Abstract
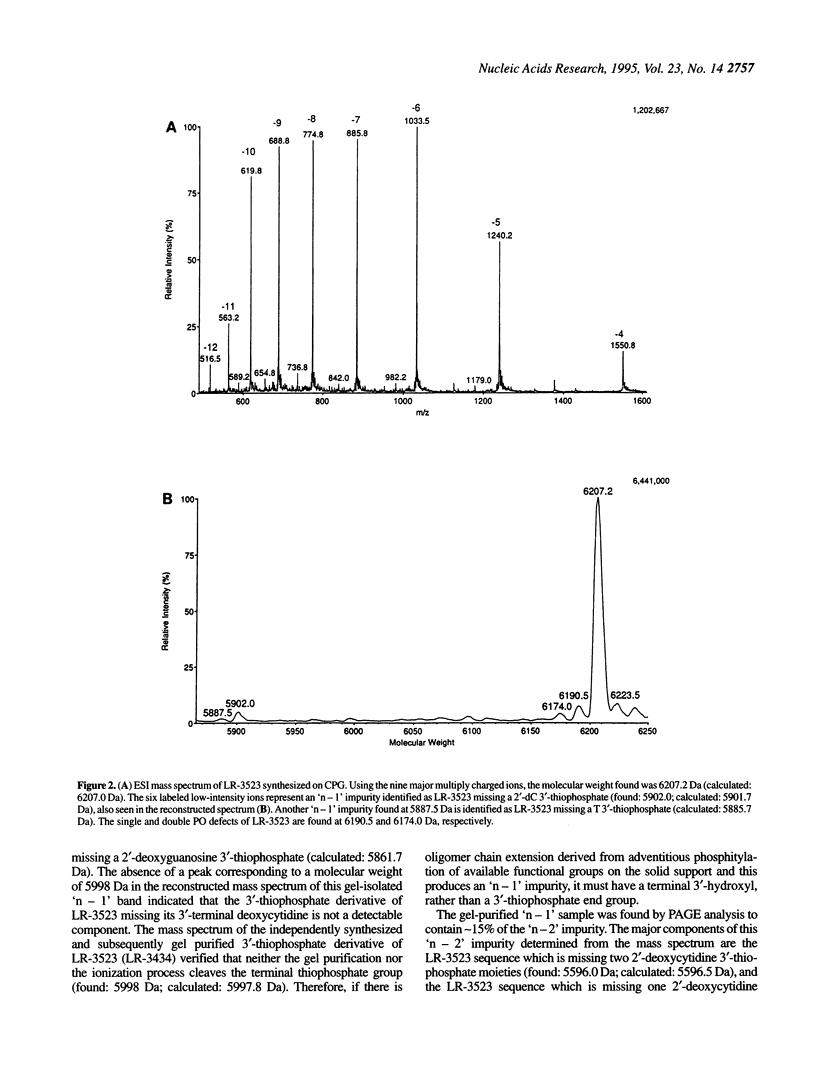
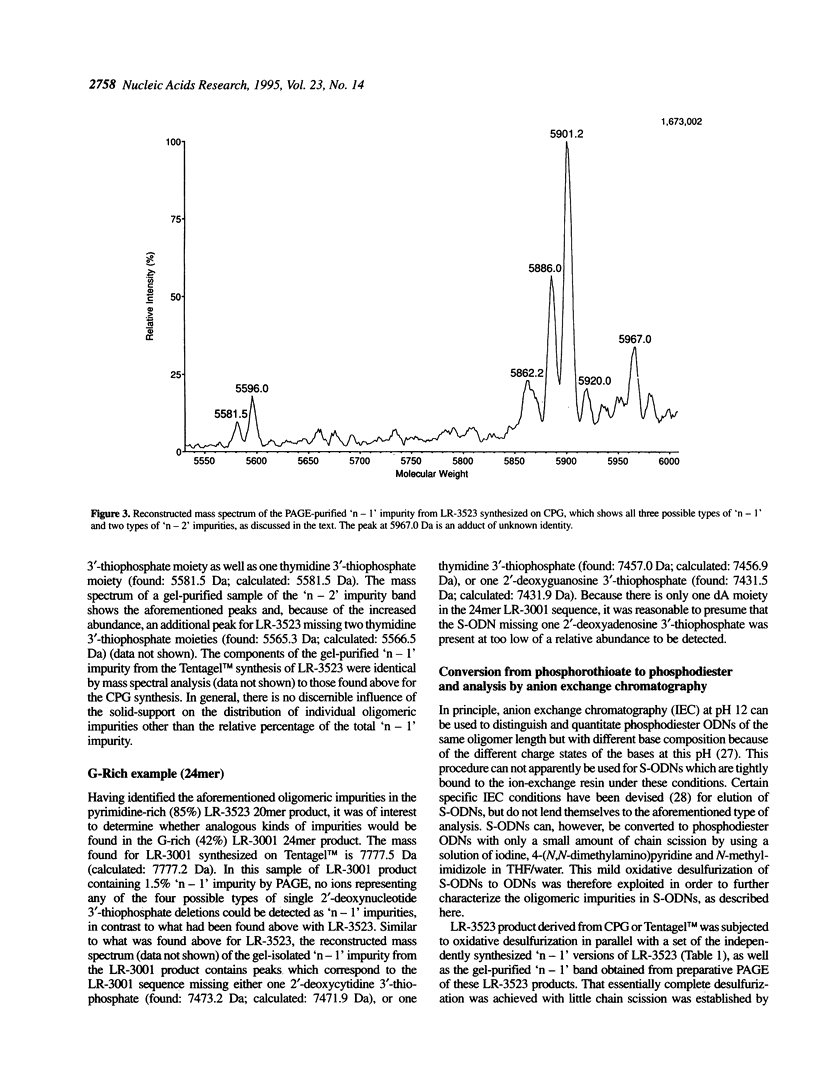
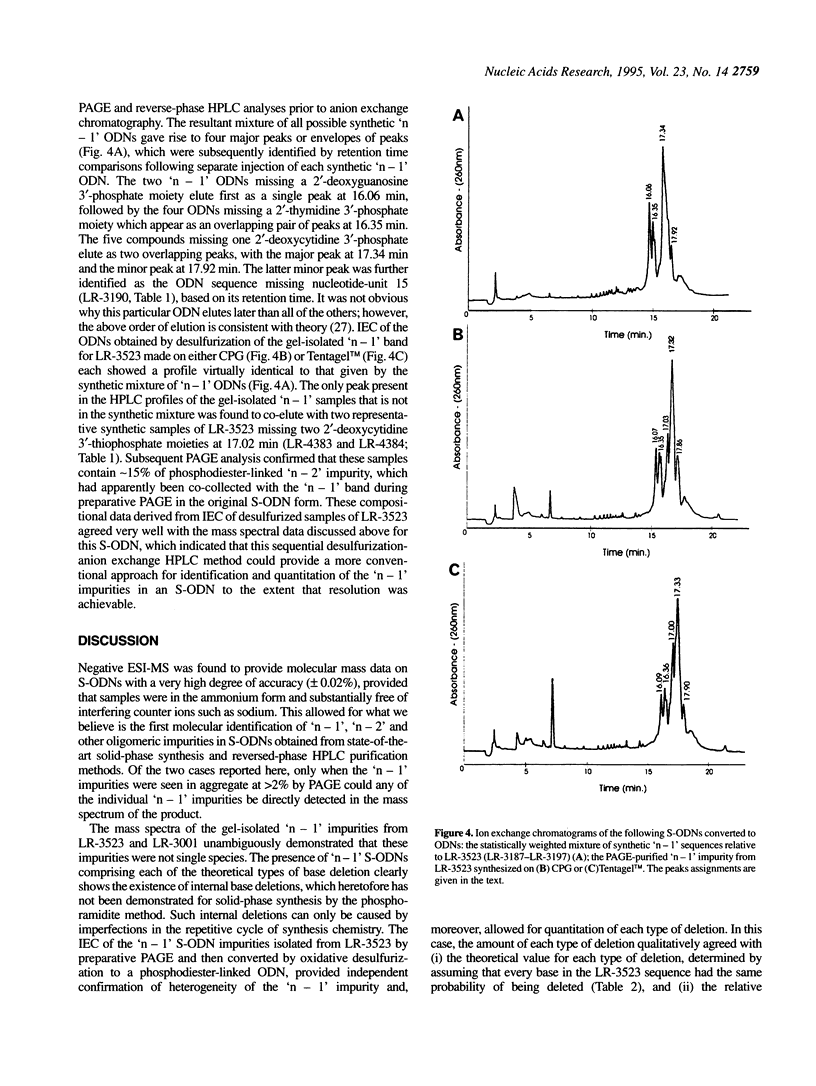
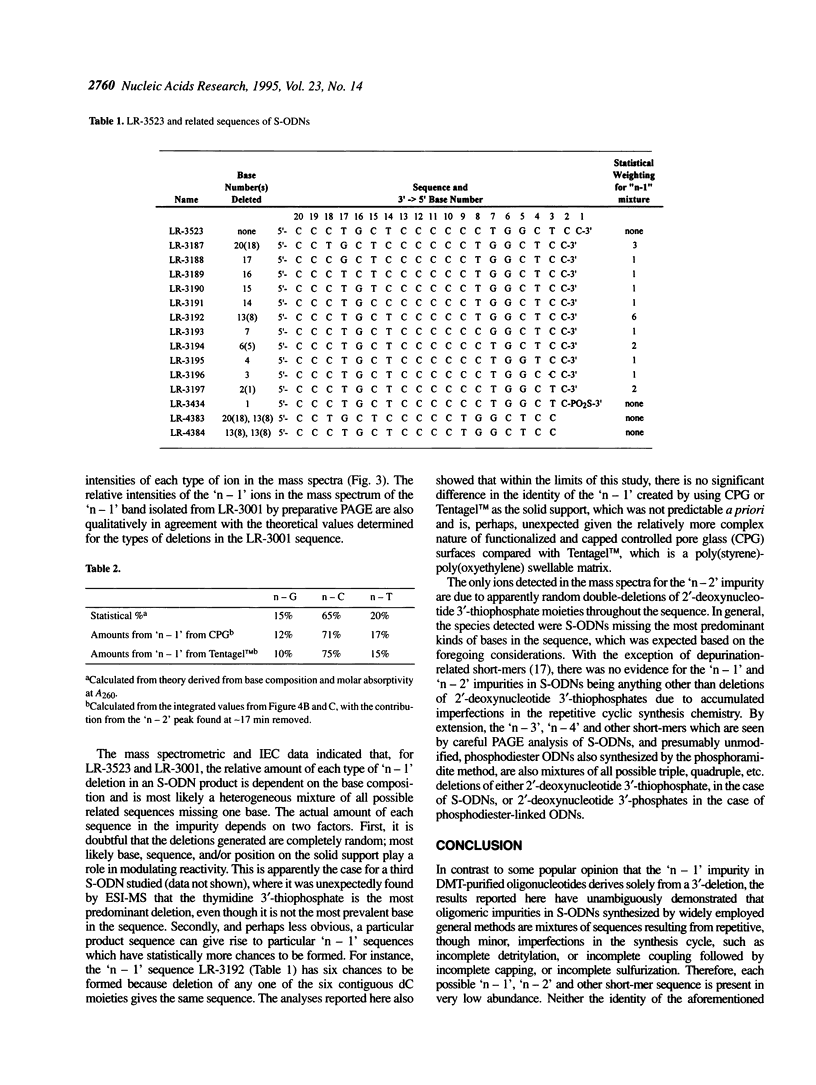
Electrospray ionization mass spectrometry (ESI-MS) of reversed-phase HPLC-purified phosphorothioate oligodeoxynucleotides (S-ODNs), and the single-('n - 1') and double-nucleotide deletion ('n - 2') impurities subsequently isolated from them by preparative polyacrylamide gel electrophoresis (PAGE), has provided direct analytical data for the identification of both S-ODN products and their major oligomeric impurities. The 'n - 1' impurity seen by PAGE consists of a mixture of all possible single deletion sequences relative to the parent S-ODN (n-mer) and results from repetitive, though minor, imperfections in the synthesis cycle, such as incomplete detritylation, or incomplete coupling followed by incomplete capping or incomplete sulfurization. Therefore each possible 'n - 1', 'n - 2', and other short-mer sequence is present only in very low abundance. The conversion of the gel-isolated 'n - 1' impurity from phosphorothioate to phosphodiester followed by base composition-dependent anion-exchange chromatography allowed for independent confirmation of its heterogeneity and quantitation of its various components. ESI-MS of both S-ODN products and their gel-isolated impurities allowed for this first molecular identification of 'n - 1', 'n - 2' and other oligomeric impurities in S-ODNs obtained from state-of-the-art solid-phase synthesis and reversed-phase HPLC purification methods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayever E., Iversen P. L., Bishop M. R., Sharp J. G., Tewary H. K., Arneson M. A., Pirruccello S. J., Ruddon R. W., Kessinger A., Zon G. Systemic administration of a phosphorothioate oligonucleotide with a sequence complementary to p53 for acute myelogenous leukemia and myelodysplastic syndrome: initial results of a phase I trial. Antisense Res Dev. 1993 Winter;3(4):383–390. doi: 10.1089/ard.1993.3.383. [DOI] [PubMed] [Google Scholar]
- Bleicher K., Bayer E. Various factors influencing the signal intensity of oligonucleotides in electrospray mass spectrometry. Biol Mass Spectrom. 1994 Jun;23(6):320–322. doi: 10.1002/bms.1200230604. [DOI] [PubMed] [Google Scholar]
- Cohen J. S. Gene-mimetic substances: drugs designed to intervene in gene expression. Adv Pharmacol. 1994;25:319–339. doi: 10.1016/s1054-3589(08)60436-6. [DOI] [PubMed] [Google Scholar]
- Covey T. R., Bonner R. F., Shushan B. I., Henion J. The determination of protein, oligonucleotide and peptide molecular weights by ion-spray mass spectrometry. Rapid Commun Mass Spectrom. 1988 Nov;2(11):249–256. doi: 10.1002/rcm.1290021111. [DOI] [PubMed] [Google Scholar]
- Froehler B. C., Matteucci M. D. Dialkylformamidines: depurination resistant N6-protecting group for deoxyadenosine. Nucleic Acids Res. 1983 Nov 25;11(22):8031–8036. doi: 10.1093/nar/11.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo K. A., Shao K. L., Phillips L. R., Regan J. B., Koziolkiewicz M., Uznanski B., Stec W. J., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. V. Synthesis and absolute configuration of Rp and Sp diastereomers of an ethyl phosphotriester (Et) modified EcoRI recognition sequence, d[GGAA(Et)TTCC]. A synthetic approach to regio- and stereospecific ethylation-interference studies. Nucleic Acids Res. 1986 Sep 25;14(18):7405–7420. doi: 10.1093/nar/14.18.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Gryaznov S. M., Letsinger R. L. Template controlled coupling and recombination of oligonucleotide blocks containing thiophosphoryl groups. Nucleic Acids Res. 1993 Mar 25;21(6):1403–1408. doi: 10.1093/nar/21.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijiya N., Zhang J., Ratajczak M. Z., Kant J. A., DeRiel K., Herlyn M., Zon G., Gewirtz A. M. Biologic and therapeutic significance of MYB expression in human melanoma. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4499–4503. doi: 10.1073/pnas.91.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn T., Urdea M. S. Solid supported hydrolysis of apurinic sites in synthetic oligonucleotides for rapid and efficient purification on reverse-phase cartridges. Nucleic Acids Res. 1988 Dec 23;16(24):11559–11571. doi: 10.1093/nar/16.24.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati R. V., Chiu Y. Y., Chen C. W., Blumenstein J. J. Regulatory concerns for the chemistry, manufacturing, and controls of oligonucleotide therapeutics for use in clinical studies. Antisense Res Dev. 1993 Winter;3(4):405–410. doi: 10.1089/ard.1993.3.405. [DOI] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]
- Ratajczak M. Z., Kant J. A., Luger S. M., Hijiya N., Zhang J., Zon G., Gewirtz A. M. In vivo treatment of human leukemia in a scid mouse model with c-myb antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11823–11827. doi: 10.1073/pnas.89.24.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorski T., Nieborowska-Skorska M., Nicolaides N. C., Szczylik C., Iversen P., Iozzo R. V., Zon G., Calabretta B. Suppression of Philadelphia1 leukemia cell growth in mice by BCR-ABL antisense oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4504–4508. doi: 10.1073/pnas.91.10.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., Loo J. A., Edmonds C. G., Barinaga C. J., Udseth H. R. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990 May 1;62(9):882–899. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Xu Y. Z., Swann P. F. Chromatographic separation of oligodeoxynucleotides with identical length: application to purification of oligomers containing a modified base. Anal Biochem. 1992 Jul;204(1):185–189. doi: 10.1016/0003-2697(92)90159-5. [DOI] [PubMed] [Google Scholar]