Abstract
In 44 consecutive patients with systemic sclerosis (SSc), plasma concentrations of von Willebrand factor (vWf) were higher than those of the vWf propeptide, but the propeptide showed less variability within patient subgroups. Higher values of the propeptide were observed in patients with early pulmonary involvement. A closer correlation of the propeptide than of vWf to biochemical markers of activity was also evident. Our results suggest that the propeptide, despite a shorter circulating half-time and lower plasma concentrations than vWf, is more useful in the assessment of disease activity in SSc.
Keywords: systemic sclerosis, von Willebrand factor, von Willebrand factor propeptide
Introduction
Systemic sclerosis (SSc) is characterized by autoimmunity, microangiopathy, and fibrosis in skin and internal organs. Endothelial activation is reflected by increased amounts of circulating endothelial proteins such as von Willebrand factor (vWf) [1], thrombomodulin [2], and endothelin [3]. Increased plasma concentrations of vWf, released from activated endothelial cells, have also been described in other vasculopathies such as hypertension [4] and diabetes [5] and are reported to predict diabetic nephropathy [6], myocardial infarction, and mortality [7,8].
However, the use of vWf as a marker of endothelial activation has several limitations. The variability is substantial even among normal subjects [9]. Concentrations of vWf can be influenced by the adhesive properties of the molecule and by changes in its clearance. For example, plasma concentrations of vWf are influenced by ABO blood groups [10,11]. Furthermore, plasma concentrations are reported to increase after exercise in normal individuals, a phenomenon which can be considered an adrenergic-type stress response [12]. Similar findings are reported in patients with rheumatoid arthritis [13] and in patients with intermittent claudication [14], possibly in both conditions an effect of a hypoxic reperfusion injury.
vWf is synthesized as a large, 360-kDa precursor, pro-vWf, which is cleaved into mature vWf and a 97-kDa propeptide [15]. Stimulation of exocytosis results in equimolar amounts of vWf and propeptide. The propeptide, which has a circulating half-time of 2-3 h, compared with >12 h [16] for vWf itself, was recently reported to be a more reliable marker of acute endothelial activation [17]. Propeptide concentrations are less influenced by factors such as blood groups, adhesive properties, and catabolism and thus show less variability among controls [11]. In a study of patients with type 1 diabetes [11], we found both vWf and propeptide to be increased in patients with overt nephropathy and in patients with microalbuminuria, whereas only the propeptide was increased in normoalbuminuric patients. This suggests that assessment of propeptide concentrations is a valuable complement in studies of endothelial activation and also in chronic vasculopathies.
The purpose of the present study was to compare the value of vWf propeptide in SSc as a marker of disease activity with that of vWf. We also related the two different vWf moieties to other markers of disease activity, including aminoterminal type III procollagen peptide (PIIINP) [18], and to internal organ involvement.
Materials and methods
Patients
Forty-four consecutive patients referred to the Department of Rheumatology, Lund University Hospital, Sweden, from January 1995 to July 1996 were included in the study. SSc was confirmed in 36 of these according to the criteria of the American College of Rheumatology (ACR) [19]. Twenty-three patients had limited cutaneous systemic sclerosis (lSSc), with skin sclerosis limited to extremities and face [20], and 13 had diffuse cutaneous systemic sclerosis (dSSc), with skin involvement of the trunk as well. The remaining eight patients were classified as having suspected systemic sclerosis (suspSSc), with Raynaud phenomenon and sclerodactyly (Supplementary Table 1). They did not fulfil the ACR criteria for SSc at the time for the assessment but did so 2 years later. No patient was at the time of assessment being treated with any putatively disease-modifying drug. Four patients were being treated with corticosteroids orally, three of them taking <5 mg per day and one taking 20 mg per day. Eighteen patients were being treated with calcium-channel blockers and three with inhibitors of angiotensin-converting enzyme. Six patients were smokers (two with dSSc, two with lSSc, and two with suspSSc). No patients had diabetes.
Supplementary Table 1.
Clinical characteristics of 44 patients with systemic sclerosis, grouped according to the skin involvement
| Disease form | dSSc | lSSc | suspSSc | ||||
| No. | 13 | 23 | 8 | ||||
| Sex (F/M) | 12/1 | 21/2 | 8/0 | ||||
| Normal | |||||||
| Median | Range (SEM) | Median | Range (SEM) | Median | Range (SEM) | values | |
| Age (y) | 49 | 27–75 (4.0) | 56 | 18–76 (3.3) | 51 | 20–67 (6) | |
| Duration (y) | 1 | 1–7 (0.46) | 2 | 1–49 (2.4) | – | ||
| Skin score (points) | 26 | 15–36 (1.7) | 5 | 2–15 (0.81) | 3 | 2–4 (0.3) | 0 |
| VC (%p) | 97 | 61–148 (6.3) | 94 | 51–120 (3.8) | 99 | 84–130 (6.0) | 80–120 |
| DLCO (%p) | 77 | 46–111 (6.0) | 77 | 40–116 (4.3) | 86 | 67–105 (5.4) | 80–120 |
| PAPsyst (mmHg) | 36 | 20–43 (2.9) | 30 | 18–65 (3.5) | 28 | 24–34 (1.6) | ≤ 32 |
| GFR (%p) | 109 | 94–130 (4.6) | 95 | 70–136 (3.8) | 101 | 93–114 (2.6) | 80–120 |
| Capillary density (loops/mm) | 5.1 | 2.9–8.3 (0.7) | 4.5 | 2.2–8.4 (0.4) | 6.9 | 5.7–7.4 (0.3) | 7.5–11.2 |
| ESR (mm/h) | 18 | 2–70 (6.3) | 14 | 4–70 (3.2) | 12.5 | 2–33 (4.3) | <20 |
| CRP (mg/l) | 6.5 | 5–88 (6.9) | 5 | 5–32 (1.2) | 5 | 5–26 (2.6) | <5 |
| Orosomucoid (g/l) | 0.94 | 0.70–1.89 (0.11) | 0.87 | 0.42–1.63 (0.53) | 0.76 | 0.45–1.13 (0.09) | 0.55–1.05 |
DLCO, carbon monoxide-diffusing capacity; GFR, glomerular filtration rate; PAPsyst, systolic pulmonary artery pressure; VC, vital capacity; %p, % of predictive value.
Based on pulmonary involvement combined with short disease duration (≤ 1 year), the 36 patients with verified SSc after the initial assessment were classified into two groups. Group A consisted of 12 patients with decreased vital capacity and/or carbon monoxide-diffusing capacity and disease duration of 1 year or less. Eight of these patients had dSSc and four had lSSc. Among the lSSc patients, pulmonary involvement was further stressed by alveolitis diagnosed by high-resolution computed tomography in two patients and pulmonary hypertension by Doppler cardiography in one patient. Group B consisted of the remaining 24 patients (Supplementary Table 2).
Supplementary Table 2.
Clinical characteristics of 35 patients with verified systemic sclerosis, grouped according to pulmonary involvement and duration of disease.
| Group A (n = 12) | Group B (n = 23) | ||||||
| Median | Range | (SEM) | Median | Range | (SEM) | ||
| dSSc/lSSc | 8/4 | 5/18 | |||||
| ACA/antiScl70 | 0/0 | 14/1 | |||||
| Age (y) | 57 | 26–75 | (4.7) | 49 | 25–76 | (2.8) | ns |
| Disease duration (y) | 1 | 1–1 | (0) | 2 | 1–49 | (2.3) | P < 0.01 |
| Skin score (points) | 26 | 3–36 | (3.2) | 8 | 2–26 | (1.6) | P < 0.01 |
| VC (%p) | 77 | 51–113 | (5.6) | 97 | 62–148 | (3.8) | P < 0.05 |
| DLCO (%p) | 62 | 40–90 | (4.9) | 85 | 47–116 | (3.7) | P < 0.01 |
| PAPsyst (mmHg) | 36 | 27–65 | (3.9) | 30 | 18–59 | (3.4) | ns |
| GFR (%p) | 103 | 89–130 | (5.4) | 96 | 70–136 | (4.1) | ns |
| Capillary density (loops/mm) | 4.2 | 2.4–6.9 | (0.6) | 5.5 | 2.2–8.4 | (0.4) | ns |
DLCO, carbon monoxide-diffusing capacity; GFR, glomerular filtration rate; PAPsyst, systolic pulmonary artery pressure; VC, vital capacity. P values indicate the difference between group A and group B. ns = Not significant.
The assays used are described briefly below, and in more detail in the Supplementary material.
Propeptide assay
The propeptide was analyzed by enzyme-linked immunosorbent assay (ELISA) as described elsewhere [17]. Each sample was tested at four dilutions. Results are expressed as percentage of a normal plasma pool (%NPP).
vWf assay
Analysis of vWf was by ELISA, using a rabbit antihuman vWf antibody (DAKO A082), a monoclonal mouse antihuman vWf antibody (DAKO M0616), and a rabbit anti-mouse Ig conjugated to alkaline phosphatase (DAKO D0314). The standard error of the mean in healthy controls was 9%. Results in this study are expressed as %NPP. The same normal plasma pool, comprising eight healthy nonsmoking persons (two male and six female), aged 36–76 years (median 54 years), was used in both methods (propeptide assay in Geneva and vWf assay in Lund). For all analyses, EDTA plasma was used. Blood samples were drawn from patients between 09.00 h and 10.00 h after 30 min rest in bed with no smoking.
Capillary abnormalities were analyzed quantitatively by a computer-based method [21]. The pulmonary artery pressure was measured in 27/44 patients by Doppler cardiography [22]. The glomerular filtration rate was determined by iohexol clearance [23] and expressed as the age-adjusted percentage of mean values for healthy controls (%p). Pulmonary function was assessed by vital capacity as measured by a dry spirometer and by carbon monoxide-diffusing capacity as measured by the single-breath method, both expressed as %p. Skin involvement was assessed by a modified Rodnan score, consisting of standardized palpation over 24 anatomical sites, using a scale of 0 to 3, with 0 being normal, 1 being thickened skin, 2 being thickened skin that cannot be pinched, and 3 being hidebound skin [24]. PIIINP was analyzed by radioimmunoassay (Orion Diagnostica, Espoo, Finland). The degree of inflammatory activity was determined by the ESR according to the Westergren method; C-reactive protein (CRP) and orosomucoid (α1 acid glycoprotein) were both determined by protein electrophoresis.
Statistics
The statistical significance of difference between two groups was calculated by the Mann–Whitney U test for unpaired observations and the Wilcoxon matched pairs signed ranks test for paired observations. A Kruskal–Wallis test was used for comparisons among three groups. The relation between two variables was calculated with Spearman's rho.
Results
Plasma concentrations of vWf in 44 SSc patients were higher than the corresponding concentrations of propeptide (P < 0.01; Supplementary Fig. 1). One outlier in vWf, with a plasma concentration nearly three times higher than any other observation, was excluded from all calculations. The range of propeptide values in each patient subgroup was narrower than that for vWf. The three subgroups (dSSc, lSSc, and suspSSc) differed in vWf (P < 0.05) but not in propeptide (P = 0.11). There was a correlation between vWf and propeptide (rho = 0.36, P < 0.05).
Supplementary Figure 1.
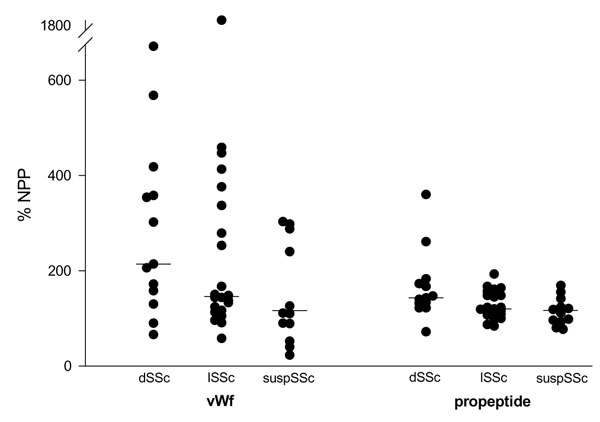
Plasma levels of vWf and its propeptide in 44 patients with SSc, grouped according to the degree of skin involvement. Medians are marked with horizontal bars.
The patients with verified SSc were subgrouped according to pulmonary involvement and short duration of disease into groups A and B. The patients in group A had higher plasma concentrations of propeptide (P < 0.02) than those in group B, whereas no significant difference was found in vWf. Table 1 shows biochemical parameters in the two groups. Orosomucoid concentrations were also higher in group A, but the difference showed less significance. No significant difference was found in CRP, ESR, or PIIINP.
Table 1.
Biochemical parameters in 35 patients with verified systemic sclerosis, grouped according to pulmonary involvement and duration of disease
| Biochemical | Group Aa | Group Ba | |
| parameter | n = 12 | n = 23 | |
| Propeptide (%NPP) | 150 | 120 | P < 0.02 |
| (87–360) | (72–167) | ||
| vWf (%NPP) | 210 | 146 | ns |
| (66–670) | (58–459) | (P = 0.32) | |
| CRP (mg/l) | 11 | 5 | ns |
| (5–88) | (5–32) | (P = 0.052) | |
| Orosomucoid (g/l) | 1.07 | 0.87 | P < 0.05 |
| (0.81–1.89) | (0.42–1.15) | ||
| ESR (mm/h) | 21 | 14 | ns |
| (2–70) | (4–70) | (P = 0.12) | |
| PIIINP (μg/l) | 5.8 | 4.0 | ns |
| (3.0–17.0) | (1.5–10.5) | (P = 0.07) |
Values are shown as median (range). P values indicate the difference between group A and group B; ns = not significant. aGroup A consisted of patients with short disease duration (≤ 1 year) and pulmonary involvement. Group B consisted of the remaining patients.
The correlation between the propeptide and vWf, respectively, and other biochemical markers of activity was calculated (Table 2). A closer relation of the propeptide than of vWf to other markers of activity was found. Both moieties correlated with skin score (rho=0.39 and 0.40, respectively) but not with pulmonary function or with kidney function. vWF but not propeptide correlated inversely with manifest vascular injury evaluated by capillary density (P < 0.05, rho=–0.41) and positively with pulmonary pressure (P < 0.05, rho=0.42). Neither parameter showed any relation to duration of disease.
Table 2.
Correlation (Spearman's rho) of plasma concentrations of vWf propeptide and vWf with various biochemical parameters of activity
| Biochemical parameter | Propeptide | vWf |
| PIIINP | 0.61** | 0.35* |
| Orosomucoid | 0.60** | 0.29 ns |
| CRP | 0.21 ns | 0.10 ns |
| ESR | 0.56** | 0.27 ns |
*P < 0.05; **P < 0.01; ns = not significant.
Discussion
Increased plasma concentrations of vWf in SSc have been described in several studies [1,25,26]. Higher concentrations in patients with a diffuse form of the disease [27,28] and in patients with more severe disease [28,29] are reported. A negative relation to the degree of capillary abnormalities is also described [25]. However, the usefulness of this marker of endothelial activation is limited by the great variability in normal values [9,11] and by the great overlap between patients and controls and between different subgroups of patients. The propeptide has been reported to be a more reliable marker of acute endothelial activation than is vWf [11,17].
In the present study of patients with SSc, we found higher plasma concentrations of vWf than of its propeptide, as would be expected in view of the known difference in the circulating half-times of the substances [16,17]. However, the propeptide showed a smaller range in each subgroup. The eight patients with suspSSc who didn't fulfil the ACR criteria at the time of the assessment but did so 2 years later are included, because they represent early cases and might be of special interest when endothelial activation is studied.
There is a lack of markers of disease activity in SSc. A lack of correlation between disease activity and CRP has been shown by Stuart et al [30]. The patients in group A had significantly shorter duration of disease and more pulmonary involvement, indicating a more serious and progressive disease. As expected, the skin score was higher in group A, because the proportion of patients with dSSc was higher in this group. The groups differed significantly in the propeptide but not in vWf. This difference was more pronounced than with other possible markers of disease activity: CRP, orosomucoid, and PIIINP. These results were further underscored by more pronounced correlations between propeptide and various markers of activity than between vWf and these markers. The absence of a correlation between vWf and CRP was also found by Blann et al [31]. The correlation of vWf and propeptide with skin score indicates a relation between endothelial activation and skin fibrosis. No correlation with pulmonary or kidney function was seen, probably because these patients had the disease for a short time and internal organ involvement was relatively discrete. In an earlier study [32], we found an inverse correlation between the glomerular filtration rate and vWf and a negative relation between pulmonary pressure and vWf in a small number of patients with isolated pulmonary hypertension. In the present study, the low proportion of patients with a decreased glomerular filtration rate (6/48) probably precludes the detection of any correlation.
This study showed lower plasma concentrations of propeptide than of vWf in patients with SSc but less variability within patient subgroups. The difference between patients with early pulmonary involvement compared with the others was more pronounced in propeptide compared with vWf and with other biochemical markers. The relation between propeptide and other markers of biochemical activity was more pronounced than for vWf. These results indicate that the propeptide concentrations might be more useful than vWf in the assessment of disease activity in patients with SSc.
Supplementary material
Materials and methods
Propeptide assay
The vWf propeptide was analyzed by ELISA as described elsewhere [17]. Briefly, 96-well plates were coated with the monoclonal antipropeptide antibodies BR5 and 3H4, 0.75 μg/ml each, in carbonate buffer (NaHCO3, 50 mM, pH 9.6). After the remaining protein binding sites were blocked with 5% (w/v) dry skimmed milk, the samples and standards, diluted in blocking solution, were added and incubated for 2 h at 37°C. The bound antigen was detected with a third, antipropeptide, monoclonal antibody (8H10) conjugated to alkaline phosphatase diluted to approximately 0.25 μg/ml. Alkaline phosphatase activity was detected using p-nitrophenyl phosphate as a substrate and measuring the optical density at 405 nm. Each sample was tested at four dilutions. Results are expressed as percentage of a normal plasma pool (%NPP).
vWf assay
vWf was analyzed by an ELISA. For this, 96-well plates (Nunc-Immuno Maxisorp, Roskilde, Denmark) were coated overnight at 4ºC with rabbit antihuman vWf antibody (DAKO A082) diluted 1:400 in phosphate-buffered saline (PBS). The plate was then washed (3 × 2 min) with PBS–Tween (0.5% Tween 20). To avoid measuring possible rheumatoid factor activity in the samples, all wells were incubated with normal rabbit IgG (DAKO X903) diluted 1:100 in PBS for 30 min at room temperature. The plates were washed, and 0.5% gelatin in PBS was added for 30 min incubation at room temperature to block remaining protein-binding sites. The plates were washed again, the samples were diluted 1:50 in PBS–Tween, added in duplicates to the plates, and incubated for 2 h at room temperature. After another washing, a monoclonal mouse antihuman vWf antibody (DAKO M0616) was added, diluted 1:2000 in PBS–Tween, and incubated for 30 min at room temperature. After washing, rabbit antimouse immunoglobulin conjugated to alkaline phosphatase (DAKO D0314) 1:1000 in PBS–Tween was added. Alkaline phosphatase activity was detected using p-nitrophenyl phosphate as a substrate and measured at dual wavelength (405 and 690 nm). At development of this ELISA, the concentration of vWf was determined against standard curves of Haemate (Hoechst, Marbourg, Germany), a commercially available vWf concentrate. The lower detection limit of the ELISA was 1.6 IE/ml. Both interassay and intra-assay variations were less than 10%. Results in the present study are expressed as %NPP. The same normal plasma pool comprising eight healthy persons (two male and six female), aged 36–76 years (median 54 years), was used in both methods (propeptide assay in Geneva and vWf assay in Lund). For all analyses, EDTA plasma was used. Blood samples were drawn from patients between 09.00 h and 10.00 h after 30 min rest in bed with no smoking.
Abbreviations
ACR = American College of Rheumatology; CRP = C-reactive protein; dSSc = diffuse cutaneous systemic sclerosis; ELISA = enzyme-linked immunosorbent assay; ESR = erythrocyte sedimentation rate; lSSc = limited cutaneous systemic sclerosis; NNP = normal plasma pool; PIIINP = aminoterminal type III procollagen peptide; suspSSc = suspected cutaneous systemic sclerosis; SSc = systemic sclerosis; vWf = von Willebrand factor.
Acknowledgments
Acknowledgements
Supported by grants from the Österlund Foundation, the Koch Foundation, The Medical Faculty of the University of Lund, the Medical Research Council (project no K97-19X-11628-02B), Sweden; and the Swiss National Science Foundation (grant 32-52667.97), Switzerland. We thank Mrs Jean Gunn for critical reading of the manuscript.
References
- Kahaleh MB, Osborn I, LeRoy EC. Increased factor VIII/von Willebrand factor antigen and von Willebrand factor activity in scleroderma and in Raynaud's phenomenon. Ann Int Med. 1981;94:482–484. doi: 10.7326/0003-4819-94-4-482. [DOI] [PubMed] [Google Scholar]
- Ohdama S, Takano S, Miyake S, Kubota T, Sato K, Aoki N. Plasma thrombomodulin as a marker of vascular injuries in collagen vascular diseases. Am J Clin Pathol. 1994;101:109–113. doi: 10.1093/ajcp/101.1.109. [DOI] [PubMed] [Google Scholar]
- Kahaleh MB. Endothelin, an endothelial-dependent vasoconstrictor in scleroderma: enhanced production and profibrotic action. Arthritis Rheum. 1991;34:978–983. doi: 10.1002/art.1780340807. [DOI] [PubMed] [Google Scholar]
- Pedrinelli R, Giampietro O, Carmassi F, Melillo E, Dell'Omo G, Catapano G, Matteucci E, Talarico L, Morale M, De Negri F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344:14–18. doi: 10.1016/s0140-6736(94)91047-2. [DOI] [PubMed] [Google Scholar]
- Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet. 1989;i:461–463. doi: 10.1016/s0140-6736(89)91365-2. [DOI] [PubMed] [Google Scholar]
- Stehouwer CDA, Fischer HRA, van Kuijk AWR, Polak BCP, Donker AJM. Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes. 1995;44:561–564. doi: 10.2337/diab.44.5.561. [DOI] [PubMed] [Google Scholar]
- Jansson J-H, Nilsson TK, Johnson O. Von Willebrand factor in plasma: a novel risk factor for recurrent myocardial infarction and death. Br Heart J. 1991;66:351–355. doi: 10.1136/hrt.66.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SG, Kienast J, Pyke SDM, Haverkate F, van de Loo JCV. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med. 1995;332:635–641. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- Ingerslev J. A sensitive ELISA for von Willebrand factor (vWF:Ag). Scand J Clin Lab Invest. 1987;47:143–149. [PubMed] [Google Scholar]
- Cox Gill J, Endres-Brooks J, Bauer PJ, Marks WJ, Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69:1691–1695. [PubMed] [Google Scholar]
- Vischer UM, Emeis JJ, Bilo HJG, Stehouwer CDA, Thomsen C, Rasmussen O, Hermansen K, Wollheim CB, Ingerslev J. Von Willebrand factor (vWf) as a plasma marker of endothelial activation in diabetes: improved reliability with parallel determination of the vWf propeptide (vWf:AgII). Thromb Haemost. 1998;80:1002–1007. [PubMed] [Google Scholar]
- Cohen RJ, Cohen LS, Epstein SE, Dennis LH. Alterations of fibronolysis and blood coagulation induced by exercise, and the role of beta-adrenergic-receptor stimulation. Lancet. 1968;ii:1264–1266. doi: 10.1016/s0140-6736(68)91760-1. [DOI] [PubMed] [Google Scholar]
- Farrel AJ, Williams RB, Stevens CR, Lawrie AS, Cox NL, Blake DR. Exercise induced release of von Willebrand factor: evidence for hypoxic reperfusion microvascular injury in rheumatoid arthritis. Ann Rheum Dis. 1992;51:1117–1122. doi: 10.1136/ard.51.10.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AT, Blann AD, Suarez-Mendez VJ, Lardi AM, McCollum CN. Systemic responses in patients with intermittent claudication after treadmill exercise. Br J Surg. 1994;81:1738–1741. doi: 10.1002/bjs.1800811211. [DOI] [PubMed] [Google Scholar]
- Vischer UM, de Moerloose P. Von Willebrand factor: from cell biology to the clinical management of von Willebrand's disease. Crit Rev Oncol Hematol. 1999;30:93–109. doi: 10.1016/s1040-8428(98)00045-6. [DOI] [PubMed] [Google Scholar]
- Borchiellini A, Fijnvandraat K, Wouter ten Cate J, Pajkrt D, van Deventer SJH, Pasterkamp G, Meijer-Huizinga F, Zwart-Huinink L, Voorberg J, van Mourik JA. Quantitative analysis of von Willebrand factor propeptide release in vivo: effect of experimental endotoxemia and administration of 1-deamino-8-D arginine vasopressin in humans. Blood. 1996;88:2951–2958. [PubMed] [Google Scholar]
- Vischer UM, Ingerslev J, Wollheim CB, Mestries J-C, Tsakiris DA, Haefeli WE, Kruithof EKO. Acute von Willebrand factor secretion from the endothelium in vivo: assessment through plasma propeptide (vWf:AgII) levels. Thromb Haemost. 1997;77:387–393. [PubMed] [Google Scholar]
- Scheja A, Åkesson A, Hørslev-Petersen K. Serum levels of aminoterminal type III procollagen peptide and hyaluronan predict mortality in systemic sclerosis. Scand J Rheumatol. 1992;21:5–9. doi: 10.3109/03009749209095054. [DOI] [PubMed] [Google Scholar]
- Masi A, Rodnan G, Medsger T, Altman RD, d'Angelo WA, Fries JF, LeRoy C, Kirsner A, MacKenzie A, McShane D, Myers A, Sharp G. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- LeRoy C, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger T, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- Scheja A, Åkesson A, Niewierowicz I, Wallin L, Wildt M, Wollheim FA. Computer based quantitative analysis of capillary abnormalities in systemic sclerosis and its relation in plasma concentration of von Willebrand factor. Ann Rheum Dis. 1996;55:52–55. doi: 10.1136/ard.55.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjaerpe T, Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986;7:704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- Krutzén E, Bäck SE, Nilsson-Ehle I, Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955–961. [PubMed] [Google Scholar]
- Åkesson A, Wollheim FA. Organ manifestations in 100 patients with progressive systemic sclerosis: a comparison between the CREST syndrome and diffuse scleroderma. Br J Rheumatol. 1989;28:281–286. doi: 10.1093/rheumatology/28.4.281. [DOI] [PubMed] [Google Scholar]
- Lee P, Norman CS, Sukenik S, Alderdice CA. The clinical significance of coagulation abnormalities in systemic sclerosis (scleroderma). J Rheumatol. 1985;12:514–517. [PubMed] [Google Scholar]
- Pagano L, Marra R, Paoletti S, Afa G, Storti S, Garcovich A, Bizzi B. Factor VIII complex in progressive systemic sclerosis. Clin Exp Rheumatol. 1986;4:319–321. [PubMed] [Google Scholar]
- Blann AD, Illingworth KJ, Jayson MIV. Raised concentrations of von Willebrand factor antigen in systemic sclerosis. Ann Rheum Dis. 1991;50:337–338. doi: 10.1136/ard.50.5.337-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL, Illingworth K, Blann A, Hay CRM, Hollis S, Jayson MIV. Von Willebrand factor, thrombomodulin, tromboxane, β-thromboglobulin and markers of fibrinolysis in primary Raynaud's phenomenon and systemic sclerosis. Ann Rheum Dis. 1996;55:122–127. doi: 10.1136/ard.55.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, Malia RG, Milford Ward A, Moult J, Holt CM, Lindsey N, Hughes P, Goodfield M, Rowell NR. Elevated von Willebrand factor antigen in systemic sclerosis: relationship to visceral disease. Br J Rheumatol. 1988;27:281–285. doi: 10.1093/rheumatology/27.4.281. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Littlewood AJ, Maddison PJ. Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol. 1995;13:17–22. [PubMed] [Google Scholar]
- Blann AD, Herrick A, Jayson MIV. Altered levels of soluble adhesion molecules in rheumatoid arthritis, vasculitis and systemic sclerosis. Br J Rheumatol. 1995;34:814–819. doi: 10.1093/rheumatology/34.9.814. [DOI] [PubMed] [Google Scholar]
- Scheja A, Eskilsson J, Åkesson A, Wollheim FA. Inverse relation between plasma concentration of von Willebrand factor and CrEDTA clearance in systemic sclerosis. J Rheumatol. 1994;21:639–642. [PubMed] [Google Scholar]


