the brains at 6 hours (panels A to C), 15 hours (panels E to G), or 24 hours (panels I to K) after unilateral HI challenge (indicated by asterisks (*)). The representative images shown for each time point in the coronal, sagittal, and horizontal views of the same brain are reconstructed from 3D in vivo T2-weighted images. The corresponding planes for sagittal (S) and horizontal (H) images are indicated. The increase of T2 signals and expansion of the affected area from 6 to 24 hours after HI, which spared the cerebellum (CB) receiving blood supply from vertebrate arteries must be noted. The hyperintensity of T2 signals in the external capsule (ec), alveus/stratum oriens (alv/Or), and the molecular layer of the dentate gyrus (MoDG) at 24 hours after HI must also be noted. (D, H, and L) Apparent diffusion coefficient (ADC) maps at 6 hours (panel D), 15 hours (panel H), or 24 hours (panel L) after HI. (M, N, and O) Quantification of ADC values in the striatum (panel M), hippocampus (panel N), and cerebral cortex (panel O) on the HI-injured (black columns) and the contralateral hemispheres (white columns) at indicated times (n=4 for each time points). Mean and variance values are shown. P-value is determined by the paired t-test. Ctx, cerebral cortex; HI, hypoxia–ischemia; Hip, hippocampus; MRI, magnetic resonance imaging; Se, the septal area; St, striatum; Th, thalamus; 3D, three dimensional.
The ADC map showed a rapid reduction of isotropic diffusion signals (caused by water accumulation intracellularly or in a tortuous extracellular space) in the striatum and cerebral cortex at all time points (P<0.05 compared with contralateral). However, the ADC map did not differentiate WM and showed little temporal progression of signal reduction. These results are consistent with the notion that ADC maps have high sensitivity in detecting cellular injury, but low specificity is resolving WM injury in acute stroke (Bhagat et al, 2008; Sakai et al, 2009).
Visualization of White Matter and Comparison of λII, λ⊥, and Fractional Anisotropy Alterations after Cerebral Hypoxia–Ischemia
Next, we used ex vivo DTI to derive 3D directionally encoded color (DEC) maps of the brains from mice killed at 6, 15, or 24 hours after HI (n=3 for each time point). The DEC maps distinguished all major nerve bundles in the forebrain, including the anterior commissure (ac), external capsule (ec), internal capsule (ic), the fimbria (fm)/fornix, and the optic tract (opt) (Figure 2A). For quantitative analysis, regions of interest were drawn in the fimbria, internal capsule, and external capsule to calculate λII, λ⊥, and FA on both sides of the brain. Previous studies have shown that although formalin fixation does not alter diffusion anisotropy, it reduces the magnitude of water diffusion coefficient in the brain (Guilfoyle et al, 2003; Sun et al, 2003, 2005). Thus, we calculated the ratio of λII, λ⊥, and FA in specific axonal tracts on the HI-injured hemisphere relative to their counterparts on the contralateral hemisphere to standardize lesion-associated changes of directional diffusivities (Table 1 ).
Figure 2.
Assessment of HI-induced WM injury by ex vivo diffusion tensor imaging (DTI). (A) Representative images of transverse/horizontal (the first column) and coronal views (from the rostral to caudal in three columns) of directionally encoded color (DEC) map of the brains at 6, 15, or 24 hours after HI. The directions of color-encoded water diffusion are indicated in the x–y–z axes. The loss of contrast on these maps in the anterior commissure (ac), the external capsule (ec), the internal capsule (ic), and the fimbria/fornix (fm) on the HI-challenged (R) side of the brain at 15 and 24 hours recovery must be noted. (B) The temporal changes of three commonly used DTI parameters (FA, fractional anisotropy; λII, axial diffusivity; λ⊥, radial diffusivity) in the fimbria/fornix, internal capsule, and external capsule. The changes are presented as the ratio of each metric to its counterpart on the contralateral hemisphere at 6, 15, and 24 hours after HI (n=3 for each time point). Mean and s.e. are shown *P<0.05 compared with the individual DTI parameters at 6 hours by ANOVA. The decrease in λII in all three axon tracts must be noted, while a steep decline of FA was only observed in the external capsule. ANOVA, analysis of variance; HI, hypoxia–ischemia; MoDG, molecular layer of the dentate gyrus; opt, optic tract; rad, stratum radiatum; WM, white matter.
Table 1. Ex vivo DTI measurements at indicated times after HI.
|
Fimbria (fm) |
Internal capsule (ic) |
External capsule (ec) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Ipsi | Cont | Ipsi/Cont (%) | Versus 6 hours | Ipsi | Cont | Ipsi/Cont (%) | Versus 6 hours | Ipsi | Cont | Ipsi/Cont (%) | Versus 6 hours |
| Six hours | ||||||||||||
| FA | 0.678 | 0.706 | 96 | 0.722 | 0.718 | 100 | 0.562 | 0.563 | 100 | |||
| (0.08) | (0.08) | (1) | (0.06) | (0.03) | (5) | (0.03) | (0.02) | (8) | ||||
| λll | 0.401 | 0.437 | 92 | 0.469 | 0.460 | 102 | 0.365 | 0.410 | 89 | |||
| (0.04) | (0.01) | (9) | (0.05) | (0.03) | (7) | (0.04) | (0.01) | (10) | ||||
| λ⊥ | 0.119 | 0.109 | 115 | 0.113 | 0.112 | 101 | 0.142 | 0.157 | 91 | |||
| (0.01) | (0.04) | (28) | (0.01) | (0.01) | (7) | (0.02) | (0.01) | (19) | ||||
| Fifteen hours | ||||||||||||
| FA | 0.765 | 0.782 | 98 | P=0.221 | 0.725 | 0.745 | 98 | P=0.946 | 0.480 | 0.538 | 88 | P=0.312 |
| (0.03) | (0.06) | (5) | (0.05) | (0.08) | (7) | (0.15) | (0.08) | (17) | ||||
| λll | 0.308 | 0.378 | 84 | P<0.01 | 0.363 | 0.394 | 95 | P<0.05 | 0.248 | 0.337 | 77 | P<0.01 |
| (0.01) | (0.07) | (16) | (0.05) | (0.09) | (24) | (0.05) | (0.08) | (28) | ||||
| λ⊥ | 0.079 | 0.086 | 92 | P<0.01 | 0.090 | 0.102 | 89 | P<0.05 | 0.119 | 0.141 | 86 | P=0.356 |
| (0.01) | (0.01) | (7) | (0.01) | (0.01) | (8) | (0.04) | (0.05) | (24) | ||||
| Twenty-four hours | ||||||||||||
| FA | 0.668 | 0.728 | 92 | P=0.876 | 0.682 | 0.713 | 96 | P=0.403 | 0.333 | 0.499 | 67 | P<0.05 |
| (0.10) | (0.08) | (11) | (0.05) | (0.04) | (3) | (0.03) | (0.06) | (14) | ||||
| λll | 0.257 | 0.416 | 63 | P<0.001 | 0.296 | 0.408 | 73 | P<0.01 | 0.229 | 0.387 | 60 | P<0.01 |
| (0.02) | (0.06) | (8) | (0.05) | (0.04) | (16) | (0.02) | (0.04) | (7) | ||||
| λ⊥ | 0.083 | 0.107 | 78 | P<0.01 | 0.084 | 0.106 | 80 | P<0.01 | 0.141 | 0.171 | 83 | P=0.965 |
| (0.01) | (0.01) | (10) | (0.01) | (0.01) | (9) | (0.02) | (0.01) | (13) | ||||
ANOVA, analysis of variance; Cont, the contralateral side to HI injury; DTI, diffusion tensor imaging; FA, fractional anisotropy; HI, hypoxia–ischemia; Ipsi, the ipsilateral side to HI injury;
Shown are mean and s.d. of FA, axial diffusivity (λII) ( × 103 mm2/sec), and radial diffusivity (λ⊥) ( × 103 mm2/sec) at indicated times after HI (n=3 for each time point). The P-value as compared with the DTI measures at 6-hour time point (versus 6 hours) was determined using one-way ANOVA with time as the factor.
Bold-faced values are the mean of ipsilateral measurements to the contralateral counterparts.
This analysis showed that axial diffusivity exhibited a steady decline in all three axonal tracts after cerebral HI, decreasing to 63%±8% (mean±s.d., relative to the contralateral side) in the fimbria, to 73%±16% in the internal capsule, and to 60%±7% in the external capsule at 24 hours recovery. These values were also significantly smaller than those at 6 hours on the ipsilateral hemisphere (P<0.01 by ANOVA). Similarly, radial diffusivity showed a gradual reduction to 78%±10% in the fimbria and to 80%±9% in the internal capsule at 24 hours recovery (P<0.01 compared with 6 hours) and a more blunted attenuation to 83%±13% at 24 hours recovery in the external capsule (P=0.965 compared with 6 hours). Our finding of concomitant reduction of axial and radial diffusivities following HI is similar to those detected in patients with acute ischemic stroke (Yang et al, 1999; Bhagat et al, 2008; Sakai et al, 2009).
In contrast to the trend of gradual decrease of axial and radial diffusivities in all three axonal tracts, a significant reduction of FA after cerebral HI was only detected in the external capsule, in which FA decreased to 67%±14% at 24 h recovery (P<0.05 compared with 6 hours). In the fimbria, FA was only reduced to 92%±11% at 24 hours recovery (P=0.876 compared with 6 hours). In the internal capsule, FA was decreased to 96%±3% at 24 hours recovery (P=0.403 compared with 6 hours). The significant decrease in FA only in the external capsule, despite general reduction of axial diffusivity in all nerve tracks examined, suggests that the more severe axonal destruction in stroke may manifest as a sizable reduction of FA.
Substantial Reduction of Fractional Anisotropy Correlates with Severe Axonal Destruction
To examine this possibility, we used EM and Thy1-YFP mice to assess the extent of axonal injury in this model of thrombotic stroke. Thy1-YFP is a line of transgenic mice that expresses the yellow fluorescent protein in a soluble form to fill the entire cellular architecture of most cortical layer V and hippocampal neurons, thus offering a global survey of axonal injury after cerebral HI (Feng et al, 2000). The analysis using Thy1-YFP mice showed severe breakdown of axonal fascicles in the external capsule (n=5; Figures 3A to 3D), but not in the fimbria/fornix and internal capsule (Figures 3A, 3B, 3G, and 3H) at 15 to 24 hours recovery. On EM following ex vivo DTI (n=3), the external capsule on the HI-injured hemisphere showed great reduction of nerve fibers and expansion of empty space between axonal bundles at 15 hours after HI (Figure 3F). In contrast, there was minimal reduction of axonal bundles in the fimbria on the HI-challenged hemisphere at 15 hours recovery (Figures 3I and 3J). Taken together, these data suggested a correlation between large FA reduction and severe axonal damage in specific nerve fiber tracts in acute cerebral ischemia.
Figure 3.
Histologic correlates of HI-induced changes in fractional anisotropy. (A to D and G to H) Sections taken from Thy1-YFP mice at 15 hours after HI (n=5) to detect axonal integrity in the external capsule (ec), internal capsule (ic), and the fimbria/fornix (fm). The selective axonal degeneration in the external capsule, but not in the internal capsule or the fimbria/fornix, on the HI-challenged side of the brain must be noted (panels B, D, and H). (E, F, I, and J) Electron microscopy study confirmed extensive axonal injury in the external capsule (panel F), but not in the internal capsule (panel J), on the HI-challenged side of the brain. The decrease in axonal bundles and increase in the empty space in the HI-injured external capsule must be noted (panel F). Scale bars: 200 μm in panels A and B; 50 μm in panels C, D, G, and H; 5 μm in panels E and F; and 1 μm in panels I and J. HI, hypoxia–ischemia.
Reduction of Radial Diffusivity after Hypoxia–Ischemia Signifies Acute Oligodendrocyte Damage
The observed early reduction of radial diffusivity in patients and our animal model of stroke stands in contrast to the slow increase in radial diffusivity in trauma- or axotomy-induced Wallerian degeneration (MacDonald et al, 2007; Zhang et al, 2009). Chronic demyelination often causes an increase in radial diffusivity (Song et al, 2002). Thus, the rapid reduction of radial diffusivity after cerebral HI may be caused by retention of myelin sheaths but expansion of the less-anisotropic extraaxonal components in WM (Beaulieu, 2002). To test this hypothesis, we used immunohistochemistry and EM to examine the relationship between myelin and axoplasma after cerebral HI.
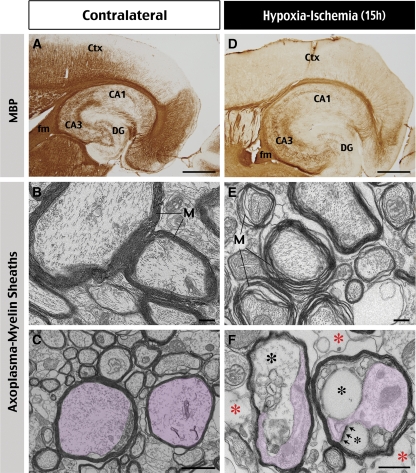
We found that HI caused greater reduction of the immunoreactivity against MBP inside the cerebral cortex than in the external capsule or the fimbria at 15 hours recovery (Figures 4A and 4D; n>6). On EM, besides severe axonal degeneration and edema (Figure 3F), two types of abnormalities were frequently observed in the less-destructed WM on the HI-challenged hemisphere. One is the swelling and separation of myelin sheaths (M in Figure 4E). The other is the appearance of large vesicles (black asterisks in Figure 4F) often containing myelin on the surface (arrows in Figure 4F), to compress the axoplasma (colored in pink in Figures 4C and 4F). There was also a trend of increased extraaxonal empty space in the WM (red asterisks in Figure 4F). These morphologic anomalies, remarkably similar to those previously described in isolated optic nerves following anoxic injury (Waxman et al, 1990, 1992), suggest rapid oligodendrocyte injury after HI.
Figure 4.
Histologic correlates of HI-induced changes in radial diffusivity. (A, D) HI causes marked reduction of myelin basic protein (MBP) staining in the cerebral cortex (Ctx) and hippocampus at 15 hours of recovery (n=6). (B to F) Representative electron micrographs of the external capsule at 15 hours after HI on the contralateral (panels B and C) and the HI-injured side of the brain (panels E and F). The separation of myelin sheaths (M), increased empty space (red asterisks in panel F), and large vesicles within a myelinated axon (black asterisks in panel F) must be noted. The vesicles often contain myelin on the surface (arrows in panel F) and compress onto the axoplasma (colored in pink in panel C and F). Scale bars: 125 μm in panels A and D; 1 μm in panels B to F. DG, dentate gyrus; fm, fimbria/fornix; HI, hypoxia–ischemia.
With regard to the mechanisms of HI-induced oligodendrocyte injury, previous studies have suggested that oligodendrocytes are very susceptible to oxidative stress owing to an insufficient antioxidant system and a high content of cellular iron (Thorburne and Juurlink, 1996; Irving et al, 1997; McCracken et al, 2000). To test this possibility, we used DHE stain to detect superoxide and found intense signals along the blood vessels on the HI-challenged hemisphere at as early as 1 hour recovery (Figures 5A; n>4). Measurement of malondialdehyde also suggested an increase in lipid peroxidation on the ipsilateral hemisphere at 6 hours after HI (Figure 5C; P<0.05 by ANOVA). These findings raised the possibility that HI-induced oxidative stress may reduce the ferric ion [Fe(III)] in oligodendrocytes to ferrous ion [Fe(II)], which in turn acts as an oxidizing agent to generate more superoxide and hydroxyl radical species (Dewar et al, 2003). Consistent with this possibility, an in vivo Turnbull's reaction histochemistry stain detected many more [Fe(II)]+ cells in the corpus callosum on the ipsilateral hemisphere (48±6 per visual field) than on the contralateral hemisphere (6±3 per visual field) at 15 hours recovery (Figures 5D; n=6) (Yu et al, 2001). In addition, the ipsilateral corpus callosum showed reduced immunoreactivity against Olig2, a marker of oligodendrocytes and their progenitors, when compared with the contralateral hemisphere (Figures 5E; n=3). On double labeling, many TUNEL+ nuclei were found adjacent to Olig2+ nuclei in the ipsilateral corpus callosum (arrows in Figure 5H), suggesting that the dying cells were oligodendrocytes in view of their typical tandem disposition in WM. On EM, the HI-challenged hemisphere also displayed many pyknotic nuclei in the corpus callosum at 15 hours after HI (Figures 5F and 5I). Taken together, these results suggest that cerebral HI rapidly induces oxidative stress in oligodendrocytes, the swelling and compression of which onto the axoplasma contributes to acute reduction of radial diffusivity in WM.
Figure 5.
Cerebral HI induces oxidative stress and oligodendrocyte injury. (A, B) Cerebral HI induces superoxide production along blood vessels in the ipsilateral brain at 1 hour of recovery, as revealed by dihydroethidium (DHE) staining. (C) HI also induces lipid peroxidation at 6 hours recovery, indicated by an increased amount of malondialdehyde (MDA) in cellular extracts of the cerebral cortex. Mean and s.d. are shown (n=4 for each). *P<0.05 compared with the other groups. (D, G) HI induces a high content of ferrous iron [Fe(II)] in oligodendrocytes in the corpus callosum (CC, arrows) (n=4). (E, H) TUNEL and Olig2 double labeling showed reduction of Olig2 immunoreactivity and the presence of many apoptotic nuclei immediately next to Olig2+ cells (arrows) in the CC on the HI-injured side of the brain at 15 hours recovery (n=3). (F, I) EM detected many pyknotic nuclei (L) of oligodendrocytes in the HI side of the CC at 15 hours. Scale bars: 100 μm in panels A and B; 5 μm in panels E and H; and 1 μm in panels F and I. Con, the contralateral side; EM, electron microscopy; HI, hypoxia–ischemia; St, striatum; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; UN, unchallenged animals.
Diffusion Tensor Imaging Captures Hypoxia–Ischemia-Induced Dendritic Degeneration in the Hippocampus
Although axons have the highest FA signals in the nervous system, in theory, any tissue with a polarized and orderly microstructure could display diffusion anisotropy (Basser et al, 1994). For example, a previous study using ex vivo DTI detected the dendrite-enriched stratum radiatum of the hippocampus in mice (Zhang et al, 2002). Consistent with this earlier report, our DTI analysis also appeared to detect the stratum radiatum inside the hippocampus (rad in Figure 2A). To verify the identities of the structures seen on DEC maps in the hippocampal subfield, we compared Nissl stain, ADC, FA, and DEC maps in the same imaging plane (Figure 6A). This cross-comparison revealed that neuronal cell bodies in the stratum pyramidal (CA (Cornu Ammonis)) and stratum granulosum (DG (dentate gyrus)) have a high isotropic diffusion (ADC) and a low anisotropic diffusion (FA) value, whereas myelinated axons in the external capsule (ec) and the molecular layer of the dentate gyrus (MoDG) behave oppositely. Examining the DEC map, we concluded that the stratum radiatum, a dendrite-rich hippocampal subfield, shows relatively high diffusion anisotropy, presumably attributed to an orderly array of apical dendrites in this region.
Figure 6.
DTI detects HI-induced dendritic degeneration in hippocampal neurons. (A) Comparison of the Nissl stain, ADC, FA, and DEC maps of the hippocampus on the same plane to determine the locations of stratum radiatum (rad) and molecular layer of the dentate gyrus (MoDG) on the DEC map. (B, C) Sections of the hippocampus taken from HI-injured Thy1-YFP mice at 15 hours recovery showed a marked reduction of apical dendrites in the stratum radiatum (st. rad) on the ipsilateral hemisphere (panel C). (D) T2-weighted MRI of an animal killed at 6 hours after HI, whose brain was examined by electron microscopy (EM), as shown in E to J. (Panels D to I) Electron micrographs of the indicated hippocampal subfields on the contralateral (panels E to G) and the HI-challenged hemispheres (panels H to J) at 6 hours after HI. The pervasive cytoskeletal dissolution inside dendrites (Den, colored in pink), swelling of the mitochondria (mit), and numerous intraaxonal vesicles (asterisks (*)) that compress onto the axoplasma (colored in green) must be noted. In contrast, there were few, if any, incidences of nuclear pyknosis inside the pyramidal neuron layer at the same time point, suggesting that dendritic and axonal histopathology precedes the apoptosis of hippocampal neurons after cerebral HI. Scale bars: 50 μm in panels B and C; 5 μm in panels E to I. ADC, apparent diffusion coefficient; DEC, directionally encoded color; DG, dentate gyrus; DTI, diffusion tensor imaging; ec, external capsule; FA, fractional anisotropy; HI, hypoxia–ischemia; Hip, hippocampus; MRI, magnetic resonance imaging.
Interestingly, close inspection of the post-HI DEC maps indicated a rapid reduction of diffusion anisotropy contrast in the stratum radiatum on the ipsilateral hemisphere (Figure 2A). By 15 hours after HI, DEC maps showed that the ipsilateral stratum radiatum was almost completely black, coinciding with severe dendritic degeneration shown in Thy1-YFP mice (Figures 6B and 6C). This finding suggests that dendrites of hippocampal neurons are highly sensitive to HI injury. To further examine this possibility, in Supplementary Figure 2, we used TUNEL, Golgi, anti-MAP2, and NeuN immunestains to compare the timing of somal-versus-dendritic injury in the hippocampus. This analysis indicated that dendritic destruction precedes the apoptosis of hippocampal neurons after cerebral HI.
Finally, to evaluate how early HI-induced dendritic degeneration occurs, we used T2-weighted MRI and EM to examine mouse brains collected at 6 hours after HI (n=3). For EM, we compared the pathology in the stratum radiatum (consisting of apical dendrites), the pyramidal cell layer (the nucleus of pyramidal neuron), and the fimbria (axons of hippocampal neurons). This analysis showed widespread dissolution of the dendritic cytoskeleton (Den in Figure 6I) and protrusion of vesicles in axons (asterisks in Figure 6J), when no obvious change was observed in the hippocampus on T2-weighted MRI (Figure 6D) and no nuclear pyknosis in pyramidal neurons on EM (Figures 6E and 6H). Taken together, these data suggest that dendrites of hippocampal neurons undergo rapid degeneration after cerebral HI, for which DTI has higher detection sensitivity than does T2-weighted MRI.
Discussion
Histopathology is the gold standard for establishing the diagnostic utility of any imaging method; however, the systematic imaging–histology correlation is not always feasible in patients. Hence, studies of animal models of the disease may illuminate the neuropathological basis of imaging findings in similar clinical settings. Previous studies have characterized the temporal evolution of diffusion tensor MRI in rodent or nonhuman primate models of cerebral ischemia at 3 and 24 hours and beyond (Carano et al, 2000; Liu et al, 2007). This study uses ex vivo DTI combined with histology and biochemical assays to examine the neuropathological basis of several DTI parameters, including axial and radial diffusivities and fraction anisotropy. Our results suggest that cross-evaluation of multiple DTI parameters may provide a fuller picture of WM and dendritic injury in acute ischemic stroke.
Reduction of Fractional Anisotropy after Ischemic Stroke Signals the Structural Breakdown of Axons
Previous studies in patients of ischemic stroke showed a rapid reduction of eigenvalues in all diffusion axes within 24 hours of symptom onset (Yang et al, 1999; Bhagat et al, 2008; Sakai et al, 2009). As FA is a measurement of the relative difference of the three eigenvalues, there was little-to-no decrease in the FA value in the hyperacute (2.5 to 7 hours) phase of ischemic stroke. Only at ∼24 hours after stroke onset did patients show reduction of FA in subcortical WM. This secondary reduction of FA has been suggested to indicate severe axonal damage, but this hypothesis of important clinical application has not been validated by histology (Bhagat et al, 2008). If validated, changes of FA values in individual axonal tracts can be used to monitor WM injury following ischemic stroke.
In this study using a murine model of HI-induced thrombotic stroke, we have reproduced the unique pattern of DTI alterations in ischemic stroke patients. This pattern includes acute reduction of eigenvalues in all three axes (thus, the concomitant reduction of axial and radial diffusivities), as well as a smaller and more variable change of FA in different axonal tracts. We found that the fimbria, internal capsule, and external capsule all had 25% to 40% reduction of axial diffusivity at 24 hours after HI, but showed a great variation in the degree of FA reduction. In the fimbria and internal capsule, there was <10% decrease in FA at 24 hours after HI, whereas the external capsule showed >30% reduction at 24 hours recovery. Importantly, a severe structural breakdown of the axons was only detected in the external capsule, but not in the fimbria or internal capsule. These results support the hypothesis that a large reduction of FA is an imaging marker of severe WM injury in acute ischemic stroke (Bhagat et al, 2008). However, more studies of the DTI–histopathology correlation are necessary to determine the threshold of FA reduction for irreversible axonal injury in acute stroke.
Oligodendrocyte Injury Likely Contributes to the Acute Reduction of Radial Diffusivity
The cellular basis of alterations of radial and axial diffusivities remains incompletely understood (Beaulieu, 2002). Axial diffusivity is believed to represent water diffusion in the lengths of axons, and its reduction could be caused by increased viscosity in the intraaxonal space (owing to cytoskeletal breakdown) or focal enlargement constriction of neuritis (‘beading') (Budde and Frank, 2010). Microtubules and myelin sheaths are generally agreed to be principally responsible for radial diffusivity, because they constrain transverse water diffusion across the axons. In trauma- or axotomy-induced Wallerian degeneration of axons, radial diffusivity slowly increases as a consequence of demyelination (MacDonald et al, 2007; Zhang et al, 2009). In contrast, clinical studies of stoke patients and our data in animal models all demonstrated an acute, concomitant reduction of axial and radial diffusivities. This disparity of DTI alterations suggested unique pathologic changes in ischemic stroke. We found that edema, separation of myelin sheaths, and protrusion of oligodendrocyte-derived vesicles compressing the axoplasma are typical WM pathologies in acute ischemic stroke. Our data of large, intramyelinic vesicles are strikingly similar to those previously described in isolated optic nerves after anoxic insults (Waxman et al, 1992), and consistent with the notion that oligodendrocyte swelling is one of the earliest morphologic changes in cerebral ischemia (Pantoni et al, 1996). It is conceivable that an increase in extraaxonal water content and compression of the axoplasma by swollen myelin sheaths may lead to reduction of radial diffusivity and even axial diffusivity in acute cerebral ischemia.
Unlike Wallerian degeneration in which the injury of oligodendrocytes is a slow process (Vargas and Barres, 2007), our data suggested that oligodendrocytes undergo rapid degeneration after hypoxic-ischemic insults. Although the exact causes of this hypersensitivity remain to be determined, our results support the notion that reactive oxidative stress and a high iron content in oligodendrocytes contribute to their increased sensitivity to hypoxic-ischemic insults (Thorburne and Juurlink, 1996; Irving et al, 1997; McCracken et al, 2000; Dewar et al, 2003). Importantly, recent studies have suggested that oligodendrocytes have a critical role in maintaining axonal function and survival (Kassmann and Nave, 2008). Hence, supply of antioxidants to oligodendrocytes may be a useful strategy for WM protection in acute ischemic stroke.
Diffusion Tensor Imaging may Detect Hypoxia–Ischemia-Induced Dendritic Degeneration in Laminated Brain Structures
This study also suggests the potential of DTI for detecting dendritic degeneration in hippocampal neurons, which may have implications beyond acute ischemic stroke. Diffusion anisotropy results from directional water flow in any tissue of a polarized intracellular structure and an orderly architecture (Basser et al, 1994). Although neural fibers are the prime example for structures with diffusion anisotropy, they are not the only such structures in the body. For example, a previous study using ex vivo DTI identified the stratum radiatum (consisting of dendrites of hippocampal neurons) in the mouse hippocampus (Zhang et al, 2002). Consistent with the earlier report, we also identified the stratum radiatum in the hippocampus by prominent diffusion anisotropy, most readily visualized on DEC maps and presumably attributed to an orderly array of apical dendrites. Furthermore, we showed that the loss of contrast on DEC maps in the stratum radiatum is associated with the degeneration of dendrites. Importantly, color-coded DEC maps have a greater sensitivity than T2-weighted MRI in detecting dendritic degeneration. Although ex vivo DTI was used in our study to increase the signal-to-noise ratio in small rodent brains, the advance of MRI technology may enable the use of in vivo DTI to detect dendritic degeneration, especially in laminated brain structures such as the hippocampus or cerebellum, in patients of ischemic stroke.
Moreover, using T2-weighted MRI followed by EM analysis, we have shown that the degeneration of hippocampal dendrites is a very early event in ischemic stroke. This conclusion is consistent with a recent in vivo two-photon confocal imaging study showing rapid degeneration of dendrites of cortical neurons in cerebral ischemia (Li and Murphy, 2008). The similarity of findings in these two studies suggests that dendrites are highly susceptible to stroke-related injury. On the basis of our results, we suggest the following sequence of pathologic events and associated imaging presentations in acute ischemic stroke:
Microscopic dendritic degeneration (only detectable by EM).
Swelling of oligodendrocytes (reduction of radial and axial diffusivities).
Massive degeneration of hippocampal dendrites (absence of diffusion contrast in DTI).
Structural breakdown of axons (at least >10% reduction of FA in DTI).
Massive accumulation of water in axon tracts (hyperintensity on T2-weighted MRI).
It is interesting to note that WM-associated T2-hyperintensity in our study resembles the hallmark of leukoaraiosis, an incident MRI finding in the elderly and an important contributor of vascular dementia (Hachinski et al, 1986; Schmidt et al, 2007). Moreover, a recent study suggested that leukoaraiosis-like WM lesions are caused by chronic hypoxia hypoperfusion (Fernando et al, 2006), which could also injure dendrites. Taken together, our results and the relevant literature raise an intriguing possibility that the decline of cognitive functions in leukoaraiosis-like vascular dementia may be in part caused by the ‘invisible' dendritic degeneration (and the consequential loss of synaptic inputs) instead of strict WM injury. If this hypothesis is correct, DTI may be a useful tool for early diagnosis of dendritic degeneration and vascular dementia in the future.
In conclusion, we have used an animal model to study the utility of anisotropic diffusion imaging in acute ischemic stroke. Although our findings with ex vivo imaging have to be confirmed by in vivo imaging, the results of our study suggest that cross-evaluation of multiple DTI parameters may provide a fuller picture of WM and dendritic injury in acute ischemic stroke.
Acknowledgments
The authors thank Drs Ton Degrauw, Joseph Broderick, Lawrence Wechsler, and Charles Dumoulin, the anonymous reviewers for critical reading of this manuscript, and Dr Steve Danzer for providing Thy1-YFP-H mice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the National Institute of Health grant NS 074559 and the Alzheimer's Association (to C-YK) and an American Heart Association fellowship (to DY).
Supplementary Material
References
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009;32:1639–1644. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Hussain MS, Stobbe RW, Butcher KS, Emery DJ, Shuaib A, Siddiqui MM, Maheshwari P, Al-Hussain F, Beaulieu C. Elevations of diffusion anisotropy are associated with hyper-acute stroke: a serial imaging study. Magn Reson Imaging. 2008;26:683–693. doi: 10.1016/j.mri.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci USA. 2010;107:14472–14477. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carano RA, Li F, Irie K, Helmer KG, Silva MD, Fisher M, Sotak CH. Multispectral analysis of the temporal evolution of cerebral ischemia in the rat brain. J Magn Reson Imaging. 2000;12:842–858. doi: 10.1002/1522-2586(200012)12:6<842::aid-jmri7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Dewar D, Yam P, McCulloch J. Drug development for stroke: importance of protecting cerebral white matter. Eur J Pharmacol. 1999;375:41–50. doi: 10.1016/s0014-2999(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G.2008The Mouse Brain in Stereotaxic Coordinates3rd edition.Academic Press: San Diego, CA [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–332. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Guilfoyle DN, Helpern JA, Lim KO. Diffusion tensor imaging in fixed brain tissue at 7.0 T. NMR Biomed. 2003;16:77–81. doi: 10.1002/nbm.814. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leukoaraiosis: an ancient term for a new problem. Can J Neurol Sci. 1986;13:533–534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- Irving EA, Yatsushiro K, McCulloch J, Dewar D. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: involvement of free radicals. J Cereb Blood Flow Metab. 1997;17:612–622. doi: 10.1097/00004647-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kassmann CM, Nave KA. Oligodendroglial impact on axonal function and survival – a hypothesis. Curr Opin Neurol. 2008;21:235–241. doi: 10.1097/WCO.0b013e328300c71f. [DOI] [PubMed] [Google Scholar]
- Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28:11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, D'Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, Pryor J, de Crespigny AJ. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–145. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- MacDonald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken E, Valeriani V, Simpson C, Jover T, McCulloch J, Dewar D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J Cereb Blood Flow Metab. 2000;20:1529–1536. doi: 10.1097/00004647-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA.1996Cerebral white matter is highly vulnerable to ischemia Stroke 271641–1646.discussion 1647 [DOI] [PubMed] [Google Scholar]
- Sakai K, Yamada K, Nagakane Y, Mori S, Nakagawa M, Nishimura T. Diffusion tensor imaging may help the determination of time at onset in cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2009;80:986–990. doi: 10.1136/jnnp.2008.163584. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–2625. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Song SK. Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med. 2003;50:743–748. doi: 10.1002/mrm.10605. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Liang HF, He YY, Schmidt RE, Hsu CY, Song SK. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn Reson Med. 2005;53:1447–1451. doi: 10.1002/mrm.20488. [DOI] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow. Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Stys PK, Ransom BR. Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Res. 1992;574:105–119. doi: 10.1016/0006-8993(92)90806-k. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Davis PK, Black JA, Ransom BR. Anoxic injury of mammalian central white matter: decreased susceptibility in myelin-deficient optic nerve. Ann Neurol. 1990;28:335–340. doi: 10.1002/ana.410280306. [DOI] [PubMed] [Google Scholar]
- Yang D, Nemkul N, Shereen A, Jone A, Dunn RS, Lawrence DA, Lindquist D, Kuan CY. Therapeutic administration of plasminogen activator inhibitor-1 prevents hypoxic-ischemic brain injury in newborns. J Neurosci. 2009;29:8669–8674. doi: 10.1523/JNEUROSCI.1117-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Tress BM, Barber PA, Desmond PM, Darby DG, Gerraty RP, Li T, Davis SM. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999;30:2382–2390. doi: 10.1161/01.str.30.11.2382. [DOI] [PubMed] [Google Scholar]
- Yu S, Iwatsuki H, Ichinohe N, Mori F, Shoumura K. In vivo perfusion Turnbull's reaction for Fe(II) histochemistry in non-anoxic/non-ischemic and anoxic/ischemic cat brains. Neurosci Lett. 2001;308:79–82. doi: 10.1016/s0304-3940(01)01944-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S, Calabresi PA. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci. 2009;29:3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci USA. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, van Zijl PC, Mori S. Three-dimensional diffusion tensor magnetic resonance microimaging of adult mouse brain and hippocampus. Neuroimage. 2002;15:892–901. doi: 10.1006/nimg.2001.1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.