Abstract
Distortion-product otoacoustic emissions (DPOAEs) were used to describe suppression growth in normal-hearing humans. Data were collected at eight f2 frequencies ranging from 0.5 to 8 kHz for L2 levels ranging from 10 to 60 dB sensation level. For each f2 and L2 combination, suppression was measured for nine or eleven suppressor frequencies (f3) whose levels varied from −20 to 85 dB sound pressure level (SPL). Suppression grew nearly linearly when f3 ≈ f2, grew more rapidly for f3 < f2, and grew more slowly for f3 > f2. These results are consistent with physiological and mechanical data from lower animals, as well as previous DPOAE data from humans, although no previous DPOAE study has described suppression growth for as wide a range of frequencies and levels. These trends were evident for all f2 and L2 combinations; however, some exceptions were noted. Specifically, suppression growth rate was less steep as a function of f3 for f2 frequencies ≤1 kHz. Thus, despite the qualitative similarities across frequency, there were quantitative differences related to f2, suggesting that there may be subtle differences in suppression for frequencies above 1 kHz compared to frequencies below 1 kHz.
INTRODUCTION
The purpose of this study was to provide a description of the growth of suppression in humans with normal hearing, based on measurements of distortion-product otoacoustic emissions (DPOAEs). Suppression growth is described for probe frequencies (f2) ranging from 0.5 to 8 kHz (in roughly 1∕2 octave steps) and for levels (L2) ranging from 10 or 20 to 50 or 60 dB sensation level (SL) relative to behavioral threshold for each subject at f2. The data presented in this paper provide a relatively complete description of the growth of DPOAE suppression in humans with normal hearing.
Suppression effects have been documented in both the mechanical responses of the basilar membrane (e.g., Rhode, 1977; Ruggero et al., 1992; Cooper and Rhode, 1996; Rhode and Cooper, 1993) and in the responses of auditory-nerve fibers (e.g., Sachs and Kiang, 1968; Abbas and Sachs, 1976; Ruggero et al., 1992; Delgutte, 1990; Pang and Guinan, 1997). These studies have increased our understanding of the nonlinear response properties of the auditory periphery in mammals. There are some general observations that can be made, based on these mechanical and electrophysiological data. One consistent observation is that suppression-growth rate depends on the relationship between suppressor frequency and characteristic frequency (CF), where CF refers either to the frequency to which a single auditory-nerve fiber has its lowest threshold or the frequency for which a specific place along the basilar membrane is most sensitive. Suppressor tones below CF by an octave or more have higher suppression thresholds, compared to suppressor tones close to CF. However, once suppression threshold is exceeded, suppressive effects grow more rapidly for suppressors lower in frequency than CF. In contrast, suppressor tones higher in frequency relative to CF also have higher suppression thresholds (depending on how much their frequency exceeds CF), but the suppressive effect grows slowly as suppressor level is increased. To a large extent, this is true regardless of whether suppression estimates are based on measurements of the basilar-membrane response or based on measurements of rate suppression in auditory-nerve fibers. These and other data have led to the view that suppression effects in peripheral-response properties reflect mechanical suppression (Ruggero et al., 1992). The combination of mechanical and single-unit measurements provides an extensive description of suppression in lower animals.
While it is reasonable to assume that similar patterns will be observed among mammals, including humans, direct description of suppressive effects in humans has potential value beyond what has been learned from studies in lower animals. At the least, a demonstration of similar effects would provide support for the use of lower animals as models of auditory nonlinear function in humans with both normal and impaired hearing. Furthermore, a comprehensive description of suppression growth in humans would extend our knowledge of suppressive effects in relation to both the frequency and level of the stimulus whose response is being suppressed. Finally, measurements of suppression growth could be used to provide an indirect estimate related to auditory response growth in humans. This description may be useful when these measurements are applied to humans, for whom abnormal response growth is a common sequela to cochlear damage underlying hearing loss. Estimates of response growth would be important in the treatment of hearing loss if it can be shown that these measurements can be made objectively, without a voluntary response from the subject, and predict changes in behavioral response growth due to cochlear damage. DPOAE measurements meet the first of these two requirements and work is currently under way to determine the extent to which they can be used to predict behavioral response growth. Objective measures of response growth are important because current signal-processing strategies to ameliorate the effects of hearing loss are limited to options that compensate for threshold elevation and for the reduced dynamic range that typically accompanies cochlear damage affecting outer hair cells, which are thought to be the generators of DPOAEs. Other than frequency compression or frequency transposition, there are no signal-processing options that attempt to address changes to frequency or temporal resolution. It should be noted, however, that even if DPOAE suppression measurements can be used to predict response growth in cases of hearing loss, it will be limited to patients with mild-to-moderate hearing loss, given the dynamic range of DPOAE measurements.
Suppression based on DPOAE measurements has been described in several studies involving both humans and lower animals (e.g., Abdala, 1998, 2001, 2004, 2005; Brown and Kemp, 1984; Gorga et al., 2002, 2008; Martin et al., 1987, 1998a, 1998b; Mills, 1998; Pienkowski and Kunov, 2001). In these and other DPOAE suppression experiments, DPOAEs are elicited by a pair of primary tones (f2, f1; f2∕f1 ≈ 1.2), whose levels are held constant while a third, suppressor tone (f3) is presented. The suppressive effect of f3 is defined as the amount by which its presence reduces the DPOAE level in response to the primary-tone pair. By varying both the frequency and the level of f3, information about the influence of the frequency relation between suppressor tone and primary tone (primarily f2) on the amount of suppression has been obtained. As a general rule, DPOAE suppression grows more rapidly for suppressor frequencies lower than f2, compared to the suppressors close to or slightly above f2. This pattern is reminiscent of the pattern that has been observed in mechanical and neural responses from lower animals.
Beyond being applicable with humans, DPOAE measurements have the added advantage that they do not require a voluntary response from subjects. This feature has been exploited in a series of studies in which DPOAE suppression measurements were made in infants and young children in efforts to describe developmental changes in peripheral auditory function (e.g., Abdala, 1998, 2001, 2003, 2004; Abdala and Chatterjee, 2003; Abdala et al., 1996).
Within individual studies of DPOAE suppression, measurements are typically restricted to a relatively small number of f2 frequencies and to a limited range of L2 levels. Rarely are data provided for more than two or three f2 frequencies and two to three L2 levels. In most cases, stimulus levels below 40 dB sound pressure level (SPL) have not been explored. However, DPOAE suppression data at low stimulus levels are of interest, since evidence suggests that DPOAE estimates of cochlear gain are greatest for low-level stimuli (Gorga et al., 2003, 2008).
Several factors may have contributed to the choice of stimulus conditions that have been explored previously, especially in humans. First, it is unreasonable to expect humans to participate in individual data-collection sessions lasting more than about 2 h. As a consequence, multiple sessions may be needed to collect data for a wide range of L2 levels, even when only one f2 is being tested. The number of sessions would increase further if data were to be collected for several f2 frequencies and for a wide range of L2 levels. It is often not possible for subjects to commit to many data-collection sessions, making it difficult to acquire data for a large combination of frequencies and levels.
A second factor relates to the influence of f2 and L2 on the reliability of the measurements [defined by the signal-to-noise ratio (SNR) in the absence of a suppressor tone]. As a general rule, noise level increases as f2 decreases because the primary sources of noise in DPOAE measurements are subject breathing and movement, which produce noise primarily at lower frequencies. This problem is compounded during DPOAE measurements because the largest distortion product in mammals is observed at a frequency equivalent to 2f1–f2, the frequency on which most DPOAE measurements are focused. However, 2f1–f2 occurs at a frequency that is about 1∕2 octave below f2 (the frequency about which predictions of cochlear function are being made), which results in a decrease in SNR for mid and low f2 frequencies due to the dependence of noise level on frequency. Noise characteristics, no doubt, have limited the extent to which low f2 frequencies have been used as stimuli during DPOAE suppression studies.
In addition, low-level stimuli produce smaller responses compared to high-level stimuli, even for high f2 frequencies, for which noise levels typically are low. Assuming the noise level remains constant regardless of stimulus level, the use of low-level stimuli results in a smaller SNR (regardless of f2), thus reducing the reliability of the measurements. The effect of suppressor tones is to further reduce the level of the response, thus reducing the SNR by decreasing the difference between DPOAE and noise levels, and making it even more difficult to reliably measure changes in response level. Indeed, it is noteworthy that Abdala and colleagues have been able to describe DPOAE suppression in neonates and young infants because these subjects have noise levels that are typically higher than those encountered when testing cooperative adults. In any case, it is for all of the above reasons that the stimulus conditions for which DPOAE suppression has been explored in humans include mostly higher f2 frequencies and no lower than moderate-level primaries.
The purpose of the present study is to describe the growth of DPOAE suppression in humans for a wide range of suppressor frequencies (f3) at each of eight f2 frequencies (0.5–8 kHz) whose levels ranged from just above behavioral threshold (10 or 20 dB SL relative to behavioral threshold at f2) to moderate stimulus levels (50 or 60 dB SL). Techniques are used that enable reliable DPOAE measurements for conditions that have not been studied previously, for the reasons described above. These data not only provide a description of suppression growth for a wide range of frequencies and levels in humans with normal hearing, but they may also provide a data set to which results from patients with mild-to-moderate hearing loss can be compared.
METHODS
Subjects
A total of 63 subjects (23 males, 40 females) participated in this study, although no subject participated in data collection at all f2 frequencies. Subjects ranged in age from 15 to 55 yr, with a mean age of 26.5 yr. Each subject had normal hearing, defined as pure-tone thresholds of 10 dB HL or better (ANSI, 2004), for standard octave and inter-octave frequencies from 0.25 to 8 kHz. This audiometric-inclusion criterion was selected in efforts to maximize the range of L2 levels over which reliable DPOAE responses could be measured in unsuppressed conditions, particularly low-level conditions. In addition, all subjects had normal 226-Hz tympanograms on each day on which DPOAE data were collected. This criterion was included as a gross measure aimed at assuring that middle-ear function was normal.
In the present experiment, data were collected at the six f2 frequencies of 1, 1.4, 2, 2.8, 5.6, and 8 kHz. Ideally, it would have been preferable to collect data at all f2 frequencies in each subject. Unfortunately, it was not possible for any subject to participate long enough for data collection at all combinations of probe frequency (f2) and level (L2). As a result, subjects were enrolled in data collection for a subset of f2 frequencies, although, once enrolled, data were collected at all L2 levels for the f2 frequency being tested with one exception. As will be described subsequently, data for f2 frequencies of 0.5 and 4 kHz were taken from a previous study (Gorga et al., 2008).1 Because no data were collected for L2 levels of 60 dB SL as part of that study, data for f2 = 4 kHz and L2 = 60 dB SL were collected on a group of subjects, which was not the same group that contributed data at lower L2 levels at this frequency. No data were collected when f2 = 0.5 kHz and L2 = 60 dB SL in the present study, due to the time commitment required to obtain data at this frequency.2
Some subjects participated in data collection for as few as one f2 frequency (at all six L2 levels), while other subjects participated in data collection for as many as five f2 frequencies for the entire range of L2 levels. For L2 levels from 20 to 60 dB SL, data were obtained from 19 to 20 subjects at every f2 frequency. At the lowest test level (L2 = 10 dB SL), it was more difficult to obtain large enough DPOAE levels (Ld) so that suppression could be reliably measured. However, reliable suppression measurements were possible in at least ten subjects when L2 = 10 dB SL.
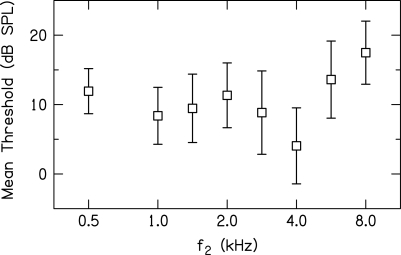
In the present study as well as in previous work from which data for f2 = 0.5 and 4 kHz were drawn (Gorga et al., 2008), a two-alternative forced-choice, transformed up-down procedure (Levitt, 1971) was used to estimate threshold at each f2 frequency in which a subject participated during DPOAE measurements. Figure 1 provides the mean threshold (dB SPL) ±1 standard deviation (SD) at each f2 for the 19–20 subjects who participated at that frequency. During suppression measurements, L2 was specified relative to each subject’s behavioral threshold at each f2 frequency for which the subject contributed data. This decision was made in an effort to assure that the input to the cochlea was nearly the same for each subject and each f2. This decision was based, in part, on the view that (to a first approximation) threshold of audibility as a function of frequency is determined by the forward transfer function of the middle ear, at least in subjects with normal hearing. By specifying L2 in dB SL, it was hoped that small differences in middle-ear transmission as a function of f2 were at least partially controlled. Thus, throughout this paper, L2 will be specified in dB SL. Of course, any small differences in absolute threshold could be a consequence of subtle differences in cochlear status; thus, our assumptions regarding equating stimulus level in each cochlea would be violated. However, subjects had no history to suggest cochlear problems and all subjects had thresholds well within the range of normal hearing. Even though data will be reported in dB SL in subsequent figures, Fig. 1 provides mean thresholds in dB SPL at each f2, providing information that can be used to convert from mean dB SL to mean dB SPL in any of the figures to follow. Note that mean behavioral thresholds range from about 4 dB SPL (4 kHz) to 17 dB SPL (8 kHz). This means that the suppression growth was measured for L2 levels that, on average, ranged from 14 to 64 dB SPL (4 kHz) and 27 to 77 dB SPL (8 kHz) for the range of SLs studied (10–60 dB).
Figure 1.
Mean behavioral threshold (dB SPL) as a function of f2 frequency (kHz). Error bars represent ±1 SD. Data from 19 to 20 normal-hearing subjects were used to derive these values, although the same subjects are not represented at each frequency.
Stimuli
DPOAEs were elicited in response to primary pairs (f1 and f2, f2∕f1 ≈ 1.2), with f2 = 1, 1.4, 2, 2.8, 5.6, and 8 kHz. The level of f2 (L2) was varied from 10 to 60 dB SL in 10-dB steps. The level of f1 (the lower frequency primary in each primary-frequency pair) was determined empirically and individually, using a paradigm in which both L1 and L2 were continuously varied, resulting in a Lissajous pattern of Ld. The L1 resulting in the largest Ld for each L2 was fit with a linear equation, which was subsequently solved to determine the L1 level for each of the six L2 levels used during DPOAE input∕output (I∕O) and suppression measurements. This paradigm was chosen because previous work suggests that individually “optimized” stimulus conditions result in the largest Ld for normal-hearing subjects (Neely et al., 2005; Johnson et al., 2006). Choosing stimulus conditions that produced the largest Ld was motivated by our desire to obtain reliable measurements of suppression for all stimulus conditions, including the low-level primary conditions (L1, L2) where Ld typically is small. In general, this approach resulted in the selection of L1 levels that were higher than those previously recommended (Kummer et al., 1998) and, unlike previous work (Kummer et al., 2000), were frequency dependent. The Lissajous procedure has been described elsewhere (Neely et al., 2005; Johnson et al., 2006), and it is identical to the one used to select primary levels in previous measurements of DPOAE I∕O functions and DPOAE suppression from our laboratory (Gorga et al., 2007, 2008). As a consequence, it will not be described here. However, using identical experimental methods in the present study and in a previous experiment (Gorga et al., 2008) allowed us to combine previously collected data at f2 = 0.5 and 4 kHz with the data collected as part of this study.
The Lissajous approach was followed for f2 frequencies ranging from 2 to 8 kHz because these frequencies are characterized by relatively low noise levels. It was not possible to use this approach when f2 = 1 or 1.4 kHz because of the higher noise levels that are associated with measurements at those frequencies. The Lissajous paradigm that was used to determine optimal levels at higher f2 frequencies (where noise levels are lower) does not include sufficient averaging time to reduce the noise to low enough levels for reliable measurements in conditions in which the noise level is high. As a result, it was inadequate for low f2 frequencies (≤1.4 kHz). However, our interest was in optimizing stimulus levels for all frequencies. To meet this need, an alternative approach was taken to determine optimal primary-level conditions. In this alternative approach, L2 was fixed at one of four levels (40, 50, 60, or 70 dB SPL), and L1 was varied (5-dB steps) over a range that allowed us to determine the L1 for each L2 that produced the largest Ld. These measurements were made with locally developed data-acquisition software (emav; Neely and Liu, 1994) that allowed for long averaging times that enabled sufficient noise reduction for reliable measurements. Like the results derived from the Lissajous-pattern paradigm, the L1, L2 combinations resulting in the largest Ld were fit with a linear equation, which was then solved to provide the L1 levels used at each of the six L2 levels in subsequent measurements. In this way, we were able to determine “optimal” primary-level conditions for each subject and frequency, including f2 frequencies for which the noise levels are high. Like the Lissajous-pattern approach, details of this procedure are provided elsewhere (Gorga et al., 2007, 2008). Notably, this is the same procedure that was used previously to select L1 levels when f2 = 0.5 kHz (Gorga et al., 2008).
For each combination of f2 and L2, 11 suppressor frequencies (f3) were used. These suppressor frequencies were chosen to extend from about 1 or 2 octaves below f2 to about 1∕4–1∕2 octave above f2 on a roughly equivalent octave scale relative to f2. Suppressor levels (L3) ranged from −20 to 85 dB SPL in 5-dB steps.
Procedures
Once the optimal primary-level conditions were determined individually for a given f2, all subsequent DPOAE measurements were made using emav (Neely and Liu, 1994) and a 24-bit soundcard (CardDeluxe, Digital Audio Labs, Chanhassen, MN). This system generated all stimuli and recorded all responses. A probe-microphone system (ER-10C, Etymōtic Research, Elk Grove Village, IL) was used to present stimuli and to record levels in the ear canal. The “receiver equalization” of the ER-10C was removed in order to increase output by as much as 20 dB. One channel of the probe system was used to present f1 while f2 and f3 (for conditions in which the suppressor was included) were presented on the second channel. A microphone housed in the probe unit was used to measure stimulus and response levels in the ear canal. Prior to data collection in each subject for each condition, a chirp was presented sequentially on each channel and the ear-canal level (in dB SPL) was measured. This reference was then used to set the level of stimuli during all DPOAE measurements. We recognize that SPL calibrations in closed ear canals are sometimes characterized by errors introduced as a result of standing waves (e.g., Siegel, 1994, 2002; Siegel and Hirohata, 1994; Scheperle et al., 2008). In this study, however, SPL calibrations were chosen in order to use conditions identical to those used in our previous study, the data from which are being combined with the present data.1 During data collection, waveforms were alternately stored in one of two buffers. The contents of the buffers were added and the level in the 2f1–f2 frequency bin was defined as DPOAE level (Ld). Subsequently, the two buffers were subtracted and the contents in the 2f1–f2 frequency bin and in the five frequency bins above and below this bin were used to provide an estimate of noise level.
Prior to data collection, cavity measurements were used to determine the level at which system distortion occurred. System distortion was level dependent and below –25 dB SPL for the low-level stimulus conditions, but was –15 dB SPL for high-level conditions. This level-dependent distortion was taken into account during data collection and analyses, but to simplify matters, the noise-level stopping rule was set to ≤−25 dB SPL during DPOAE data collection.
Following audiometric and tympanometric testing and the determination of optimal stimulus levels, a DPOAE I∕O function was measured prior to suppression measurements at each f2 frequency for which the subject participated. These data represent the equivalent of control conditions, in which no suppressor was present and were useful in determining that there was a sufficient SNR in control conditions so that suppressive effects could be measured over a wide range for a given subject.
For each subject, an f2 was selected and its level was fixed at one of six levels (10–60 dB SL, 10-dB steps). The only exception to this rule occurred at 0.5 kHz, a frequency for which no attempt was made to collect data at 10 dB SL due to the noise levels (Gorga et al., 2008) and for which no data were collected at 60 dB SL. Next, a suppressor frequency was selected and presented in increasing level from −20 to 85 dB SPL (5-dB steps). Prior to and just following the presentation of a suppressor-level series for each f3, a control condition was included in which no suppressor was presented. If Ld for the two controls differed by more than 6 dB when L2 = 10 or 20 dB SL or by 4 dB for higher L2 levels, the condition was repeated. The Ld for these two control conditions was averaged and the Ld in the presence of the suppressor was subtracted from this average to provide an estimate of the amount of suppression produced by the suppressor (referred to as decrement). Once data collection was complete for one f3, another f3 was selected and this process was repeated until decrement vs suppressor-level functions were measured at each of 11 f3 frequencies. Following the collection of suppression data for all f3 frequencies at a given L2, another L2 (for the same f2) was selected and the entire process was repeated until suppression data were collected at all six L2 levels. The order of suppressor frequency varied across L2 (and across subjects). This process was completed for each f2 frequency. In this way, suppression growth was estimated at each of 11 suppressor frequencies, eight f2 frequencies, and as many as six L2 levels.
In the present study, data-collection time ranged from about 3 h per subject for conditions in which the SNR was favorable (e.g., f2 = 5.6 kHz) to as much as 20 h per subject for conditions in which the SNR was low (f2 = 1 kHz). In total, it took 950 h to complete data collection on six f2 frequencies (see Footnote 1 for information regarding data collection when f2 = 0.5 and 4 kHz). The overall long data-collection time was a consequence of our use of measurement-based stopping rules, which were chosen in the hope that this would increase our chances of obtaining reliable data for a wide range of stimulus conditions. Data collection for each stimulus condition (i.e., the measurement of DPOAE I∕O functions and DPOAE suppression measurements) continued until one of the following conditions was met: (1) the noise level ≤−25 dB SPL, (2) the SNR ≥20 dB, or (3) 210 s of artifact-free averaging time had expired. These rules were used for data collection for f2 frequencies ≥1.0 kHz, including the previously collected data at 4 kHz (Gorga et al., 2008) which are included in this paper. Because of the higher noise levels when f2 = 0.5 kHz, the SNR stopping rule was reduced to 12 dB. This compromise was necessary in the interest of data-collection time. The data when f2 = 0.5 kHz, like the previously collected data at 4 kHz, are combined with the present data to provide a description of suppression growth from 0.5 to 8 kHz. For most f2 frequencies, averaging stopped either on the noise-floor or SNR criterion. At 0.5 kHz in the previous study, testing seldom stopped on the noise-floor criterion, even after 210 s of averaging time. At this frequency, averaging stopped on SNR criterion in some cases (i.e., for high L2 levels), but mostly on the averaging-time criterion. At 1 and 1.4 kHz in the present study, averaging time required to reach the SNR or noise-floor stopping criteria was greater than for higher f2 frequencies. Thus, disproportionate amounts of data-collection time were devoted to f2 = 0.5 kHz in our earlier study and to f2 = 1 and 1.4 kHz in the present study. Even so, these stopping rules resulted in reliable data for a wide range of f2, L2 combinations for every suppressor frequency.
RESULTS AND DISCUSSION
Control conditions
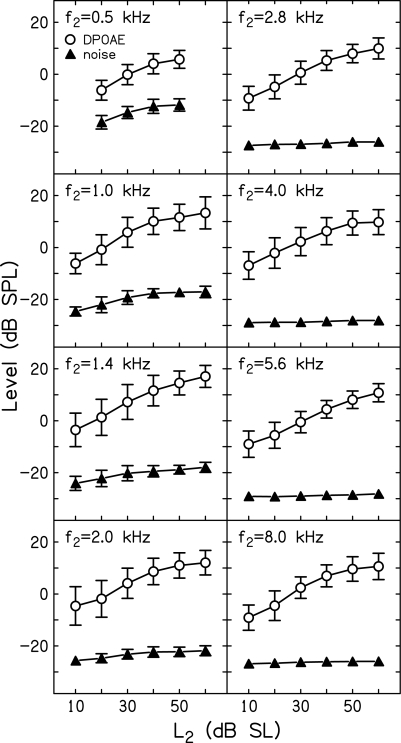
Figure 2 plots mean (±1 SD) DPOAE (Ld) and noise levels as a function of L2 (dB SL) for the subjects who participated in data collection at each of the f2 frequencies for which data are available. In this and all subsequent figures, data from the present study (f2 = 1, 1.4, 2, 2.8, 5.6, and 8 kHz) are combined with data from a previous study (f2 = 0.5 and 4 kHz).1 With 11 f3 frequencies per L2, f2 combination (there were nine f3 frequencies when f2 = 0.5 kHz) and with a control condition preceding and following each L3 series, the mean Ld and noise levels for each subject were derived from 18 to 22 measurements. These individual means were then used to calculate the grand means and SDs plotted in Fig. 2. The mean SNR for control conditions (those in which no suppressor was presented) can be ascertained at each f2 from the dB difference between Ld and noise levels at each L2. As can be seen from the data summarized in this figure, the smallest SNR was observed when f2 = 0.5 kHz, which is to be expected because noise levels are high at this frequency, despite averaging for as long as 210 s. Even so, Ld levels were, on average, at least 12 dB above the noise floor, including low-level stimulus conditions at this frequency. At higher f2 frequencies and∕or at higher L2 levels, the SNR was larger, and, in some cases, exceeded 30 dB. With such large SNRs for control conditions, it was possible to reliably measure DPOAE suppression over a wide range of suppressor levels.
Figure 2.
Mean level (dB SPL) ±1 SD as a function of L2 (dB SL). Open circles represent mean DPOAE level (Ld), while filled triangles represent mean noise levels. These data represent the mean levels measured during control conditions when no suppressor was present.
Even among subjects with the same auditory thresholds, DPOAE levels are variable and the factors contributing to that variability are not well understood (e.g., Garner et al., 2008). The SDs for Ld in Fig. 2 reflect this intersubject variability. On the other hand, the SDs for noise levels were smaller and error bars are often obscured by the symbol representing the mean. This occurred when f2 > 2 kHz, and is a consequence of our stopping rule, which allowed averaging to continue until the noise level was ≤−25 dB SPL. For f2 frequencies above 2 kHz, averaging almost always stopped on the noise-floor rule, resulting in little variability in noise levels.
In contrast, there was greater variability in mean absolute Ld across subjects, as reflected in the larger error bars for Ld in Fig. 2, regardless of f2. This intersubject variability is perhaps less important than intrasubject variability for control conditions because we were interested in measuring the extent to which the presence of a suppressor reduced the DPOAE response relative to the response that was measured during control conditions for each subject. This makes within-subject variability in the control condition a more important estimate of the reliability of suppression measurements. Table TABLE I. provides a summary relevant to this issue. This table includes the mean standard deviations (MeanSD) for the control conditions for each f2, L2 combination across subjects, along with the SD of these standard deviations (SDSD). Assuming that the SDs from individual control conditions provide an estimate of intrasubject variability, the MeanSD describes the average variability of the control condition across subjects. In every case, the MeanSD was less than 3 dB and, in fact, was less than 2 dB in 40 of 46 conditions.2 The SDSD provides information on the consistency with which these SDs were obtained. In 42 of 46 conditions, the SDSD was less than 1 dB, and, in the other four cases, it was less than 1.25 dB. The summary provided in Table TABLE I. indicates that across the subjects who contributed data at each f2, L2 combination, the control conditions were consistent. In total, the data shown in Fig. 2 and Table TABLE I. suggest that the use of measurement-based stopping rules (as opposed to terminating measurements after a fixed number of averages or after a fixed amount of time regardless of response conditions) resulted in reliable measurements for all conditions of interest. No doubt, terminating averages when the noise floor was ≤−25 dB SPL and∕or allowing averages to continue for 210 s when this noise floor was not achieved added to data-collection time, but also contributed to the reliability of the measurements.
Table 1.
MeanSD and SDSD for the control conditions for all eight f2 frequencies (kHz) and six L2 levels (dB SL).
| f2 (kHz) | 10 dB | 20 dB | 30 dB | 40 dB | 50 dB | 60 dB | |
|---|---|---|---|---|---|---|---|
| 0.5 | MeanSD | — | 2.75 | 2.13 | 2.01 | 2.10 | — |
| SDSD | — | 0.81 | 0.47 | 0.52 | 0.93 | — | |
| 1.0 | MeanSD | 2.25 | 1.44 | 1.25 | 1.21 | 1.20 | 1.14 |
| SDSD | 1.23 | 0.53 | 0.40 | 0.56 | 0.42 | 0.34 | |
| 1.4 | MeanSD | 1.74 | 1.41 | 1.21 | 1.06 | 0.93 | 0.90 |
| SDSD | 0.80 | 0.23 | 0.26 | 0.26 | 0.32 | 0.37 | |
| 2.0 | MeanSD | 1.46 | 1.38 | 1.11 | 1.11 | 0.97 | 0.91 |
| SDSD | 0.37 | 0.35 | 0.25 | 0.37 | 0.41 | 0.36 | |
| 2.8 | MeanSD | 1.51 | 1.28 | 1.05 | 1.11 | 1.07 | 1.05 |
| SDSD | 0.40 | 0.36 | 0.43 | 0.42 | 0.46 | 0.43 | |
| 4.0 | MeanSD | 2.18 | 1.89 | 1.90 | 1.74 | 1.76 | 0.44 |
| SDSD | 0.84 | 0.76 | 1.13 | 1.08 | 1.16 | 0.24 | |
| 5.6 | MeanSD | 1.57 | 1.44 | 1.23 | 1.24 | 1.21 | 1.24 |
| SDSD | 0.46 | 0.46 | 0.47 | 0.52 | 0.59 | 0.46 | |
| 8.0 | MeanSD | 1.50 | 1.23 | 1.06 | 0.91 | 0.97 | 1.11 |
| SDSD | 0.42 | 0.42 | 0.47 | 0.46 | 0.42 | 0.47 |
Conversions to decrements, data transformation, and suppression-threshold estimates
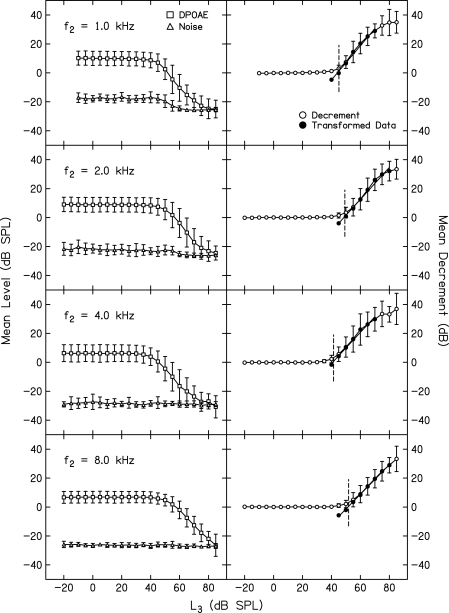
Figure 3 shows examples of DPOAE suppression measurements, the conversion of these data into decrements, transformation of these data, simple linear fits to the transformed data, and finally the determination of suppression thresholds that were used to construct suppression tuning curves (STCs) that are the focus of a companion paper (Gorga et al., 2011). Each row shows data for a different f2, all of which were presented at an L2 of 40 dB SL. In each case, the suppressor frequency (f3) was slightly higher than f2, and was defined as the “on-frequency” condition. It is not possible to present a suppressor at exactly the same frequency as f2 because it needs to be separable from f2 in order to have a level (L3) that is distinct from L2. The left column plots mean Ld (squares) and noise levels (triangles), along with SDs, all of which are based on data from 19 or 20 subjects. The SDs associated with Ld in the left column of this figure reflect the variability in absolute Ld even among subjects with normal hearing. The smaller SDs for noise levels are a consequence of the measurement-based stopping rules, the most important of which caused data collection to terminate when noise achieved a level of ≤−25 dB SPL. Note that for L3 levels up to about 40–50 dB SPL (levels roughly equivalent to L2), there was no suppression. As L3 increased above 40–50 dB SPL, Ld decreased, and by the time L3 was 70–80 dB SPL, the response was completely suppressed into the noise floor. The variability in Ld was slightly dependent on L3, in that it increased as the response was suppressed. Under these circumstances, the contribution of noise variance to Ld increased because the SNR decreased, resulting in greater variability in Ld.
Figure 3.
Left column: Mean Ld (squares) and noise levels (triangles) as a function of L3 (dB SPL). Error bars represent ±1 SD. Data for a different f2 are shown in each row. In all four rows, L2 = 40 dB SL (relative to each subject’s behavioral threshold at f2). Right column: Decrement in dB (open circles) obtained by subtracting the Ld in the presence of a suppressor from the average of two control conditions, in which no suppressor was present (see text for additional details). Only those points that showed at least 1 dB decrements and SNR ≥3 dB were included in a transformation (see text) and were subsequently fit with a straight line. The transformed data points are shown as filled circles. The fits to the transformed data provided the estimate of slope and were solved for the L3 resulting in 3 dB of suppression, which was then used to construct the STCs in a companion paper (Gorga et al., 2011).
The right column of Fig. 3 represents the same conditions as shown in the left column, only here the data were converted into decrements (open circles), which is the amount by which Ld was reduced as a consequence of the presence of the suppressor. This conversion was accomplished by averaging the Ld for control conditions (conditions in which no suppressor was present) just prior to and just following an L3 series for a given f3, and then subtracting the Ld during the presentation of the suppressor from the average Ld for the control conditions. There are several advantages of converting the data into decrements. Decrements represent the measurement of interest (amount of suppression) and they reduce the influence of variability in Ld. This is evident in the small SDs (completely obscured by the symbols when the suppressor has no effect on the response) for low L3 levels, and is still evident when L3 begins to cause suppression.
To estimate the slope of these functions and to determine suppression thresholds for the purposes of constructing STCs, which is the focus of the companion paper (Gorga et al., 2011) any data point for which the decrement was zero (or negative) was eliminated from further analyses. In addition, only data points for which the SNR was ≥3 dB were included, which eliminated those points for which the response was completely suppressed. In the right column, these data are represented by the open circles at high L3 levels. At the outset, we established an inclusion rule, such that only data points for which the decrement increased monotonically were included in the analyses. However, non-monotonicities were never observed in any of the examples shown in Fig. 3 or any of the other mean decrement-vs-L3 functions. Apparently, averaging across subjects eliminated the rare non-monotonicities in the data from individual subjects. Once the set of data points was reduced by the application of these three inclusion criteria, remaining data points were transformed by the following equation:
| (1) |
The transformed data points (means and SDs) are represented as filled symbols in the right column of panels. The transformed data were fit with a simple linear regression (SLR), providing slopes of the suppression-growth functions. The regressions also were solved for the L3 resulting in a decrement of 3 dB (D = 0), which was defined as suppression threshold when constructing STCs in the companion paper (Gorga et al., 2011). The short vertical dashed lines in the right column represent the suppression threshold so derived. A comparison of the SDs around the decrement at this suppression threshold, compared to the equivalent SDs around the absolute Ld at the same L3 in the left column demonstrates one advantage of converting to decrements, which is to reduce the influence of variability in Ld by having each subject serve as his or her own control. This is important for the conditions that will be used to define the suppression thresholds and plotted as STCs in the companion paper. The entire process described above was completed for all f3 frequencies, of which there were 11, and at all combinations of f2 and L2.1
Examples of decrement vs L3 functions for all f3 frequencies
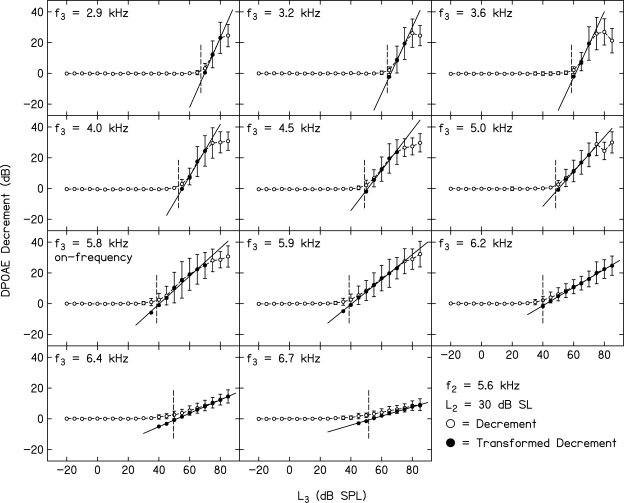
Figure 4 shows mean decrement (±1 SD) vs L3 functions for all 11 f3 frequencies when L2 was set to 30 dB SL and f2 = 1 kHz. Figure 5 provides similar data when f2 = 5.6 kHz and L2 = 30 dB SL. In both figures, data from 20 normal-hearing subjects are included, although not necessarily the same 20 subjects at both frequencies. These two f2 frequencies were selected to provide examples of decrement functions for a relatively low f2 and a relatively high f2 from the set of f2 frequencies for which suppression was measured in the present study (similar examples of decrement functions for f2 = 0.5 and 4 kHz were provided in Gorga et al., 2008). Although not shown in this figure, on average, the noise level was about 8–10 dB higher for f2 = 1 kHz, compared to when f2 = 5.6 kHz (see Fig. 2). Still, a sufficient SNR was achieved in both cases for reliable measurements of suppression for a wide range of f3 frequencies and L3 levels. In fact, the patterns across f3 for these two f2 frequencies are sufficiently similar that we will describe the data in these two figures at the same time.
Figure 4.
Mean decrement (dB) as a function of suppressor level, L3 (dB SPL). f2 = 1 kHz and L2 = 30 dB SL. Each panel represents data for a different f3. Open circles represent the mean difference in Ld between control conditions and the conditions in which a suppressor was present. Error bars represent ±1 SD. Filled circles represent the decrements after they were transformed using Eq. 1 (see text). Lines represent best fits to the transformed decrements. The fits to the transformed data provided the estimate of slope and were solved for the L3 resulting in 3 dB of suppression, which was then used to construct the STCs in a companion paper (Gorga et al., 2011).
Figure 5.
Same as Fig. 4, only here f2 = 5.6 kHz and L2 = 30 dB SL.
Open circles represent the mean decrements (±1 SD) as a function of L3. As stated above, the conversion to decrements reduces the influence of differences in absolute Ld across subjects. With the exception of cases in which f3 > f2, the variability (as estimated by the SD) increased as L3 increased. Even for f3 > f2, the SD increased at high L3 levels. This is to be expected because the SNR decreased as L3 increased because Ld decreased (due to suppression) while noise level remained the same (i.e., it was unaffected by the suppressor), resulting in greater variability due to the increased influence of noise variance on estimates of Ld. There was a smaller effect of L3 on variability when f3 > f2 because the reduction in Ld was small, meaning that the contribution of noise variance to variability in Ld was also small.
Of interest in both Figs. 4 and 5 is the relative frequency dependence (f3 relative to f2) of the slope of the decrement function (growth rate of suppression) as a function of L3. This can be seen in the raw decrement data (open circles), as well as in the transformed data to which a line was fit. An examination of the data shown in these two figures indicates that the trends at 1 and 5.6 kHz are similar. Specifically, low-frequency suppressors relative to f2 require a high L3 in order for suppression to occur (see upper left panel in both figures), but once suppression starts to occur, it grows rapidly with further increases in L3. Both suppression threshold and the rate at which it grows decreased as f3 increased. In general, the lowest suppression threshold occurred for the f3 that was closest to but slightly higher than f2. For convenience, this f3 is referred to as the “on-frequency” suppressor. As f3 increased above this frequency, the rate at which suppression grew decreased systematically with f3. “Suppression threshold” increased slowly as f3 increased above f2, compared to what might be expected for single-unit rate-vs-level functions sharing similar frequency relationships (e.g., Sachs and Abbas, 1974). This effect, however, is consistent with the observation of wider STCs, compared either to excitation tuning curves at the single-unit level or differences between simultaneous and forward-masking psychophysical tuning curves in humans (e.g., Sachs and Kiang, 1968; Ruggero and Temchin, 2005; Jesteadt and Norton, 1985; Moore et al., 1984; Shannon, 1976). For the L2 condition shown in these two figures (L2 = 30 dB SL), it was possible to completely suppress the response (as evident both in the asymptotic portions of some of the decrement functions and in the open symbols at high L3 levels, indicating that the SNR <3 dB). Complete suppression occurred for some, but not all, f3 frequencies. This was especially true on the high-frequency side of f2 because there were insufficient suppressor levels to result in complete suppression (see the panels describing suppression for the three highest f3 frequencies at both f2 frequencies). These general patterns were observed at other f2 frequencies and L2 levels, including the data for f2 = 0.5 and 4 kHz, which were taken from a previous paper (Gorga et al., 2008).1
Examples of decrement vs L3 functions for all f2 frequencies
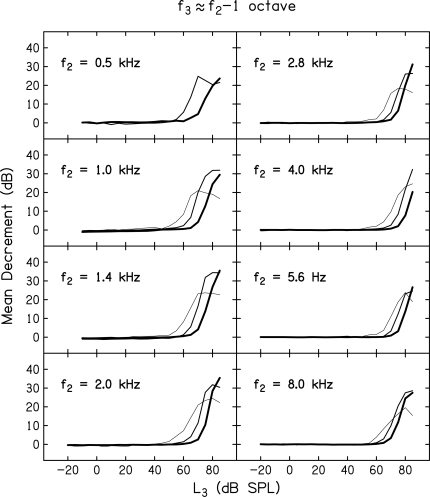
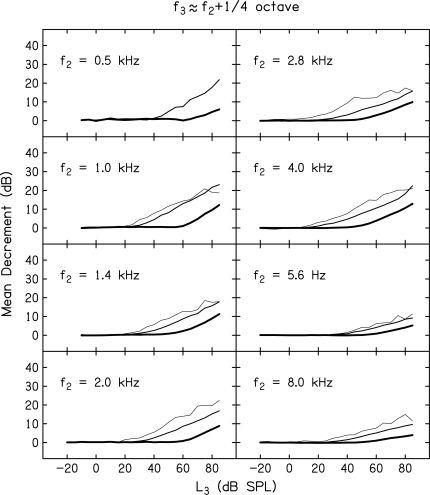
Whereas, Figs. 4 and 5 provide mean decrement vs L3 functions for all 11 f3 frequencies, but for only two f2 frequencies and one L2 level, Figs. 678 provide mean decrement vs L3 functions for all f2 frequencies, but only for subsets of f3 frequencies and L2 levels. The f3 frequencies were chosen, in part, because they represent frequencies that have been of interest in other measures of cochlear nonlinearity, including suppression. Each figure provides data for a single f3 relative to f2 while separate panels within each figure provide data for one of eight f2 frequencies. Data are shown for f3 ≈ f2 – 1 octave (Fig. 6), the on-frequency case when f3 ≈ f2 (Fig. 7), and when f3 ≈ f2 + octave (Fig. 8).
Figure 6.
Mean decrement (dB) as a function of suppressor level, L3 (dB SPL). Data for a different f2 are represented in each panel. Within each panel, data are shown for L2 levels of 10, 30, and 50 dB SL, with the thinnest line representing data for the 10-dB SL condition and progressively heavier lines representing data for L2 = 30 and 50 dB SL. In all panels, f3 ≈ f2 – 1 octave.
Figure 7.
Same as Fig. 6, only here f3 ≈ f2.
Figure 8.
Same as Fig. 6, only here f3 ≈ f2 + octave.
In all three figures, the results for different L2 levels are represented by different line weights, with the results for the lowest L2 (10 dB SL) shown with the thinnest line, and progressively thicker lines for the higher two L2 levels (30 and 50 dB SL). There are no data for the case when f2 = 0.5 kHz and L2 = 10 dB SL because the high noise levels for this f2 combined with the low Ld at this L2 prevented reliable DPOAE measurements and, therefore, prevented reliable measurements of suppression. As a result, there are only two functions in the panels depicting results when f2 = 0.5 kHz.
First, consider the low-frequency suppressor results in Fig. 6. Note that for all f2 frequencies and the three L2 levels for which data are shown, suppression threshold was relatively high, but only slightly dependent on L2. For a 40-dB range in L2 (10–50 dB SL), suppression threshold changed by 20 dB or less. However, once suppression threshold was reached, the decrement increased rapidly with further increases in L3. In many (but not all) cases, these low-frequency suppressors resulted in complete suppression of the response, as indicated by the asymptotic decrements at high L3 levels. Not surprisingly, this was more likely to occur when L2 = 10 or 30 dB SL, but did not occur when L2 = 50 dB SL because the response at this level was too large to be completely suppressed, even for the highest L3 levels.
Figure 7 provides examples of mean decrement vs L3 functions when f3 ≈ f2. Note that the L3 at which decrements first occurred was lower in this case, compared to the results shown in Fig. 6, meaning that suppression thresholds were lower when f3 was close to f2. Unlike the results shown in Fig. 6, suppression threshold appeared to be more dependent on L2, such that there was about a 20-dB increase in suppression threshold for each 20-dB increase in L2. As expected, complete suppression was a more common occurrence when f3 ≈ f2, which is evident in the larger number of conditions for which asymptotic decrements occurred at high L3 levels.
Figure 8 provides examples of decrement vs L3 functions when f3 ≈ f2 + octave. Suppression thresholds were higher in this case, compared to when f2 ≈ f3 (Fig. 7), and once suppression threshold was reached, the amount of suppression grew slowly with increases in L3. With the exception of the condition in which f2 = 1 kHz and L2 = 10 dB SL, there was no indication of complete suppression, even for the highest L3.
Like the more detailed comparisons in Figs. 4 and 5, the summaries provided in Figs. 678 indicate that, while there are differences across f2 frequencies, these differences are relatively small and the general trends are the same regardless of frequency and level. These data are consistent with previous growth of suppression data based on auditory-nerve fiber responses in lower animals (e.g., Abbas and Sachs, 1976; Delgutte, 1990; Pang and Guinan, 1997) and are also consistent with measurements of suppression in basilar-membrane responses (e.g., Ruggero et al., 1992; Cooper and Rhode, 1996; Rhode and Cooper, 1993). Similar patterns have been observed in previous DPOAE suppression data as well (e.g., Abdala, 1998, 2001; Abdala and Fitzgerald, 2003; Abdala and Charterjee, 2003; Gorga et al., 2002, 2003; Kummer et al., 1995). Specifically, these previous studies showed that suppression grows most rapidly for suppressors lower in frequency than the signal frequency and that the growth of suppression decreases as suppressor frequency increases. In many respects, it is easier to interpret the single-unit and mechanical data from lower animals because fewer assumptions are needed regarding the stimuli and the location at which the response is generated in the cochlea. With DPOAE measurements in humans, two tones are used to represent the “signal,” so presumably the suppression of the representation of one or the other tone might result in a reduction in Ld. The distortion measured in the ear canal is complex (in that it may include contributions from both a distortion and a reflection source (e.g., Shera and Guinan, 1999); theoretically, suppression of either source might result in a phase-dependent reduction or enhancement of Ld. Finally, it is not possible in humans to know the exact location of the generator site within the cochlea, and, in fact, the generator sites might be dispersed along regions basal to the characteristic place of f2 (e.g., Martin et al., 2010), although others have questioned this view (Withnell and Lodde, 2006). Despite these limitations, the human data in Figs. 45678, as well as previous DPOAE suppression data, are consistent with results obtained in lower animals using more invasive procedures. Thus, we take these data to suggest that DPOAE suppression measurements in humans are, at least to a first approximation, describing the same underlying processes that have been described more directly in lower animals.
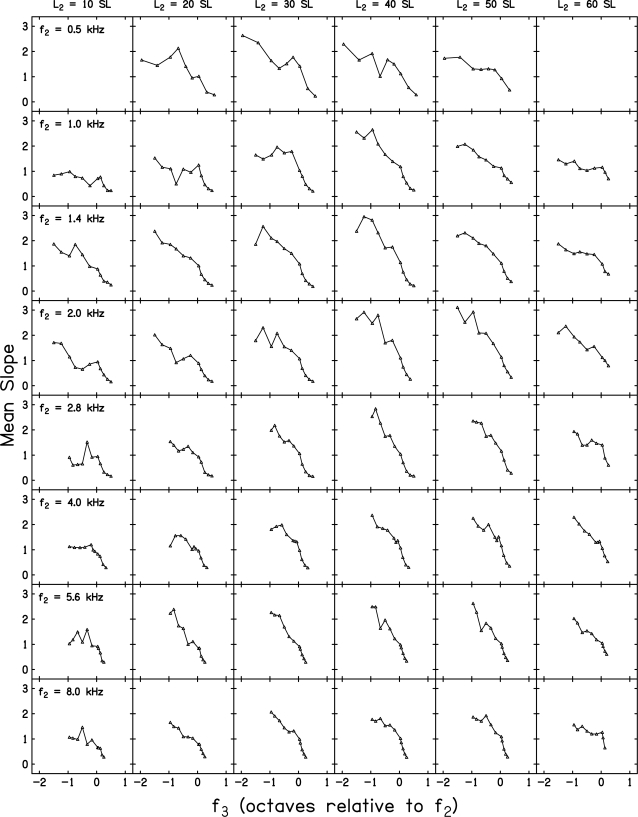
Slopes of decrement vs L3 functions
Figure 9 plots the slope of mean decrement vs L3 functions for all eight f2 frequencies, as many as six L2 levels, and as many as 11 f3 frequencies. In all cases, data from at least 10 but no more than 20 subjects are included in each panel (as few as ten subjects provided reliable data when L2 = 10 dB SL for some f2 frequencies), but data are not from the same subjects in all 46 panels. As in other figures, there are no data for f2 = 0.5 kHz and L2 = 10 or 60 dB SL because these conditions were not measured.2 These slopes were calculated based on SLR, in which decrement functions were fit independently for each f3, L2 combination. Data for a different f2 are shown in each row while the results for each L2 are shown in separate columns. In this figure, f3 is plotted on an octave scale relative to f2, which is a form of normalization that may be preferred for comparison across f2.
Figure 9.
Slope of the decrement function as a function of f3 in octaves relative to f2. Data for a different f2 are shown in each row and data for a different L2 are shown in each column. Slopes are estimated from a SLR in which only L3 was a variable.
Clearly, there were differences across frequency and level. For example, there appeared to be a range of f3 frequencies for which the slope is relatively constant when f2 = 0.5 and 1 kHz. This trend was either less evident or absent for higher f2 frequencies. Part of this observation relates to the fact that it was possible to suppress the response to lower f2 frequencies with f3 frequencies in some cases as much as 1.5–2 octaves below f2 (0.5 and 1 kHz). While this was true for several low-frequency f2 frequencies, it was seldom possible to produce suppression with f3 frequencies < f2 – 1 octave at higher f2 frequencies. One factor contributing to this effect may be the output limitations in our hardware, but it is unlikely that this alone accounted for the more limited range of low f3 frequencies for which suppression was produced for higher f2 frequencies. Thus, this may represent a difference in DPOAE suppression effects between low and high f2 frequencies. Qualitatively, this frequency difference is not unlike similar effects observed in single-unit measurements (Delgutte, 1990) and in derived measurements from otoacoustic emission (OAE) latencies (Shera et al., 2010). There also was evidence that the slope of the decrement functions sometimes decreased non-monotonically with f3, but this effect was not a systematic function of frequency, as it was evident for both high and low f2 frequencies for some L2 levels and it did not occur at the same f3 relative to f2 as might be expected if the non-monotonicities were caused by the complex interaction of distortion and reflection sources.
These differences across f2 notwithstanding, the general trends were similar across f2 frequency and L2 level. That is, the steepest slopes were observed for conditions in which f3 < f2, slope decreased as f3 increased, and the shallowest slopes were observed for conditions in which f3 > f2. When f3 ≈ f2, the slope of the decrement function (i.e., the suppression-growth rate) was close to 1.
The data summarized in Figs. 4567 are consistent with previous descriptions of DPOAE suppression in humans (Abdala, 1998, 2001; Abdala et al., 1996; Abdala and Chatterjee, 2003; Abdala and Fitzgerald, 2003; Gorga et al., 2002a,b, 2003; Brown and Kemp, 1983; Kummer et al., 1995). Data from these studies showed the same dependence of growth of suppression on the relation between f3 and f2. The present paper differs from these previous efforts in terms of the range of f2 frequencies and L2 levels that are described herein. For example, L2 levels ranged from as low as 10 to as high as 60 dB SL in the present study. On the low end (10 dB SL), this translates into L2 levels in dB SPL that ranged from about 14 dB SPL at 4 kHz to about 28 dB SPL at 8 kHz (see Fig. 1). Few studies of DPOAE suppression report data for L2 levels as low as these levels and it is not common to find suppression data for 5–6 L2 levels. Finally, there are few papers that include data from 0.5 to 8 kHz. Thus, the present work provides a description of suppression growth for an unusually wide range of frequencies and levels.
Other differences exist between previous data and the results reported here. In addition to the use of the present measurement-based stopping rules, data were converted into decrements, specific inclusion criteria were applied to determine the data points for further analysis, these data points were transformed, and then fit with a linear equation. As a consequence, only qualitative comparisons seem appropriate. Even so, it is noteworthy that the many previous studies in humans have described similar frequency dependence of the slope of the suppression functions.
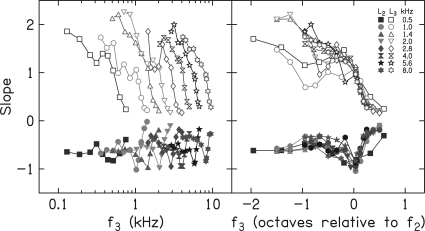
Multivariate linear regressions
Whereas, Fig. 9 provides estimates of slope based on SLRs in which only L3 was an input variable, Fig. 10 provides estimates of slope based on multiple linear regressions (MLRs), in which both L2 and L3 served as input variables. Although it covered a smaller range and was varied with less precision during data collection, compared to L3, L2 still varied from 10 or 20 to 50 or 60 dB SL in 10-dB steps. The left panel of Fig. 10 plots slope on a log-frequency scale, while the right panel plots slope on an octave scale relative to f2. The open symbols in each panel provide slope estimates when L3 is the variable and filled symbols in each panel describe slopes when L2 is the variable. In the left panel, it can be seen that slope functions for both L3 and L2 moved toward higher frequencies as f2 increased. This panel allows one to discern the slope of these decrement functions for each f2, which appear to be similar (although not identical) across f2. Given the summaries in earlier figures, this is the expected outcome. There is less variation in slope when L2 is the variable of interest, as evident in the functions at the bottom of the left panel. However, there is a local minimum on each of these functions, which occurs when f3 ≈ f2, and the slope is close to −1. This means that when f3 ≈ f2, every 10-dB increase in L2 was accompanied by about a 10-dB increase in L3.
Figure 10.
(Color online) Slopes from multivariate linear regressions in which L3 and L2 were included. Left panel: f3 is plotted on a log-frequency scale. Right panel: f3 plotted on an octave frequency scale relative to f2. Within each panel, the parameter is f2. Open symbols represent slopes as a function of L3, while filled symbols represent slopes as a function of L2.
The right panel of Fig. 10 plots these same slope values, only here an octave-frequency scale relative to f2 is used. On this x-axis scale, both similarities and differences as a function of f3 are emphasized. For example, the growth of suppression was greatest when f3 was an octave or more below f2, decreased as f3 increased, and had a minimum for f3 frequencies higher than f2. With the exceptions of f2 = 0.5 and 1 kHz, these slopes overlapped for all f2 frequencies. When L2 is the variable, there was more uniformity of slope across f2. The minimum L2 slope occurred when f3 ≈ f2 regardless of f2. The slopes based on MLR and plotted on an octave scale suggest that growth of suppression is similar across a wide range of frequencies in humans. The exceptions to this observation occurred when f2 = 0.5 and 1 kHz, but even in these cases, the overall pattern was at least qualitatively similar to what was observed at other f2 frequencies.
The results for f2 frequencies of 0.5 and 1 kHz are reminiscent of stimulus frequency otoacoustic emission (SFOAE) data reported by Shera et al. (2010). They measured SFOAE latencies in humans, and noted that the function relating latency to frequency appeared to have different slopes for frequencies >1 kHz, compared to frequencies ≤1 kHz. The underlying mechanism responsible for this change in slope is not obvious, but the results shown in the right panel of Fig. 10 may be consistent with the findings of Shera et al. in suggesting different behavior below 1 kHz. The slope of the DPOAE decrement functions when f2 = 0.5 and 1 kHz change more gradually as f3 frequency increases, compared to the results for higher f2 frequencies. These results also share similarities with auditory-nerve data reported by Delgutte (1990). For example, Delgutte reported a more gradual change in suppression-growth rate as suppressor frequency increased for fibers having CFs <2 kHz, and a more abrupt change in slope with frequency for fibers with CFs >2 kHz. In addition, he observed more rapid growth in suppression for low-frequency suppressors relative to CF, even when suppressor frequency was normalized (see Fig. 9; Delgutte, 1990). Comparisons of auditory-nerve data to DPOAE data are complicated by many factors, not the least of which is the differences in the stimuli that are used to elicit these different responses. For example, single-fiber response properties can be probed by presenting a single signal frequency, whereas DPOAE measurements must use a “signal” that consists of two pure tones whose relative levels add complexity to the stimulus paradigm. We do not intend to minimize these differences, but it is unlikely that the similarities and consistencies in the present data, relative to data from lower animals, are coincidental. To the extent that f2 frequency can be considered as an approximation of CF, the results shown in Fig. 10 of the present study share similarities with the auditory-nerve data reported by Delgutte. Delgutte provided a detailed description of growth of suppression based on changes in auditory-nerve fiber responses and there were differences in how suppression was quantified in the present study, compared to the approach taken by Delgutte. However, the present results are consistent with the results based on auditory-nerve data and cover a wide range of frequencies (although not as wide as the animal work). Thus, the present study provides a description of DPOAE suppression in humans, which shares at least qualitative similarities with data from lower animals.
In this study (as in virtually all other studies of DPOAE suppression), we did not use methods in which the influence of complex source contributions on suppressive effects was evaluated. There are several reasons for this, including potentially complex stimulus conditions when attempting to control the reflection source and the observation in many studies of DPOAE suppression (including this study) that evidence of the influence of source contribution is not obvious. Still, DPOAEs include both a distortion source (presumably located near the f2 place) and a reflection source (at the 2f1–f2 place), both of which contribute to the Ld measured in the ear canal (e.g., Shera and Guinan, 1999). Depending on the relative level and phase of the components from these two sources, either constructive or destructive interference will occur. In the context of the present study, one might predict that the decrement functions would be altered when f3 was close to 2f1–f2. However, we observed no evidence to suggest that this was occurring, at least not in the mean data. This observation alone does not necessarily indicate that there were no contributions from the reflection source because any effects might average out, due to variations in phase for the component coming from that source.
SUMMARY AND CONCLUSIONS
DPOAE suppression is reported for f2 frequencies ranging from 0.5 to 8 kHz and for L2 levels ranging from 10 or 20 to 50 or 60 dB SL. For each f2, L2 combination, suppression was measured for up to 11 suppressor frequencies ranging in level from −20 to 85 dB SPL. With the exception of 0.5 kHz where no data were collected when L2 = 10 or 60 dB SL, data were collected from between 10 and 20 subjects with normal hearing. Measurement techniques were used that reduced noise levels to as low as −25 dB SPL, thus permitting reliable estimates for a wide range of conditions. The growth of suppression was greatest for f3 frequencies below f2, decreased as f3 increased, and was slowest for f3 frequencies above f2. This dependence of suppression growth on the relation between f3 and f2 was observed for all f2 frequencies and all L2 levels, although the behavior at 0.5 and 1 kHz differed from what was observed at higher frequencies. These results are consistent with similar data obtained from invasive techniques in lower animals and are similar to previous DPOAE suppression data from both lower animals and humans. The results are unique in that they cover a wider range of frequencies and levels than previously reported.
Controversy remains as to what OAE measurements like the present measurements tell us about cochlear function. Of necessity, these measurements rely on indirect techniques, complex stimulus paradigms, and interpretations that are based on the assumption that these data are describing underlying processes based on the similarity in patterns, compared to more direct measurements in lower animals. It is impossible to perform direct measurements in humans, but the measurements described here could be used to obtain data in lower animals that could then be compared to data obtained from more invasive measurements in the same animal. Such a comparison would provide a more direct test of the extent to which OAE measurements describe underlying cochlear processes.
ACKNOWLEDGMENTS
We would like to thank Rachel Scheperle, Natalie Lenzen, Elizabeth Searing, Michelle Parks, Megan Thorson, and Rachel Tomasek for their help with data collection. We also thank Megan Thorson for her help with figure preparation. We thank Sandy Estee for her help in subject recruitment, especially among members of minority communities. Finally, we thank Brenda Lonsbury-Martin, Caroline Abdala, and one anonymous reviewer for helpful suggestions on an earlier version of this paper. This work was supported by the NIH (NIDCD R01 2251 and P30 4662).
Footnotes
Data when f2 = 0.5 and 4 kHz from a previous study (Gorga et al., 2008) were combined with the data collected as part of this study. Because the conditions from the present study are identical in all respects with those used previously, it was decided to combine the previously collected data for f2 = 0.5 and 4 kHz with the presently collected data at an additional six f2 frequencies, thus providing a description of DPOAE suppression from 0.5 to 8 kHz. This seemed reasonable because identical subject-selection criteria and experimental procedures were used in the previous and present data-collection efforts. Furthermore, the collection of data for f2 = 0.5 and 4 kHz consumed approximately 800 h of data-collection time (the majority of which was devoted to data collection when f2 = 0.5 kHz). Repeating that effort, in addition to the 950 h of data-collection time that was needed for the six new f2 frequencies with which those data are now being combined, would have been prohibitive and perhaps an inappropriate use of resources. Even in the present study, no subject participated in data collection at all six f2 frequencies because to do so would have required an unacceptable and unachievable time commitment from most subjects. In our previous efforts, it was not possible to collect data when f2 = 0.5 kHz and L2 = 10 dB SL because the SNR was not sufficient for reliable suppression measurements for that condition even after as much as 210 s of artifact-free averaging time. We did not collect suppression data at 0.5 kHz when L2 = 60 dB SL, due to limitations in the amount of time subjects were willing to dedicate to data collection. These are the only frequency and level combinations for which no data were collected in either study. Instead, we focused our data-collection efforts on L2 levels from 20 to 50 dB SL when f2= 0.5 kHz.
Even though there were eight f2 frequencies and six L2 levels, there are only 46 conditions because we did not measure suppression when f2 = 0.5 kHz for L2 = 10 and 60 dB SL. Recall that the data for f2 = 0.5 and 4 kHz came from a previous paper (Gorga et al., 2008). At that time, data were not collected for either of these two f2 frequencies at L2 = 60 dB SL. In the previous study, we attempted to collect suppression data when f2 = 0.5 kHz and L2 = 10 dB SL, but the measurements were unreliable for the reasons stated in Footnote 1. However, we were able to obtain data when f2 = 4 kHz and L2 = 10 dB SL previously, and those data are included here. In the course of the present study, additional data were collected for f2 = 4 kHz and L2 = 60 dB SL because those data could be collected quickly. It was decided not to collect additional data when f2 = 0.5 kHz and L2 = 60 dB SL because this would have increased data-collection time, which already was extensive in this study.
References
- Abbas, P. J., and Sachs, M. B. (1976). “Two-tone suppression in auditory-nerve fibers: Extensions of a stimulus-response relationship,” J. Acoust. Soc. Am. 59, 112–122. 10.1121/1.380841 [DOI] [PubMed] [Google Scholar]
- Abdala, C. (1998). “A developmental study of distortion product otoacoustic emission (2f1-f2) suppression in humans,” Hear. Res. 21, 125–138. 10.1016/S0378-5955(98)00073-2 [DOI] [PubMed] [Google Scholar]
- Abdala, C. (2001). “Maturation of the human cochlear amplifier: Distortion product otoacoustic emission suppression tuning curves recorded at low and high primary levels,” J. Acoust. Soc. Am. 110, 1465–1476. 10.1121/1.1388018 [DOI] [PubMed] [Google Scholar]
- Abdala, C. (2003). “A longitudinal study of distortion product otoacoustic ipsilateral suppression and input/output characteristics in humans,” J. Acoust. Soc. Am. 114, 3239–3250. 10.1121/1.1625930 [DOI] [PubMed] [Google Scholar]
- Abdala, C. (2004). “DPOAE (2f1-f2) suppression in three-month-old infants: Evidence for postnatal maturation in children with sensorineural hearing loss,” J. Acoust. Soc. Am. 116, 3572–3580. 10.1121/1.1811472 [DOI] [PubMed] [Google Scholar]
- Abdala, C. (2005). “Effects of aspirin on distortion product otoacoustic emission suppression in human adults: A comparison with neonatal data,” J. Acoust. Soc. Am. 118, 1566–1575. 10.1121/1.1985043 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Chatterjee, M. (2003). “Maturation of cochlear nonlinearity as measured by distortion product otoacoustic emission suppression growth in humans,” J. Acoust. Soc. Am. 114, 932–943. 10.1121/1.1590973 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Fitzgerald, T. S. (2003). “Ipsilateral distortion product otoacoustic emission (2f1-f2) suppression in children with sensorineural hearing loss,” J. Acoust. Soc. Am. 114, 919–931. 10.1121/1.1587147 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Sininger, Y., Ekelid, M., and Zeng, F. G. (1996). “Distortion product otoacoustic emission suppression tuning curves in human adults and neonates,” Hear. Res. 98, 38–53. 10.1016/0378-5955(96)00056-1 [DOI] [PubMed] [Google Scholar]
- American National Standards Institute, ANSI S3.6-1996 (2004). Specifications for Audiometers (American Institute of Physics, New York: ). [Google Scholar]
- Brown, A. M., and Kemp, D. T. (1984). “Suppressibility of the 2f1-f2 stimulated acoustic emissions in gerbil and man,” Hear. Res. 13, 29–37. 10.1016/0378-5955(84)90092-3 [DOI] [PubMed] [Google Scholar]
- Cooper, N. P., and Rhode, W. S. (1996). “Two-tone suppression in apical cochlear mechanics,” Aud. Neurosci. 3, 289–299. [Google Scholar]
- Delgutte, B. (1990). “Two-tone suppression in auditory-nerve fibers: Dependence on suppressor frequency and level,” Hear. Res. 49, 225–246. 10.1016/0378-5955(90)90106-Y [DOI] [PubMed] [Google Scholar]
- Garner, C. A., Neely, S. T., and Gorga, M. P. (2008). “Sources of variability in distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 124, 1054–1067. 10.1121/1.2939126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dierking, D., Dorn, P. A., Hoover, B. M., and Fitzpatrick, D. (2003). “Distortion product otoacoustic emission tuning curves in normal-hearing and hearing-impaired human ears,” J. Acoust. Soc. Am. 114, 263–278. 10.1121/1.1575751 [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dierking, D., Kopun, J., Jolkowsik, K., Groenenboom, K., Tan, H., and Stiegemann, B. (2007). “Low-frequency and high-frequency cochlear nonlinearity in humans,” J. Acoust. Soc. Am. 122, 1671–1680. 10.1121/1.2751265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Dierking, D., Kopun, J., Jolkowsik, K., Groenenboom, K., Tan, H., and Stiegemann, B. (2008). “Low-frequency and high-frequency distortion product otoacoustic emission suppression in humans,” J. Acoust. Soc. Am. 123, 2172–2190. 10.1121/1.2839138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. G., Neely, S. T., Konrad-Martin, D., and Dorn, P. A. (2002). “The use of DPOAE suppression as an estimate of response growth,” J. Acoust. Soc. Am. 111, 271–284. 10.1121/1.1426372 [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Kopun, J., and Tan, H. (2011). “Distortion-product otoacoustic emission suppression tuning curves in humans,” J. Acoust. Soc. Am. 129, 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesteadt, W., and Norton, S. J. (1985). “The role of suppression in psychophysical measures of frequency selectivity,” J. Acoust. Soc. Am. 78, 365–374 . 10.1121/1.392500 [DOI] [PubMed] [Google Scholar]
- Johnson, T. A., Neely, S. T., Garner, C. A., and Gorga, M. P. (2006). “Influence of primary-level and primary-frequency ratios on human distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 119, 418–428. 10.1121/1.2133714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer, P., Janssen, T., and Arnold, W. (1995). “Suppression tuning curves of the 2f1-f2 distortion-product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 98, 197–210. 10.1121/1.413747 [DOI] [PubMed] [Google Scholar]
- Kummer, P., Janssen, T., and Arnold, W. (1998). “The level and growth behavior of the 2f1-f2 distortion-product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss,” J. Acoust. Soc. Am. 103, 3431–3444. 10.1121/1.423054 [DOI] [PubMed] [Google Scholar]
- Kummer, P., Janssen, T., Hulin, P., and Arnold, W. (2000). “Optimal L1-L2 primary tone level separation remains independent of test frequency in humans,” Hear. Res. 146, 47–56. 10.1016/S0378-5955(00)00097-6 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Jassir, D., Stagner, B. B., and Lonsbury-Martin, B. L. (1998a). “Effects of loop diuretics on the suppression tuning of distortion-product otoacoustic emissions in rabbits,” J. Acoust. Soc. Am. 104, 972–983. 10.1121/1.423340 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Jassir, D., Stagner, B. B., Whitehead, M. L., and Lonsbury-Martin, B. L. (1998b). “Locus of generation for the 2f1-f2 vs 2f2-f1 distortion-product otoacoustic emissions in normal-hearing humans revealed by suppression tuning, onset latencies, and amplitude correlations,” J. Acoust. Soc. Am. 103, 1957–1971. 10.1121/1.421347 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Lonsbury-Martin, B. L., Probst, R., Sheinin, S. A., and Coats, A. C. (1987). “Acoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure-tone exposures,” Hear. Res. 28, 191–208. 10.1016/0378-5955(87)90049-9 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Stagner, B. B., and Lonsbury-Martin, B. L. (2010). “Evidence for basal distortion-product otoacoustic emission components,” J. Acoust. Soc. Am. 127, 2955–2972. 10.1121/1.3353121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, D. M. (1998). “Interpretation of distortion product otoacoustic emission measurements. II. Estimating tuning characteristics using three stimulus tones,” J. Acoust. Soc. Am. 103, 507–523. 10.1121/1.421101 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Glasberg, B. R., and Roberts, B. (1984). “Refining the measurement of psychophysical tuning curves,” J. Acoust. Soc. Am. 76, 1057–1066. 10.1121/1.391425 [DOI] [PubMed] [Google Scholar]
- Neely, S. T., Johnson, T. A., and Gorga, M. P. (2005). “Distortion-product otoacoustic emissions with continuously varying stimulus level,” J. Acoust. Soc. Am. 117, 1248–1259. 10.1121/1.1853253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, S. T., and Liu, Z. (1994). “EMAV: Otoacoustic emission average,” Tech. Memo No. 17 (Boys Town National Research Hospital, Omaha, NE: ). [Google Scholar]
- Pang, X. D., and Guinan, J .J. (1997). “Growth rate of simultaneous masking in cat auditory-nerve fibers: Relationship to the growth of basilar-membrane motion and the origin of two-tone suppression,” J. Acoust. Soc. Am. 102, 3564–3575. 10.1121/1.420147 [DOI] [PubMed] [Google Scholar]
- Pienkowski, M., and Kunov, H. (2001). “Suppression of distortion product otoacoustic emissions and hearing thresholds,” J. Acoust. Soc. Am. 113, 1574–1586. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S. (1977). “Some observations on two-tone interaction measured with the Mossbauer effect,” in Psychophysics and Physiology of Hearing, edited by Evans E. F. and Wilson J. P. (Academic Press, London: ), pp. 27–41. [Google Scholar]
- Rhode, W. S., and Cooper, N. P. (1993). “Two-tone suppression and distortion production on the basilar membrane in the hook region of cat and guinea pig cochleae,” Hear. Res. 66, 31–45. 10.1016/0378-5955(93)90257-2 [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., Robles, L., and Rich, N. C. (1992). “Two-tone suppression in the basilar membrane of the cochlea: Mechanical basis of auditory-nerve rate suppression,” J. Neurophysiol. 68, 1087–0199. [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., and Temchin, A. N. (2005). “Unexceptional sharpness of frequency tuning in the human cochlea,” Proc. Natl. Acad. Sci. 102, 18614–18619. 10.1073/pnas.0509323102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs, M. B., and Abbas, P. J. (1974). “Rate versus level functions for auditory-nerve fibers in cats: Tone-burst stimuli,” J. Acoust. Soc. Am. 56, 1835–1847. 10.1121/1.1903521 [DOI] [PubMed] [Google Scholar]
- Sachs, M. B., and Kiang, N. Y. S. (1968). “Two-tone inhibition in auditory-nerve fibers,” J. Acoust. Soc. Am. 43, 1120–1128. 10.1121/1.1910947 [DOI] [PubMed] [Google Scholar]
- Scheperle, R. A., Neely, S. T., Kopun, J. G., and Gorga, M. P. (2008). “Influence of in-situ sound level calibration on DPOAE variability,” J. Acoust. Soc. Am. 4, 288–300. 10.1121/1.2931953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, R. V. (1976). “Two-tone unmasking and suppression in a forward masking situation,” J. Acoust. Soc. Am. 59, 1460–1470. 10.1121/1.381007 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J. (1999). “Evoked otoacoustic emission arise by two fundamentally different mechanisms: A taxonomy of mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., Guinan, J. J., and Oxenham, A. J. (2010). “Otoacoustic estimates of cochlear tuning: Validation in the chinchilla,” J. Assoc. Res. Otol. 11, 343–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, J. H. (1994). “Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes,” J. Acoust. Soc. Am. 95, 2589–2597. 10.1121/1.409829 [DOI] [Google Scholar]
- Siegel, J. H. (2002). “Calibrating otoacoustic emission probes,” in Otoacoustic Emissions: Clinical Applications, 2nd ed., edited by Robinette M. S. and Glattke T. J. (Thieme Medical Publishers, Inc., New York, NY: ), pp. 416–441. [Google Scholar]
- Siegel, J. H., and Hirohata, E. T. (1994). “Sound calibration and distortion product otoacoustic emissions at high frequencies,” Hear. Res. 80, 146–152. 10.1016/0378-5955(94)90106-6 [DOI] [PubMed] [Google Scholar]
- Withnell, R. H., and Lodde, J. (2006). “In search of basal distortion product generators,” J. Acoust. Soc. Am. 120, 2116–2123. 10.1121/1.2338291 [DOI] [PubMed] [Google Scholar]