Abstract
Development of small footprint, disposable, fast, and inexpensive devices for pathogen detection in the field and clinic would benefit human and veterinary medicine by allowing evidence-based responses to future out breaks. We designed and tested an integrated nucleic acid extraction and amplification device employing a loop-mediated isothermal amplification (LAMP) or reverse transcriptase-LAMP assay. Our system provides a screening tool with polymerase-chain-reaction-level sensitivity and specificity for outbreak detection, response, and recovery. Time to result is ~90 min. The device utilizes a swab that collects sample and then transfers it to a disc of cellulose-based nucleic acid binding paper. The disc is positioned within a disposable containment tube with a manual loading port. In order to test for the presence of target pathogens, LAMP reagents are loaded through the tube’s port into contact with the sample containing cellulose disc. The reagents then are isothermally heated to 63°C for ~1 h to achieve sequence-specific target nucleic acid amplification. Due to the presence of a colorimetric dye, amplification induces visible color change in the reagents from purple to blue. As initial demonstrations, we detected methicillin resistant Staphylococcus aureus genomic DNA, as well as recombinant and live foot-and-mouth disease virus.
Index Terms: Device, disposable, methicillin resistant Staphylococcus aureus (MRSA), point-of-care testing (POCT), triage, virus
I. INTRODUCTION
EARLY detection of infectious diseases impacting public health and/or veterinary medicine requires cost-effective, robust, and specific assays. Due to these requirements, such assays traditionally have been conducted in centralized laboratories offering large footprint, fragile and/or expensive equipment (centrifuges, vortexers, thermocyclers, microscopes, incubators, external power supplies, etc.), and highly trained technicians, rather than close to the affected patient or animal.
A few companies have successfully designed highly specific point-of-care testing (POCT) equipment relevant to the detection of pathogens. POCT promises to bring the test to the test subject, in either the field or the clinic, providing more rapid detection with potential benefit to both the test subject and to public health.
High-specificity POCT equipment for pathogen detection typically relies on polymerase chain reaction (PCR), conducted in the field. Although PCR systems provide excellent sensitivity and specificity, they also greatly increase costs and require very clean samples as compared to other less sensitive assays, such as enzyme-linked immunosorbent assays (ELISAs).
We have designed and tested a radically simplified POCT system for the detection of infectious diseases. The system mitigates a need for the substantial upfront investment of PCR-based systems. Furthermore, it is contained and disposable, reducing a risk of cross contamination via the testing equipment.
We successfully conducted proof-of-concept validation of our POCT system by amplifying and detecting methicillin resistant Staphylococcus aureus (MRSA). S. aureus is a gram-positive bacterium that causes serious community- and hospital-acquired infections. In developed countries, MRSA constitutes up to 60% of isolated S. aureus infections [1].
We also amplified and detected serotype O (most prevalent of seven different serotypes) [2] foot-and-mouth disease virus (FMDV) reference strain O1 Manisa. FMD is a highly infectious viral disease of cloven-hoofed animals, including cows, sheep, goats, and pigs.
II. METHODS AND MATERIALS
A. Prototype Tubes and Assay
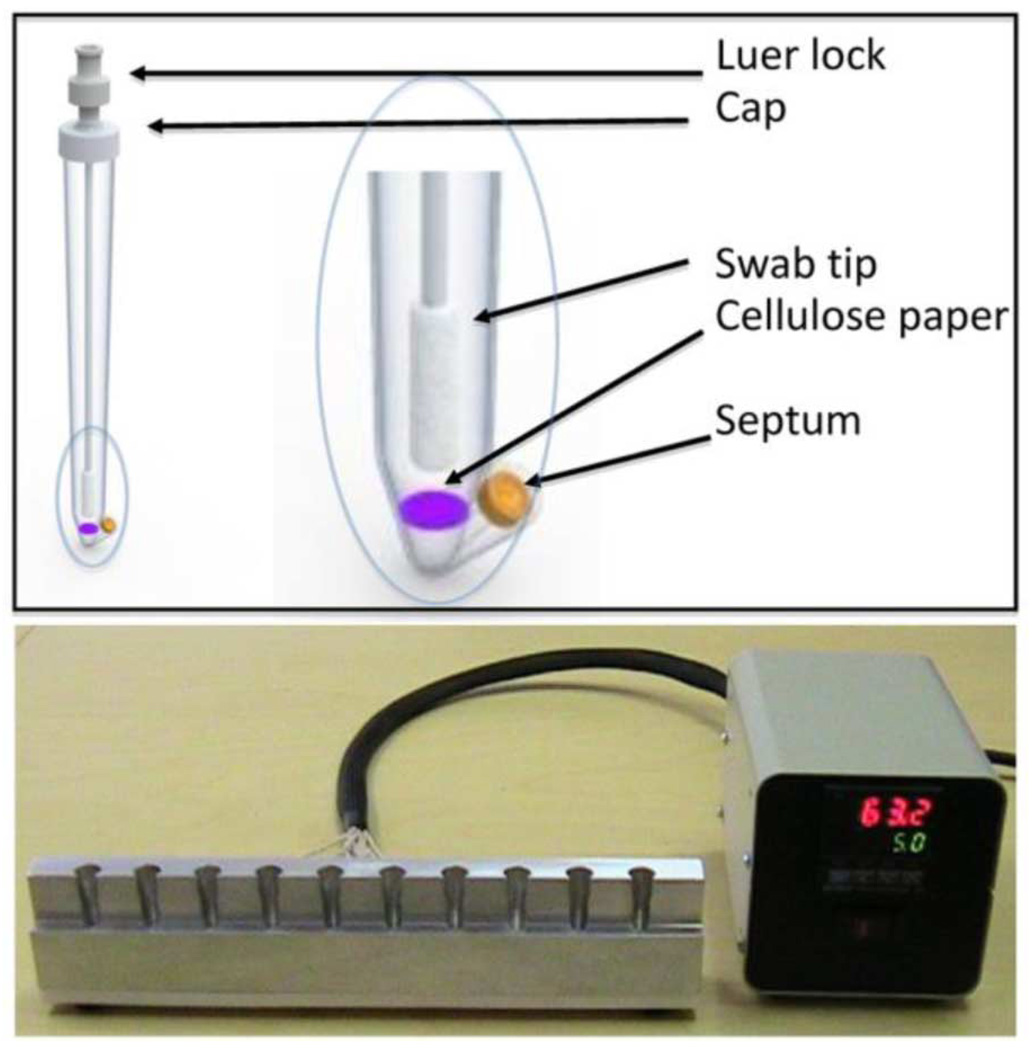
We have designed a POC assay that encompasses sample acquisition, sample preparation/nucleic acid extraction, amplification, and detection in a single, disposable tube. A rendering of our prototype assay device, designed at Lawrence Livermore National Laboratory (Livermore, CA) and fabricated at Symbient Product Development (Vista, CA), is shown in Fig. 1. The platform includes the sealable polypropylene tube, a hollow polyester swab coupled to a tube cap, a 4-mm disc of cellulose FTA disc (Whatman, Kent, U.K.) positioned at the base of the tube, reagents including dye for conducting loop-mediated isothermal amplification (LAMP), and a heater. The swab facilitates sample acquisition from surfaces, oral or nasal cavities, or lesions. This technique is a modification of previously published work accomplished by direct transfer of swab to FTA card [3], [4].
Fig. 1.
Schematic rendering of (top) disposable prototype device and (bottom) photograph of heater, both built by Symbient Product Development.
Sample is collected by wiping material of interest with the hollow swab. After sample collection, the swab is returned to the tube, and the tube cap is secured in a manner that precludes reopening it. A first syringe containing 4 mL of Purification Reagent (Whatman) is attached to a Luer lock fitting on the cap, and then 2 mL of the Purification Reagent is delivered through the swab lumen, immersing the sample. The tube is swirled and set in a rack for approximately 2 min, followed by waste removal through the tube’s lower, polyisoprene septum via a pipette. Purification Reagent steps then are repeated.
The first syringe is decoupled from the Luer lock, a second syringe containing 4-mL TE buffer (Ambion, Austin, TX) is attached, and 2-mL TE buffer is delivered through the swab lumen to rinse the sample. After swirling of the tube, waste is removed, and the TE buffer steps are repeated. At this point, sample has transferred from swab to FTA disc and cleaned.
Next, LAMP/reverse transcriptase (RT-LAMP) master mix plus enzymes are pipetted into the evacuated tube via the polyisoprene septum, immersing the FTA card. One or more such assay tubes are then placed on the custom thermal heater rack (see Fig. 1), which is maintained at an isothermal temperature of about 63°C for 45 min to 1 h, to conduct LAMP/RT-LAMP amplification. Positive amplification is determined by a visible color change in the reagents from purple to blue, due to the presence of colorimetric dye.
In our current prototype system, Purification Reagent and buffer streams are introduced through the swab via syringe, while reagents are introduced through the septum via pipette. Furthermore, waste streams are evacuated through the septum. While such introduction and evacuation of solutions are appropriate in a laboratory setting, our planned next-generation prototype system will reduce platform complexity and facilitate field deployment by fully enclosing wash and reagent solutions and by fully containing waste streams.
B. Primers, Template, and Virus
MRSA primers were designed using LAVA-LAMP software [5] and were purchased from Biosearch Technologies. MRSA primers are part of a publication in progress. S. aureus strain FPR3757/USA300 genomic DNA (BAA-1556D-5, American Type Culture Collection Manassas, VA), used as positive MRSA template, was received as lyophilized powder and dissolved in 1X TE buffer (10 mM Tris-HCl, pH 8.0, 1.0 mM EDTA, pH 8.0, sterile solution, (#T0226, Teknova, Inc., Hollister, CA) to an initial concentration of approximately 100 ng/µL. The exact DNA concentration was measured by the PicoGreen assay (P11496, Invitrogen Corp., Carlsbad, CA) on a Qubit fluorometer (Invitrogen Corp., Carlsbad, CA), and 1 ng/µL stock solutions were prepared and stored at −20°C. Our lowest level of detection, 0.05 pg, represents 17 copies of DNA.
The primers for pan-serotypic detection of FMDV and the performance of this assay (analytical sensitivity) have been previously published [6]. Recombinant template was used for initial FMDV experiments. For live virus testing, an epithelial homogenate (10% suspension in PBS) was prepared under BSL3 (UK SAPO4 containment—Specified Animal Pathogens Order) conditions from tongue tissue collected from cattle experimentally infected with O1-Manisa strain FMDV. The performance of the LAMP assay system was evaluated using prototype swabs that were dipped in 100 µL of the suspension at 10−1, 10−2, and 10−5 dilutions. Presence of FMDV in this material was verified by automated real-time RT-PCR targeting the 3-D region of the FMDV genome [7].
C. LAMP and RT-LAMP
LAMP is a robust, isothermal nucleic acid amplification method. Master mix and enzymes were added to prototype tubes via the septum and heated to 63°C for between 45 min and 1 h. Reactions included primer solutions prepared by combining 40 µL each of 100 µM FIP and 100 µM BIP, 5 µL each of 100 µM F3 and 100 µM B3, and 20 µL each of 100 µM LF and 100 µMLB, and 370 µl of TE buffer, resulting in 500 µL of combined primer solution. reaction volumes of 100 µL for MRSA assays comprised 25 µL of combined primer solution plus 70-µL base mix [1.4 mM each dNTPs (Roche Diagnostics, Basel, Switzerland), 0.8 M betaine (Sigma, St. Louis, MO), 4.1 mM MgSO4 (New England Biolabs, Ipswich, MA), 1X Thermopol buffer (New England Biolabs), and 100 µM hydroxynaphthol blue (HNB) (Dojindo Laboratories) in DEPC water (Ambion)] and 5 µL BST polymerase (New England Biolabs).
FMDV Master Mix was slightly modified and included 4 µL BST polymerase, as well as 0.3 µL Thermoscript RT (Invitrogen, Carlsbad, CA) per 100-µL reaction volume.
HNB indicates target amplification to the unaided eye via a color shift that stems from changes in the concentration of Mg2+ in solution: free Mg2+ in the reaction solution binds to pyrophosphate that is generated as deoxynucleotide triphosphates are added to growing amplification product, forming magnesium pyrophosphate [8]. This is in contrast to fluorescent dyes, which fluoresce upon intercalation into amplification products but require an excitation source and electronic detection system.
III. RESULTS
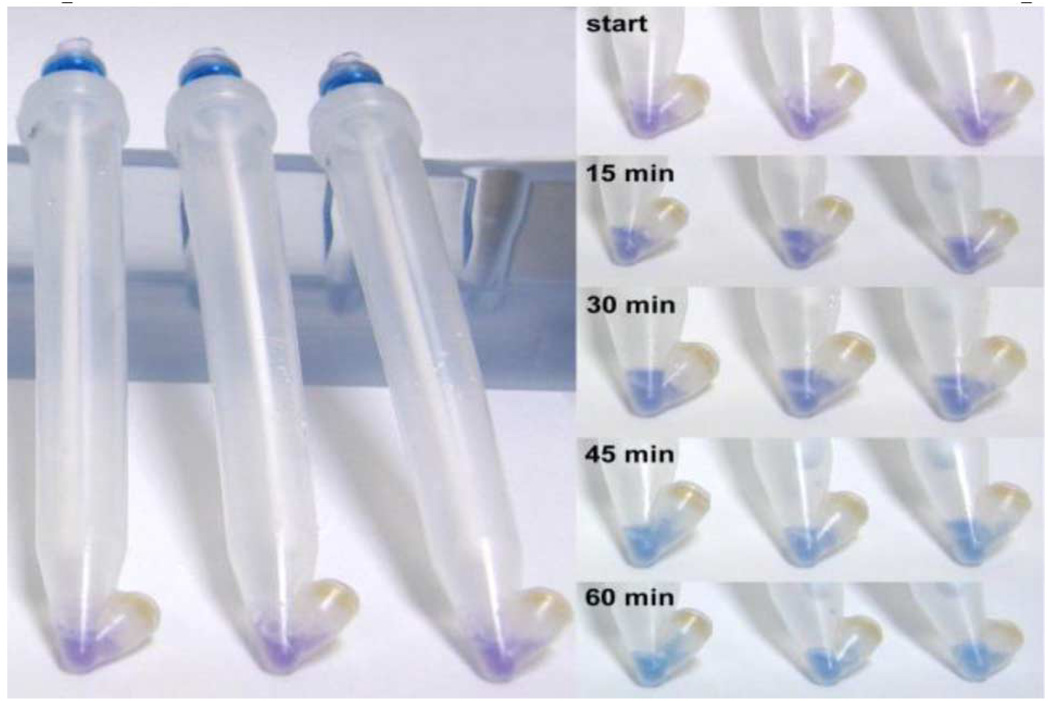
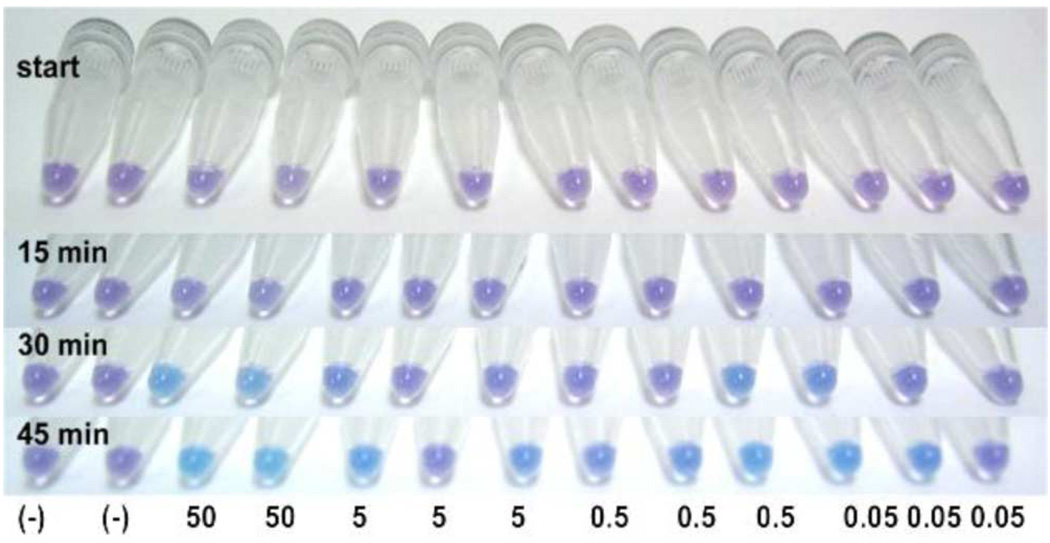
We successfully tested our POCT device under BSL1 conditions for MRSA genomic DNA. Fig. 2 shows time series images of MRSA assay in prototype tubes. Genomic DNA transferred from swabs to filter paper during sample preparation and amplification was performed on material embedded in the paper. Fig. 3 shows a 45-min MRSA assay in microcentrifuge tubes in order to assess preliminary assay sensitivity. Data indicate that the colorimetric assay can detect ~17 genomic copies of MRSA. Water served as no template control (NTC). Four percent agarose gels corroborated sequence amplification in solutions that turned blue, and lack of amplification in solutions that remained purple.
Fig. 2.
Time series images of initial MRSA assay in prototype tubes. Prototype swabs were dipped in 100 µL of 10 pg/µL DNA.
Fig. 3.
Time series images of MRSA assay in microcentrifuge tubes. (−) represents no template controls. Numbers indicate pg DNA per reaction. Most dilute MRSA genomic DNA samples on right contain ~17 copies of DNA.
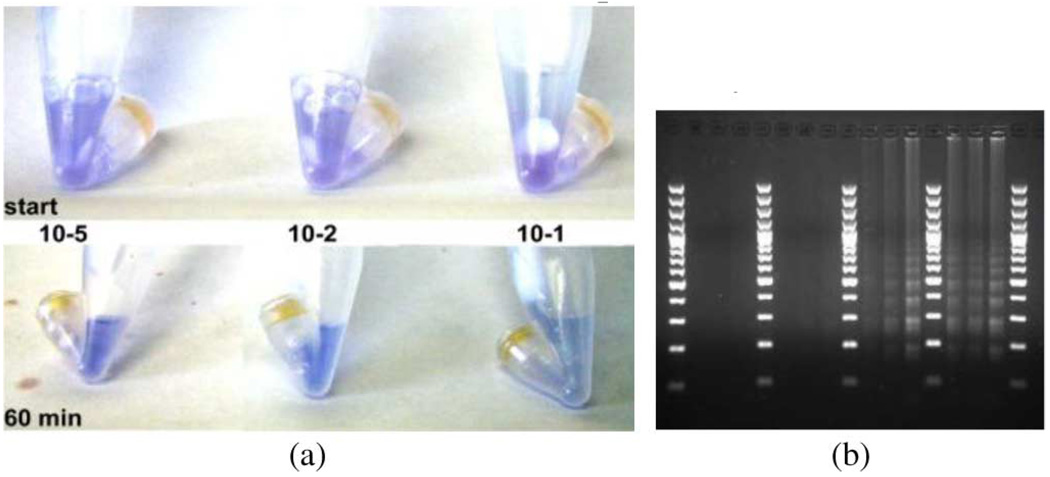
POCT testing on recombinant FMDV, and clinical isolate tissue homogenate (live virus, BSL3, Pirbright, UK) was also performed. Fig. 4 shows images of a dilution series of live FMDV taken at start and 60 min. A gel was run to confirm the colorimetric results. Our results indicate that, while performing the entire FMDV assay in a single disposable tube, we can colorimetrically detect virus diluted down to 10−5 dilution in about 45 min. Parallel testing of these samples (in triplicate) by real-time RT-PCR generated CT values (±standard deviation) of 15.7 ± 0.1, 19.6 ± 0.3, and 31.0 ± 0.1 for the 10−1, 10−2, and 10−5 dilutions, respectively.
Fig. 4.
(a) Images of live FMDV dilution series from tissue homogenate run in prototype reaction tubes. Top panel indicates starting reaction colors, and lower panel indicates reaction mix colors at 60 min. Reaction mixture results indicate a violet purple to blue transition with increasing virus concentration (more virus yields → lighter blue). (b) Time sequence gel electrophoresis results from samples run in prototype reaction tubes. Lanes represent 15, 30, 45, and 60 min time points across 10−5, 10−2, and 10−1 dilution series. A 100-bp DNA marker was used. LAMP typically produces a laddered agarose gel pattern representing multiple copies of the same sequence in the product. All virus dilutions amplified.
IV. DISCUSSION
POCT efforts, whether field or clinic based, strive to convert testing of samples from slower culture based or rapid and qualitative screening methods, such as ELISAs, to diagnostic tests, such as PCR, without significant loss of time. As O’Shea describes present field-based biological detection capabilities, “The devices are split between quick and easy, and specific and not so easy” [9]. O’Shea further describes affordable assays (Advnt’s single BADD assay is $27) as those that can grant “bronze”-level detection, but reports that higher specificity or “gold”-level detection is based on costly PCR to provide more accurate results.
Sample preparation is another limiting step in the development of POCT assays. Some sample preparation kits, such as Qiagen RNA extraction kits, are easy to use and effective. However, these protocols require a centrifuge, precluding incorporation of such kits into hand-held POCT devices. Current fully integrated technologies for pathogen detection via nucleic acid amplification are prohibitively expensive for routine clinical or veterinary use at the POC.
Our prototype system provides a cost-effective, field-deployable POCT pathogen-screening tool with PCR-level sensitivity by providing sample acquisition, preparation, amplification, and detection all in one disposable tube. The only component of our system requiring power is an isothermal heater, and we anticipate that the heater could be simplified and run via battery power.
We have incorporated a simple and convenient sample preparation technology into our device: a purification reagent cleans up complex tissue matrix sample and separates out genetic material, while a Whatman FTA card retains the material. FTA cards are filter papers containing chemicals that lyse cells, denature proteins, and protect nucleic acids from nucleases, oxidative, and UV damage [10]. While clinical studies have amplified nucleic acid from human papillomavirus (HPV) and Pseudomonas aeruginosa eluted from FTA cards [11], [12], other studies have successfully amplified nucleic acid via LAMP directly on filter paper bound samples [13], [14].
In contrast to PCR, LAMP-based amplification enables sensitive and continuous amplification of genetic material under isothermal conditions. This greatly reduces instrumentation complexity, and the LAMP technique increasingly is being used in bench-top assays for rapid detection and typing of emerging viruses [6], [15], [16]. Denatured template is not required [17], and LAMP can withstand more contaminants than PCR. Previous reports indicate that PCR has lower sensitivity for amplification of DNA on filter papers compared to LAMP [14]; it is thought that this is due to blood components, which do not affect the BST polymerase used in LAMP [18].
Modifying detection technologies from traditional fluorescence readouts to those that rely on the naked eye as detector also simplifies instrumentation/device requirements and therefore reduces costs. Goto reported using HNB as a colorimetric end point indicator consistent with the requirements of LAMP in 2009 [8]. Recently, Ma reported use of HNB in conjunction with a H1N1 assay [19]. HNB, first used for titration of calcium ions, is stable in solution for months and can be added to master mix prior to amplification.
Our current prototype successfully demonstrates sample preparation through detection in a single tube. Current direct costs per disposable prototype are $15, and mass production would reduce significantly costs. Future work will focus on creating a simplified “add sample and heat” disposable device. This work includes fully enclosing wash and reagents, and fully containing waste streams for safety and quality control, adding an internal, positive control and adding an optional and inexpensive spectrometer to increase detection robustness. While additional studies and system development, such as further reduction in assay time, are deemed necessary, the simplicity, sensitivity, and cost effectiveness of our system hold significant promise for both the field and the clinic.
ACKNOWLEDGMENT
The authors would like to thank B. Hindson, P. Hullinger, N. Ferris, G. Hutchings, U. Mueller-Doblies, G. Varns, and K. Michlitsch for their efforts and insights. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
This work was supported in part by Lawrence Livermore National Laboratory under Project 08-ERD-044 and under Contract DE-AC52–07NA27344, in part by the National Institute of Biomedical Imaging and Bioengineering under Award U54EB007959, and in part by the U.K. Department for Environment, Food and Rural Affairs under Project SE1124.
Contributor Information
Jane P. Bearinger, Email: jane@llnl.gov, Lawrence Livermore National Laboratory, Livermore, CA 94550 USA
Lawrence C. Dugan, Email: dugan3@llnl.gov, Lawrence Livermore National Laboratory, Livermore, CA 94550 USA
Brian R. Baker, Email: baker69@llnl.gov, Lawrence Livermore National Laboratory, Livermore, CA 94550 USA
Sara B. Hall, Email: hall79@llnl.gov, Lawrence Livermore National Laboratory, Livermore, CA 94550 USA
Katja Ebert, Email: katja.ebert@bbsrc.ac.uk, Institute for Animal Health, Pirbright Laboratory, Pirbright GU24 0NF, U.K..
Valerie Mioulet, Email: valerie.mioulet@bbsrc.ac.uk, Institute for Animal Health, Pirbright Laboratory, Pirbright GU24 0NF, U.K..
Mikidache Madi, Email: mikidache.madi@bbsrc.ac.uk, Institute for Animal Health, Pirbright Laboratory, Pirbright GU24 0NF, U.K..
Donald P. King, Email: donald.king@bbsrc.ac.uk, Institute for Animal Health, Pirbright Laboratory, Pirbright GU24 0NF, U.K.
REFERENCES
- 1.Misawa Y, Yoshida A, Saito R, Yoshida H, Okuzumi K, Ito N, Okada M, Moriya K, Koike K. Application of loop-mediated isothermal amplification technique to rapid and direct detection of methicillin-resistant Staphylococcus aureus (MRSA) in blood cultures. J. Infect. Chemother, CA. 2007 Jun.vol. 13:134–140. doi: 10.1007/s10156-007-0508-9. [DOI] [PubMed] [Google Scholar]
- 2.Grubman MB, Baxt B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004 Apr.vol. 17:465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClure MC, McKay SD, Schnabel RD, Taylor JF. Assessment of DNA extracted from FTA cards for use on the Illumina iSelect BeadChip. BMC Res. Notes. 2009 Jun.vol. 2:107–110. doi: 10.1186/1756-0500-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckett SM, Laughton SJ, Pozza LD, McCowage GB, Marshall G, Cohn RJ, Milne E, Ashton LJ. Buccal swabs and treated cards: Methodological considerations for molecular epidemiologic studies examining pediatric populations. Am. J. Epidemiol. 2008 May;vol. 167:1260–1267. doi: 10.1093/aje/kwn012. [DOI] [PubMed] [Google Scholar]
- 5.Torres CL, Vitalis EA, Baker BR, Gardner SN, Torres MW, Dzenitis JM. LAVA: An open-source approach to designing LAMP (loop mediated isothermal amplification) DNA signatures. BMC Bioinform. doi: 10.1186/1471-2105-12-240. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dukes JP, King DP, Alexandersen S. Novel reverse transcription loop-mediated isothermal amplification for rapid detection of foot-and-mouth disease virus. Arch. Virol. 2006 Jun.vol. 151:1093–1106. doi: 10.1007/s00705-005-0708-5. [DOI] [PubMed] [Google Scholar]
- 7.Reid SM, Ebert K, Bachanek-Bankowska K, Batten C, Sanders A, Wright C, Shaw AE, Ryan ED, Hutchings GH, Ferris NP, Paton DJ, King DP. Performance of real-time RT-PCR for the detection of foot-and-mouth disease virus during field outbreaks in the United Kingdom in 2007. J. Vet. Diagn. Invest. 2009 May;vol. 21:321–330. doi: 10.1177/104063870902100303. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009 Mar.vol. 46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea B. The search for the holy fail(safe) CBRNe World. 2008:52–54. [Google Scholar]
- 10.FTA Cards. Buckinghamshire, U.K.: GE Healthcare U.K. Ltd.; 2010. Feb., Data File 51613. [Google Scholar]
- 11.Gustavsson I, Lindell M, Wilander E, Strand A, Gyllensten U. Use of FTA card for dry collection, transportation and storage of cervical cell specimen to detect high-risk HPV. J. Clin. Virol. 2009 Oct.vol. 46:112–116. doi: 10.1016/j.jcv.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Williams HL, Turnbull L, Thomas SJ, Murphy A, Stinear T, Armstrong DS, Whitchurch CB. A diagnostic PCR assay for the detection of an Australian epidemic strain of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2010 Jul.vol. 9:18–25. doi: 10.1186/1476-0711-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhassan A, Thekisoe OM, Yokoyama N, Inoue N, Motloang MY, Mbati PA, Yin H, Katayama Y, Anzai T, Sugimoto C, Igarashi I. Development of loop-mediated isothermal amplification (LAMP) method for diagnosis of equine piroplasmosis. Vet. Parasitol. 2007 Jan.vol. 143:155–160. doi: 10.1016/j.vetpar.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Kobuki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. Loop-mediated isothermal amplification for detection of African trypanosomes. J. Clin. Microbiol. 2003 Dec.vol. 41:5517–5524. doi: 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong TC, Mai QL, Cuong DV, Parida M, Minekawa H, Notomi T, Hasebe F, Morita K. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004 May;vol. 42:1956–1961. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James HE, Ebert K, McGonigle R, Reid SM, Boonham N, Tomlinson JA, Hutchings GH, Denyer M, Oura CA, Dukes JP, King DP. Detection of African swine fever virus by loop-mediated isothermal amplification. J. Virol. Methods. 2009 Mar.vol. 164:68–74. doi: 10.1016/j.jviromet.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Nagamine K, Watanabe K, Ohtsuka K, Hase T, Notomi T. Loop-mediated isothermal amplification reaction using a nondenatured template. Clin. Chem. 2001 Sep.vol. 47:1742–1743. [PubMed] [Google Scholar]
- 18.Grab JD, Lonsdale-Eccles J, Inoue N. LAMP for tadpoles. Nat. Methods. 2005 Sep.vol. 2:635–636. doi: 10.1038/nmeth0905-635a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma XJ, Shu YL, Qin M, Wang DY, Gao RB, Wang M, Wen LY, Han F, Zhou SM, Zhao X, Cheng YH, Li DX, Dong XP. Visual detection of pandemic influenza A H1N1 Virus 2009 by reverse-transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. J. Virol. Methods. 2010 Aug.vol. 167:214–217. doi: 10.1016/j.jviromet.2010.03.027. [DOI] [PubMed] [Google Scholar]