Abstract
The mammalian central nervous system (CNS) is generally believed to be completely dependent on the presence of oxygen (O2) to maintain energy levels necessary for excitability. However, previous studies on CNS white matter (WM) have shown that a large subset of CNS-myelinated axons of mice aged 4 to 6 weeks remains excitable in the absence of O2. We investigated whether this surprising WM tolerance to anoxia varied with age. Acutely isolated mouse optic nerve (MON), a purely myelinated WM tract, was studied electrophysiologically. Excitability in the MONs from 1-month-, 4-month-, and 8-month-old mice was assessed quantitatively as the area under the supramaximal compound action potential (CAP). Anoxia-resistant WM function declined with age. After 60 minutes of anoxia, ∼23% of the CAP remained in 1-month-old mice, 8% in 4-month-old mice, and ∼0 in the 8-month-old group. Our results indicated that although some CNS axons function anaerobically in young adult animals, they lose this ability in later adulthood. This finding may help explain the clinical impression that favorable outcome after stroke and other brain injuries declines with age.
Keywords: anoxia, CNS axon, cyanide, energy metabolism, glycolysis
Introduction
The central nervous system (CNS) is generally believed to be a tissue which requires oxygen for its function and energy metabolism in adult animals. To our knowledge, this principle is unchallenged with regard to the gray matter (GM), those areas of the CNS that contain neuron cell bodies and synapses (Goldberg et al, 1987). The white matter (WM), which contains axons and glia, but no synapses, has a lower metabolic rate than does the GM (Clarke and Sokoloff, 1999), consuming only ∼50% as much O2 as the GM (Nishizaki et al, 1988). This lower metabolic demand in the WM may be a reason why a subset of axons in 1-month-old mice is completely resistant to anoxia and continues to function normally for extended periods of time without oxygen (Tekkok et al, 2003; Tekkok and Ransom, 2004). These axons, of course, must be able to generate sufficient ATP (adenosine triphosphate) from anaerobic glycolysis to maintain the steep ion gradients of Na+ and K+ necessary for action potential discharge.
Age is an important variable regarding metabolic function. Enzymes for the anaerobic catabolism of glucose are more highly expressed in the neonatal brain, whereas aerobic enzymes develop postnatally. As might be anticipated from these facts, neonatal mammals, compared with adults, are highly resistant to anoxia-induced injury (Duffy et al, 1975; Kabat, 1940). In addition, functional outcomes after comparable periods of ischemia or anoxia tend to be better in young animals. This principle has been substantiated by recent experimental work on the WM showing that advanced age increases vulnerability to experimental ischemia (i.e., oxygen and glucose deprivation) (Baltan et al, 2008).
The purpose of this study was to determine the effect of animal age on CNS axon function during anoxia. To date, anoxia effects on the WM have only been studied in young animals (i.e., 4-week-old rodents). We hypothesized that anaerobic function in the WM would decrease with animal age. To test this hypothesis, we measured axon excitability in optic nerves (a completely myelinated WM tract) from mice of various ages, in the presence and absence of oxygen (or before and after blocking oxidative metabolism). We found that these myelinated CNS axons gradually lost their ability to function in the absence of oxygen as a function of age. This was not an artifact of larger optic nerve diameter in the older nerves nor was it a consequence of inadequate glucose supply during anoxia. These experiments establish proof of principle that some CNS axons, at least the myelinated variety, lose their ability to function in the absence of oxygen by 8 months of age. The implications of this unanticipated change in the energy requirements for CNS axon excitability are not clear. At a minimum, this helps to explain why the CNS WM is more tolerant of anoxia during the first few months of life ((Fern et al, 1998; Tekkok et al, 2003) and (Hamner and Ransom, unpublished observations)).
Materials and methods
All mouse procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Washington.
The mouse optic nerves (MONs) were obtained from C57BL/6 mice or Swiss Webster mice of different ages: 1 (28 to 40 days), 4 (112 to 133 days), and ∼8 months (210 to 294 days) of age. The optic nerves were dissected free, placed in an interface perfusion chamber (Medical Systems, Greenvale, NY, USA), and superfused with artificial cerebrospinal fluid with the following composition (in mmol/L): 124 NaCl, 3.0 KCl, 2.0 CaCl2, 2.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 20 sucrose. In some experiments, glucose was increased to 30 mmol/L, deleting sucrose to maintain normal osmolarity. The chamber containing the nerves was continuously aerated by a humidified gas mixture of 95% O2/5% CO2, unless otherwise stated (see below), whereas all artificial cerebrospinal fluid solutions supplied to the chamber were continuously bubbled with 95% N2/5% CO2. In addition, the gas flow rate in the chamber was set at a level which ensured that the tissue would only be under the influence of the chamber gas (i.e., neither ambient air nor dissolved oxygen in the solutions had an appreciable effect on chamber gas composition). All experiments were performed at 37°C, unless otherwise noted.
Two sets of suction electrodes were placed in the bath to allow recording from two optic nerves at the same time. Suction electrodes were back-filled with artificial cerebrospinal fluid. The stimulating electrode was attached to the rostral end of the nerve, and the recording electrode was attached to the caudal end of the nerve to record the compound action potential (CAP), ensuring orthodromic stimulation. Each pair of electrodes had its own pulse generator (Isostim 520; WPI, Sarasota, FL, USA) and Axoprobe 1-A amplifier (Axon Instruments, Union City, CA, USA). The recorded signal was amplified 50 times, filtered at 30 kHz (Standford Research Systems, Model SR 560, Sunnyvale, CA, USA), and acquired at 20 to 30 kHz. The stimulus pulse (30-μsecond duration) strength was adjusted to evoke the maximum CAP possible, and then increased by 25% to ensure supramaximal stimulation. During experiments, the supramaximal CAP was elicited every 30 seconds. The MONs were allowed to equilibrate for 30 to 60 minutes in the chamber in normal artificial cerebrospinal fluid to establish a stable baseline for the supramaximal CAP.
Anoxia was induced by switching the chamber gas mixture from 95% O2/5% CO2 to 95% N2/5% CO2. The standard period of anoxia was 60 minutes. After anoxia, O2 was restored and CAPs were recorded for up to 3 hours (reperfusion period). Chemical anoxia was induced by applying a bath solution containing 2 mmol/L sodium cyanide (CN) for 60 minutes (Leppanen and Stys, 1997; Malek et al, 2005; Tekkok et al, 2003), followed by a recovery in normal bath solution. Some of the chemical anoxia experiments were performed at room temperature (25°C) as noted in the text.
Optic nerve diameter was measured using a calibrated hemocytometer (Olympus, Tokyo, Japan). The nerves were gently ‘blotted' using an absorbent tissue to remove moisture, placed on the hemocytometer, and the diameter was read using a stereomicroscope.
Optic nerve function was quantitatively estimated by integrating the area under the CAP. Compound action potential recordings were normalized to the baseline CAP area, which was determined by averaging the CAP area for 15 minutes under control conditions; this value was set at 1.0. As such, the normalized CAP area at any time is roughly proportional to the number of functioning axons (Buchtal, 1966; Cummins et al, 1979). Changes in the CAP area, of course, cannot be precisely related to changes in the number of functioning axons because of the fact that different classes of axons may contribute differentially to the currents constituting the CAP (Buchtal, 1966; Cummins et al, 1979). In general, however, at a baseline value of 1.0, ∼100% of the viable axons are functioning. If the normalized CAP decreases to 0.5, roughly half of the baseline axons are now functioning. For statistical comparisons, normalized data from several optic nerves under the same conditions were averaged together. All data points are presented as the average normalized CAP±s.e.m. The anaerobic capacity of the optic nerve was defined as normalized CAP after 60 minutes of continuous anoxia. Statistical significance was determined by unpaired two-tailed Student's t-test. P-values <0.05 were considered significant.
Results
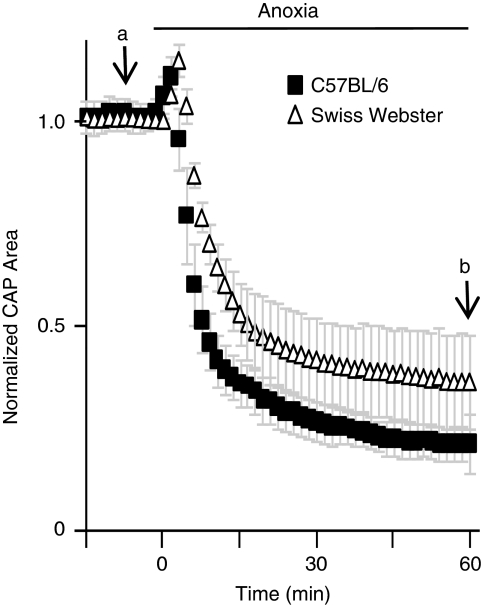
Earlier studies on the effects of anoxia on WM function have used a wide variety of mouse and rat strains, thereby hindering a direct comparison of results. To better align our results with genomic and aging-related studies, we performed our experiments primarily on C57BL/6 mice, the mouse strain with the most reference data available (Jackson, 2009; NIA, 2010). Swiss Webster mice (1-month of age) were used in the previous study showing that some MON axons were resistant to anoxia (Tekkok et al, 2003). To validate that anaerobic capacity was not strain specific, we compared the effect of anoxia on optic nerve function in 1-month-old C57BL/6 and Swiss Webster mice. At the onset of anoxia, the CAP area increased briefly because of a transient decrease in extracellular space volume (Brown et al, 2001), and then rapidly declined for ∼10 minutes. After 30 to 45 minutes of anoxia, the normalized CAP area achieved a new steady state between 0.25 and 0.30 of the original CAP area (Figure 1). After 60 minutes of anoxia, there was no statistical difference between the normalized CAP areas of the two mouse strains (C57BL/6: 23.0%±4.07% and Swiss Webster: 34.95%±11.3%, P=0.054), although a trend existed suggesting that the Swiss Webster strain had a greater percentage of axons with anaerobic capacity. In both mouse strains, some optic nerve axons remained excitable in the complete absence of oxygen.
Figure 1.
Anaerobic capacity of the MON from 1-month-old mice is similar between Swiss Webster and C57BL/6 strains. Anaerobic activity, measured as a function of normalized CAP after 60 minutes of anoxia (b), is approximately 34.95%±11.3% in Swiss Webster MONs (N=5) versus 23.0%±4.07% in C57BL/6 (N=7), compared with baseline (a). Differences in anaerobic function were not significantly different (P=0.054). CAP, compound action potential; MON, mouse optic nerve.
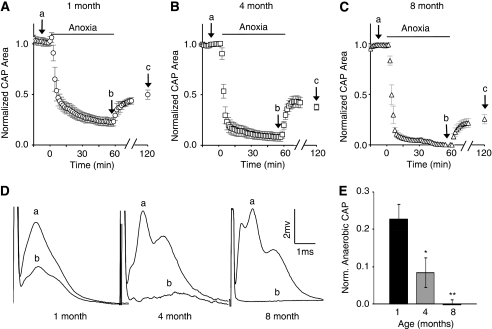
To test whether animal age affects axon capacity for anaerobic function, we measured the CAP area during anoxia in the MONs from 4-month- and 8-month-old mice. Anoxia-resistant WM function declined with age. The percentage of axons functioning during anoxia, approximated by change in the CAP area, decreased to 8% in 4-month-old mice, and was ∼0 in the 8-month-old group (Figures 2A to 2D). The remaining extent of CAP function after 60 minutes of anoxia is shown as a function of age in Figure 2E.
Figure 2.
Axons lose their ability to function anaerobically with age. (A) In the MONs, from 1-month-old mice, 23.0%±4.07% (n=7) of the CAP remains after 60 minutes of anoxia. The CAP recovered to 48.2%±4.81% after 60 minutes of reoxygenation. It must be noted that the points, labeled a, b, and c, refer to control CAP, anaerobic CAP, and CAP recovery levels, respectively (see D below and text for discussion). (B) In the MONs from 4-month-old mice, 8.48%±3.94% (n=5; P=0.02 versus 1-month-old) of the CAP remains during anoxia, and (C) in 8-month-old mice, no significant anaerobic function was found (n=8, 0.01%±0.01% P<0.00001 versus 1-month-old mice and P=0.04 versus 4-month-old mice). After anoxia, CAP recovered to 37.7%±3.03% and 30.8%±3.97% in 4-month- and 8-month-old animals, respectively. (Panel D) Representative CAPs from 1-month-, 4-month-, and 8-month-old animals are shown at baseline (a) and after 60 minutes of anoxia (b). (E) A bar graph showing anaerobic CAP function (i.e., after 60 minutes of anoxia, corresponding to point ‘b') as a function of age, normalized to baseline. Anaerobic function at 1 month compared with that at 4 and 8 months: *P<0.05 and **P<0.00001. CAP, compound action potential; MON, mouse optic nerve; Norm., normalized.
As shown in Figures 2A to 2C (time point ‘c'), CAP recovery after anoxia varied with animal age. Recovery was measured at 60 minutes after the end of anoxia because it reached a maximum value at that point (Stys et al, 1991). Compound action potential recovery was 48.4%±6.0%, 36.8%±5.0%, and 28.8%±5.5% of baseline in 1-month-, 4-month-, and 8-month-old animals, respectively. Compound action potential recoveries from 4-month- and 8-month-old animals were significantly less than recovery in 1-month-old animals (P<0.001 and P<0.005, respectively). Therefore, the extent of CAP recovery was directly correlated with that of anaerobic capacity, and like anaerobic capacity, this declined with age.
Compound action potentials in mature rodent optic nerves are typically composed of three peaks (Stys et al, 1991). After 60 minutes of anoxia, the CAP area was reduced (roughly proportional to the number of axons that are no longer excitable (Stys et al, 1991)) and only the MON from 1-month-old mice maintained the typical three peaks (Figure 2D). Anaerobic CAPs from 4-month-old mice appeared to have only one peak, whereas there was little or no anaerobic CAPs measured in the MONs from 8-month-old mice.
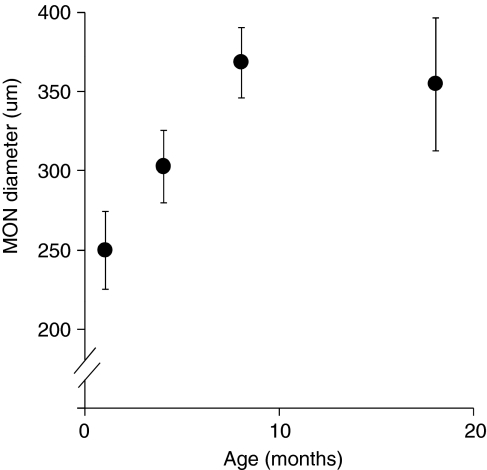
Previously, we found that the larger size of the rat optic nerve, compared with the size of the MON, can significantly reduce the diffusion of glucose (Tekkok et al, 2003). To assess whether changes in MON diameter could contribute to age-related changes in anoxia-induced CAP decrease, we measured MON diameter in each of the three age groups tested with anoxic exposure, and at 18 months of age. The diameter of the optic nerve increased significantly with age (250±24.8 μm, 303±22.9 μm (P=1.6 × 10−4 versus 1-month-old mice) and 369±22.2 μm (P=4.48 × 10−5 versus 4-month-old mice) for 1-month-, 4-month-, and 8-month-old mice, respectively; Figure 3). In other words, the diameter of the optic nerves from 8-month-old animals was ∼48% greater than that of 1-month-old animals. There was no significant difference in optic nerve diameter between 8-month- and 18-month-old animals (Figure 3).
Figure 3.
MON diameter increases with age. On average, the MON diameter increases to 48% from 1-month- to 8-month-old mice. There is no statistical increase in size after 8 months of age. MON, mouse optic nerve.
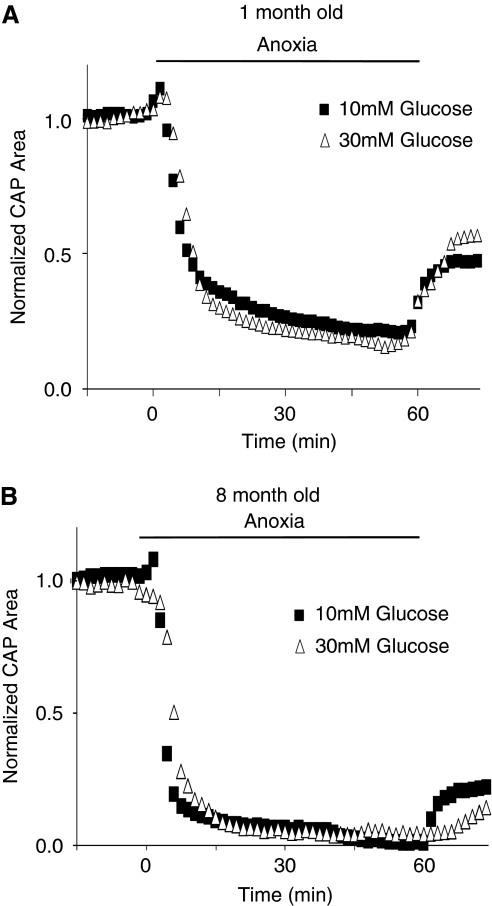
Experiments were designed to consider the possibility that optic nerve diameter might contribute to age-related differences in response to anoxia by affecting the diffusion of glucose to axons of the optic nerve. We reasoned that if glucose diffusion became rate limiting for sustaining ATP levels during anoxia in older nerves with greater diameters, this could be overcome by increasing the bath concentration of glucose (Tekkok et al, 2003). However, the decrease in the CAP area during anoxia was not altered when carried out in 30 mmol/L rather than in 10 mmol/L glucose, in the MONs from 1-month- or 8-month-old mice (Figure 4). These results imply that limited glucose diffusion is not the explanation for the increased vulnerability of older MONs to loss of excitability during anoxia.
Figure 4.
Increased glucose concentration does not affect anaerobic CAP amplitude in the optic nerves from 1-month- or 8-month-old animals. MON function, measured as area under the CAP, is not statistically different after 60 minutes of anoxia regardless of glucose concentration (i.e., 10 mm or 30 mmol/L) in (A) 1-month-old and (B) 8-month-old animals. Traces represent the average area under the CAP (N=5 to 7 for 1-month-old animals, and N=6 to 8 for 8-month-old animals). CAP, compound action potential; MON, mouse optic nerve.
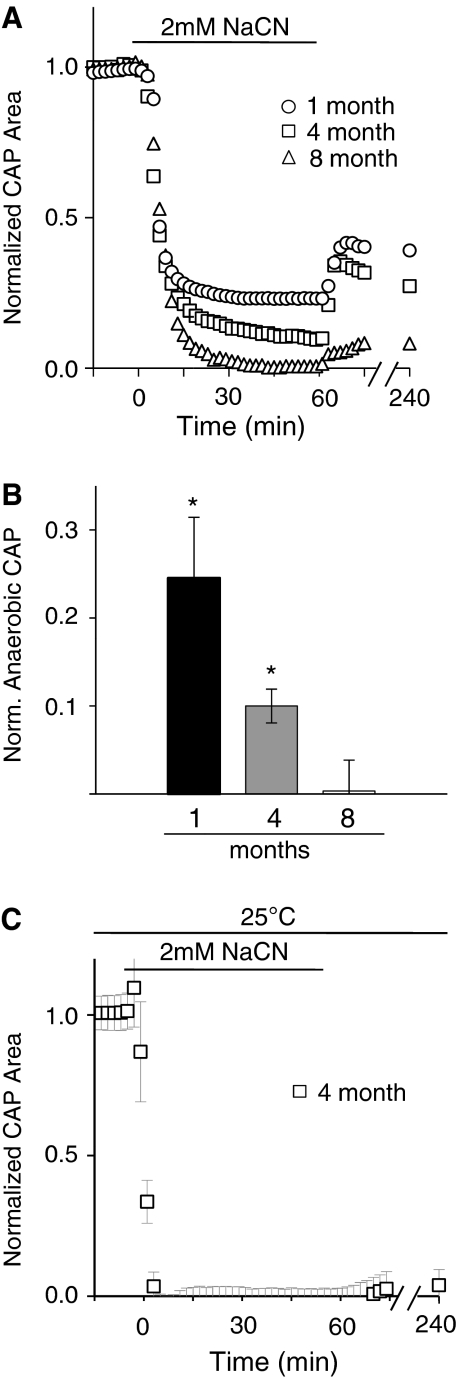
To strengthen and confirm our findings on MON excitability during anoxia, a second protocol was used to achieve a state of anaerobic metabolism in the MONs. In particular, we used CN to create a state of ‘chemical anoxia', a strategy that has been used successfully by other investigators (Leppanen and Stys, 1997; Malek et al, 2005; Tekkok et al, 2003). Sodium cyanide completely blocks all aerobic energy metabolisms by irreversibly binding to complex IV in the electron transport chain, preventing oxidative phosphorylation. Sodium cyanide (2 mmol/L) was added to the superfusate containing 10 mmol/L glucose for 60 minutes. Chemical anoxia caused age-related decreases in the CAP area that were similar in time course and magnitude to those seen with anoxia (Figures 5A and 5B; cf. Figures 2A to 2C). After 60 minutes of chemical anoxia, the CAP area decreased to normalized values of 24.5%±6.91%, 10.0%±1.92%, and 0.01%±2.29%, for 1-month-, 4-month-, and 8-month-old animals, respectively. The 8-month value was significantly less than the 1-month value (P=0.003), and significantly less than the 4-month value (P=0.002).
Figure 5.
Chemical anoxia confirms loss of anaerobic activity with age. (A, B) The amount of anaerobic capacity after 60 minutes of 2 mmol/L NaCN is similar to that seen after 60 minutes of anoxia for the nerves of different ages (compare with Figure 2E). Anaerobic CAP after 60 minutes of anoxia is 24.5%±6.91%, 10.0%±1.92%, and 0.01%±2.29% for 1 month, 4 months, and 8 months, respectively. *P<0.005 vs. 8 month old. (C) At room temperature, there is no significant CAP recovery after 60 minutes of 2 mmol/L NaCN (0.048%±0.56% cf. to panel A; see text). CAP, compound action potential.
We observed modest recovery of function after CN was removed from the bath, especially in 1-month- and 4-month-old animals (Figure 5A; function remained stable for at least 3 hours). This is surprising because CN is generally believed to be an irreversible blocker of oxidative phosphorylation. However, it is known that the naturally occurring enzyme rhodanese can metabolize and inactivate CN in the brain and other tissues (Llenado and Rechnitz, 1972). To determine whether the enzymatic removal of CN through rhodanese could account for CAP recovery after CN exposure, these experiments were repeated at room temperature. Rhodanese has greatly diminished enzymatic capacity at room temperature compared with normal physiologic temperature, i.e., 37°C (Llenado and Rechnitz, 1972). Therefore, at 25°C, the rate of CN inactivation should be slowed and its effect more persistent. At 25°C, CAP decreased slightly faster at the onset of anoxia compared with those experiments performed at 37°C. After removal of CN from the perfusate, no statistically significant recovery occurred (Figure 5C; function remained stable for at least 2 hours), suggesting that enzyme removal of CN may be behind the partial CAP recovery seen after CN removal at 37°C.
Discussion
This study showed that the ability of the mammalian WM to function anaerobically (i.e., remain excitable) decreased and finally vanished with advancing age. White matter anaerobic function was measured over an age range of 1 month to 8 months, and was tested using two different anoxia-producing conditions: O2 deprivation and cyanide application. We estimated that ∼23% of MON (C57BL/6) axons remained functional during anoxia in 1-month-old animals, in agreement with previous studies (Tekkok et al, 2003; Tekkok and Ransom, 2004). We eliminated the remote possibility that this was a strain-dependent phenomenon by examining both Swiss Webster and C57BL/6 mice; we found no statistical difference between these strains in terms of their capacities for anaerobic function. Anaerobic function was significantly diminished by 4 months of age and was completely lost by 8 months of age (Figure 2). These results provide further insights into the link between animal age and vulnerability to anoxic, or ischemic, WM injury, specifically, the well-known tendency for young animals to have less brain injury from brief periods of anoxia compared with their adult counterparts (Duffy et al, 1975; Kabat, 1940). In fact, the extent of irreversible injury was clearly greater in older animals with less anaerobic capacity (i.e., Figures 2A to 2C). Gray matter function, as assessed by synaptic transmission, shows little if any anaerobic tolerance in the age range studied herein (Tekkok and Ransom, 2004), thus suggesting that it is the age-related change in WM anoxic vulnerability that underlies better neurologic outcomes after anoxia in younger mammals, including humans.
It is essential to point out that the GM does not exhibit any significant anaerobic capability in young adult animals (Goldberg et al, 1987). This emphasizes a crucial difference between synaptic and nonsynaptic areas of the mammalian CNS, and explains why the WM, but not the GM, showed substantial recovery after brief periods of anoxia or ischemia (e.g., Tekkok and Ransom, 2004). Current and earlier studies have focused on two WM areas, the optic nerve and the corpus callosum. It will be important to determine whether other WM and GM areas behave in a similar manner. This cannot be assumed. Earlier studies have shown, for example, that different WM tracts that vary in their percentage of myelinated axons (i.e., the corpus callosum and the optic nerve) behave somewhat differently with regard to ischemia (cf. Tekkok et al (2003) and Tekkok and Goldberg (2001)). The time course of functional recovery from ischemia is multiphasic in the corpus callosum, but is monophasic in the optic nerve.
The mechanism(s) underlying our observed age-related decline in WM anaerobic function is not known. This result was not caused by an increase in optic nerve diameter, which increases substantially with age and can act as a diffusion barrier for glucose (Tekkok et al, 2003). The smaller optic nerve diameters in the mouse (<370 μmol/L), compared with those in the rat (>500 μmol/L), did not pose a functionally significant diffusion barrier. An obvious hypothesis for age-related decline in anaerobic function is changes in axon glucose metabolism. Perhaps the glycolytic metabolic rate is downregulated while aerobic ATP production becomes more efficient. In fact, PFK1 (phosphofructokinase-1), the rate-limiting enzyme in glycolysis, switches from the L-M to M-C isozyme form during aging resulting in a 20% loss in activity (Dunaway and Kasten, 1988). The decrease in activity plateaus ∼12 months of age (Kasten et al, 1993). In addition, the regulatory sugar, fructose-2,6-P2, which allosterically activates PFK1, decreases with age in the brain and may also contribute to deceased glycolytic activity (Kasten and Dunaway, 1993). The rate of glucose phosphorylation by hexokinase could also affect glycolytic rate, but activity of this enzyme actually increases in the brain during postnatal development (Beitner et al, 1982; Cimino et al, 1994; Leong et al, 1981).
All axons in the MONs are myelinated and their capacity to conduct action potentials depends on the integrity of their myelin. Anoxia is associated with retraction of the perinodal oligodendrocytic loops that might lead to electrical shunting and to failure of saltatory conduction (Waxman et al, 1994). Therefore, it is also possible that age-related changes in the anaerobic capacity of oligodendrocytes might have contributed to our results. Unfortunately, little information is available on this question.
Conceivably, axons with anaerobic capacity constitute a unique population within the optic nerves of young mice. If true, selective loss of this population in 8-month-old animals could explain our finding of decreased anaerobic function with age. We are unaware of any study that directly measures total axon number as a function of age in the MONs. The number of axons in rat optic nerve decreases in very old animals but is relatively stable between 3 and 12 months of age, showing only a 3% decline (Cavallotti et al, 2003). To the extent that these rat data are relevant to the mouse, the decline in optic nerve number seems too small to account for our findings.
In addition to removing oxygen directly, we also induced ‘chemical anoxia' using CN (Figure 5). Cyanide and similar drugs halt oxidative phosphorylation and have been used previously to study the effects of anoxia on WM function (Leppanen and Stys, 1997; Tekkok et al, 2003). Our finding that some recovery occurred after cyanide treatment was surprising in light of the fact that cyanide irreversibly binds to the fourth complex in the electron transport chain. We postulated that this recovery was most likely caused by the enzyme rhodanese, which removes CN from the electron transport chain by converting it to thiocyanate. Rhodanese is a mitochondrial enzyme that has been shown to be present in the brain (Wrobel et al, 1996). Like many enzymes, rhodanese activity is highly temperature dependent and is far less active at temperatures below the physiologic range (Llenado and Rechnitz, 1972). We tested this interpretation by carrying out the chemical anoxia experiment at room temperature (25°C). We reasoned that at room temperature, rhodanese would be relatively inactive and thus unable to catalyze CN removal. Our results supported this hypothesis, as statistically significant recovery failed to occur after CN removal at room temperature (Figure 5C).
Acknowledgments
The authors thank Kass Klemz for her help in editing this manuscript.
The authors declare no conflict of interest.
Footnotes
This study was supported by the University of Washington Neurosurgery Training Grant, NIH NS15589 (BRR), and the Sidney Gift Fund.
References
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner R, Klein S, Nordenberg J. The participation of glucose-1,6-diphosphate in the regulation of hexokinase and phosphoglucomutase activities in brains of young and adult rats. Int J Biochem. 1982;14:195–199. doi: 10.1016/0020-711x(82)90138-0. [DOI] [PubMed] [Google Scholar]
- Brown AM, Westenbroek RE, Catterall WA, Ransom BR. Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J Neurophysiol. 2001;85:900–911. doi: 10.1152/jn.2001.85.2.900. [DOI] [PubMed] [Google Scholar]
- Buchtal F, Rosenfalck A. Evoked action potentials and conduction velocity in human sensory nerves. Brain Res. 1966;3:1–122. [Google Scholar]
- Cavallotti C, Cavallotti D, Pescosolido N, Pacella E. Age-related changes in rat optic nerve: morphological studies. Anat Histol Embryol. 2003;32:12–16. doi: 10.1046/j.1439-0264.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- Cimino M, Marini P, Colombo S, Cattabeni F, Bianchi M, Magnani M. Localization and age-dependent expression of hexokinase mRNA in the rat brain. Brain Res Mol Brain Res. 1994;25:1–6. doi: 10.1016/0169-328x(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L.1999Circulation and energy metabolism of the brain Basic Neurochemistry: Molecular Cellular and Medical Aspects6th ed. (Siegel GS, Agranoff B, eds),Philadelphia, PA: Lippincott-Raven Pubs.637–669. [Google Scholar]
- Cummins KL, Perkel DH, Dorfman LJ. Nerve fiber conduction-velocity distributions. I. Estimation based on the single-fiber and compound action potentials. Electroencephalogr Clin Neurophysiol. 1979;46:634–646. doi: 10.1016/0013-4694(79)90101-9. [DOI] [PubMed] [Google Scholar]
- Duffy TE, Kohle SJ, Vannucci RC. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1975;24:271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Dunaway GA, Kasten TP. Physiological implications of the alteration of 6-phosphofructo-1-kinase isozyme pools during brain development and aging. Brain Res. 1988;456:310–316. doi: 10.1016/0006-8993(88)90233-8. [DOI] [PubMed] [Google Scholar]
- Fern R, Davis P, Waxman SG, Ransom BR. Axon conduction and survival in CNS white matter during energy deprivation: a developmental study. J Neurophysiol. 1998;79:95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Weiss JH, Pham PC, Choi DW. N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J Pharmacol Exp Ther. 1987;243:784–791. [PubMed] [Google Scholar]
- Jackson L.C57BL/6J (JAX Mice database). http://jaxmice.jax.org/strain/000664.html , 2009
- Kabat H. The greater resistance of very young animals to arrest of the brain circulation. Am J Physiol. 1940;130:588–599. [Google Scholar]
- Kasten TP, Dunaway GA. Fructose 2,6-bisphosphate: changes during neonatal maturation and aging of rat and potential role in regulation of glucose utilization. Mech Ageing Dev. 1993;68:37–45. doi: 10.1016/0047-6374(93)90138-h. [DOI] [PubMed] [Google Scholar]
- Kasten TP, Mhaskar Y, Dunaway GA. Regulation of brain 6-phosphofructo-1-kinase: effects of aging, fructose-2,6-bisphosphate, and regional subunit distribution. Mol Cell Biochem. 1993;120:61–68. doi: 10.1007/BF00925985. [DOI] [PubMed] [Google Scholar]
- Leong SF, Lai JC, Lim L, Clark JB. Energy-metabolizing enzymes in brain regions of adult and aging rats. J Neurochem. 1981;37:1548–1556. doi: 10.1111/j.1471-4159.1981.tb06326.x. [DOI] [PubMed] [Google Scholar]
- Leppanen L, Stys PK. Ion transport and membrane potential in CNS myelinated axons. II. Effects of metabolic inhibition. J Neurophysiol. 1997;78:2095–2107. doi: 10.1152/jn.1997.78.4.2095. [DOI] [PubMed] [Google Scholar]
- Llenado RA, Rechnitz GA. Rhodanese enzyme determination using ion-selective membrane electrodes. Anal Chem. 1972;44:1366–1370. doi: 10.1021/ac60316a055. [DOI] [PubMed] [Google Scholar]
- Malek SA, Adorante JS, Stys PK. Differential effects of Na-K-ATPase pump inhibition, chemical anoxia, and glycolytic blockade on membrane potential of rat optic nerve. Brain Res. 2005;1037:171–179. doi: 10.1016/j.brainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- NIA Aged Rodent Colonies Handbook . http://www.nia.nih.gov/ResearchInformation/ ScientificResources/AgedRodentColoniesHandbook/ , 2010
- Nishizaki T, Yamauchi R, Tanimoto M, Okada Y. Effects of temperature on the oxygen consumption in thin slices from different brain regions. Neurosci Lett. 1988;86:301–305. doi: 10.1016/0304-3940(88)90500-9. [DOI] [PubMed] [Google Scholar]
- Stys PK, Ransom BR, Waxman SG. Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Res. 1991;546:18–32. doi: 10.1016/0006-8993(91)91154-s. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Ransom BR. Axon function persists during anoxia in mammalian white matter. J Cereb Blood Flow Metab. 2003;23:1340–1347. doi: 10.1097/01.WCB.0000091763.61714.B7. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Ransom BR. Anoxia effects on CNS function and survival: regional differences. Neurochem Res. 2004;29:2163–2169. doi: 10.1007/s11064-004-6890-0. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Ransom BR, Stys PK. Anoxic injury of rat optic nerve: ultrastructural evidence for coupling between Na+ influx and Ca(2+)-mediated injury in myelinated CNS axons. Brain Res. 1994;644:197–204. doi: 10.1016/0006-8993(94)91680-2. [DOI] [PubMed] [Google Scholar]
- Wrobel M, Wlodek L, Srebro Z. Sulfurtransferases activity and the level of low-molecular-weight thiols and sulfane sulfur compounds in cortex and brain stem of mouse. Neurobiology (Bp) 1996;4:217–222. [PubMed] [Google Scholar]