Abstract
Th17 cells play a significant role in inflammatory and autoimmune responses. Although a number of molecular pathways that contribute to the lineage differentiation of T cells have been discovered, the mechanisms by which lineage commitment occurs are not fully understood. Transcription factors play a k role in driving T cells toward specific lineages. We have identified a role for the transcription factor Kruppel-like factor (KLF) 4 in the development of IL-17–producing CD4+ T cells. KLF4 was required for the production of IL-17, and further, chromatin immunoprecipation analysis demonstrated binding of KLF4 to the IL-17 promoter, indicating a direct effect on the regulation of IL-17. Further, KLF4-deficient T cells upregulated expression of retinoic acid-related orphan receptor γt similar to wild-type during the polarization process toward Th17, suggesting that these two transcription factors are regulated independently.
Naive T cells differentiate into Th1, Th2, Th17, or regulatory T cells (Tregs), based on their activation of molecular pathways. Previous studies have shown that expression of specific transcription factors leads to skewing of T cells toward a specific phenotype. Specifically, the induction of T-bet leads to differentiation into Th1 (1), GATA-3 into Th2 (2), retinoic acid-related orphan receptor (ROR) γt into Th17 (3), and Foxp3 into Tregs (4). However, other transcription factors may play a significant role in the differentiation of T cells into these subsets, and identifying additional factors may help to further elucidate mechanisms by which T cell lineage fate decisions are made.
The Kruppel like family (KLF) of transcription factors contains a large number of members, many with overlapping functions. KLF4 was originally cloned by two independent groups and named to reflect the two different cell types from which it was cloned; one group identified it as gut-enriched KLF because of its intestinal location (5), whereas another group cloned it from differentiated skin epithelium and thus named it epithelial zinc finger (6). KLFs exert effects on different cell types and at different stages of differentiation. For example, KLF1 is necessary for erythrocyte differentiation (7), whereas KLF2 directs the maturation of T cells, prevents apoptosis, and helps maintain quiescence (8–10). Thus, these transcription factors produce significant effects on cell development, survival, and cell cycle progression.
Whereas RORγt is required for the induction of Th17 cells, additional cofactors may also be necessary. We have discovered a role for KLF4 in the differentiation of naive cells into Th17 cells. To assess the mechanism by which IL-17 production was affected, we evaluated whether KLF4 was responsible for a direct regulation of gene expression through promoter modulation. Results of our analyses showed that KLF4 binds to the IL-17 promoter, which indicates a direct effect of KLF4 on expression of IL-17.
Materials and Methods
Mice
Mice were purchased from the National Cancer Institute (Bethesda, MD) or bred at The Johns Hopkins University (Baltimore, MD). All procedures were conducted under approved protocols. Adoptive transfer experimental autoimmune encephalomyelitis (EAE) was conducted as previously described (11), and mice were scored as we have described (12).
Fetal liver transplantation
As KLF4−/− mice die a few days after birth, bone marrow chimeras were generated to investigate the development of the hematopoietic system. Fetal liver cells were isolated from embryonic day 14.5 KLF4−/− and wild-type (wt) littermates and genotyped by PCR (both on a C57BL/6 CD45.2+) background (as previously described in Ref. 13). To generate hematopoietic chimeras, 2 × 106 KLF4−/− or wt fetal liver cells (CD45.2+) were transplanted i.v. into 6–8-wk-old lethally irradiated (850 cGy) CD45.1+ B6 congenic hosts. Engraftment (typically >90% after 3 mo) was verified by the identification of CD45.1/CD45.2 cells, and analyses for donor were gated on CD45.2+ cells.
Perfusion and immunostaining
Mice were processed as previously described in Ref. 12 with minor modifications as noted below. Mice were anesthetized, and the forebrain and cerebellum were removed. CNS tissue was digested and enriched for mononuclear cells. Collected cells were then resuspended in FACS buffer and stained with Abs against CD4-PECy7, CD8-APC, CD25-PE, and CD44-FITC (BD Biosciences, San Jose, CA). For intracellular cytokine staining, cells were isolated as described and stimulated in PMA (50 ng/ml; Sigma-Aldrich, St. Louis, MO), ionomycin (1 µg/ml; Sigma-Aldrich), and monensin (4 µl/6 ml; GolgiStop, BD Biosciences, San Jose, CA) at 37°C for 4 h. Staining for Foxp3 was performed with the eBioscience Foxp3 kit (eBioscience, San Diego, CA). The cells were stained with IL-17-APC (Biolegend, San Diego, CA), IFN-γ–FITC (eBioscience), and corresponding isotype controls.
Statistical analysis
All statistics shown were conducted using the GraphPad Prism program (GraphPad, San Diego, CA).
In vitro polarization assay
CD4+ T cells were isolated from splenocytes with the EasySep Mouse CD4+ T Cell Enrichment Kit (StemCell Technologies, Vancouver, British Columbia, Canada). CD4+ T cells were activated with Dynal Mouse T activator anti-CD3/anti-CD28 beads (Invitrogen, Oslo, Norway). Naive CD4+ T cells were incubated with IL-12, IL-2, and anti–IL-4 to induce Th1 differentiation, with anti–IFN-γ, anti–IL-4, TGF-β, IL-6, and IL-23 for Th17, or with TGF-β alone for Treg induction. Four days later, cells were analyzed.
Chromatin immunoprecipitation
The chromatin immunoprecipitation (ChIP) assay was conducted as previously described (14). Briefly, CD4+ T cells were isolated from a C57BL/6 mouse, and Th17 differentiation was induced for 24 h as described above. One percent of sheared DNA was reserved for the input control. Two rabbit Abs specific for KLF-4 (obtained from either Abcam, Cambridge, MA or Santa Cruz Biotechnology, Santa Cruz, CA) were tested. Normal rabbit IgG Ab (Upstate Biotechnology, Waltham, MA) was used as a negative control. PCR was performed with primers flanking KLF-4 binding sites in the IL-17 promoter. A 180 bp region of the IL-17 promoter was amplified spanning 2 KLF-4 binding sites at nucleotide position −1097 to −1083: forward, 5′-GGGTATTATCCCAAGGGTATCC-3′ and reverse, 5′-ATGCAGCATGAGGTGGACCGAT-3′.
Results and Discussion
KLF4 is necessary for polarization toward Th17 in vitro
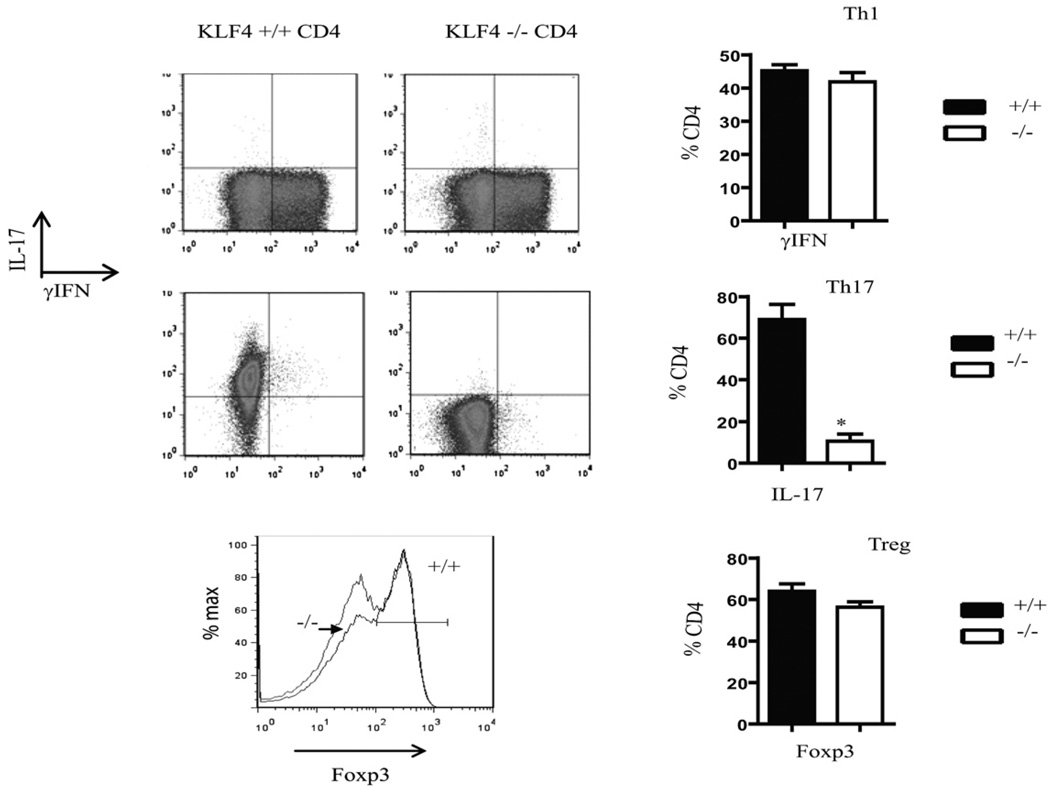
To determine the role of KLF4 in the differentiation of T cells, we assessed the ability of naive KLF4−/− T cells to polarize toward Th17, Tregs, and Th1 subsets. Wild-type and KLF4−/− T cells were cultured under polarizing conditions and then assessed for numbers and percentages of cells expressing IFN-γ, IL-17, and Foxp3. Whereas differentiation into Th1 and Tregs was not significantly changed, the KLF4−/− T cells did not effectively differentiate into Th17 populations when compared with T cells derived from wt controls (Fig. 1). Poststimulation, numbers of wt and KLF4−/− T cells were similar (not shown), indicating that survival and proliferation were unaffected, which suggests that the block in the differentiation pathway toward the Th17 pathway was selective.
FIGURE 1.
KLF4 is required for IL-17 production. Naive T cells were isolated from either wt or KLF4−/− mouse spleens, and then cultured for 4 d under polarizing conditions. Cells were then harvested, counted, and stained for IFN-γ, IL-17, and Foxp3. Shown are a representative FACS plot and a graph with summary of three independent experiments. In all figures,+/+ refers to wt and−/− refers to KLF4−/−. t tests were conducted for statistical analysis; those with statistical difference (p < 0.05) are marked with *.
Interestingly, the loss of IL-17 production did not correlate with an increased conversion to Tregs, and no increase in Foxp3 expression was observed, which is distinct from the role of RORγt/Foxp3 and T-bet/GATA-3, in which the loss of these factors led to a conversion toward the opposing axis (i.e., Th17/Treg and Th1/Th2, respectively) (2, 15). CD4+ T cells lacking KLF4 instead remained in a more uncommitted state. This change in phenotype is consistent with the role of this transcription factor in monocytes in which it was a key regulator of monocyte differentiation. Those studies showed that its absence induced a block in differentiation, whereas a forced expression drove precursors toward a monocytic lineage (16), indicating that induction of differentiation may be an important role of KLF4 in the hematopoietic system. Further, KLF4 was shown to be required for the inflammatory cytokine secretion by macrophages (17). These findings may indicate that a combination of factors is necessary to drive cells toward mature and/or inflammatory lineages and that in the absence of one or more, either a delay in maturation or an arrest in a less mature state might occur.
KLF4 is necessary for polarization toward Th17 in vivo and for development of adoptive transfer EAE
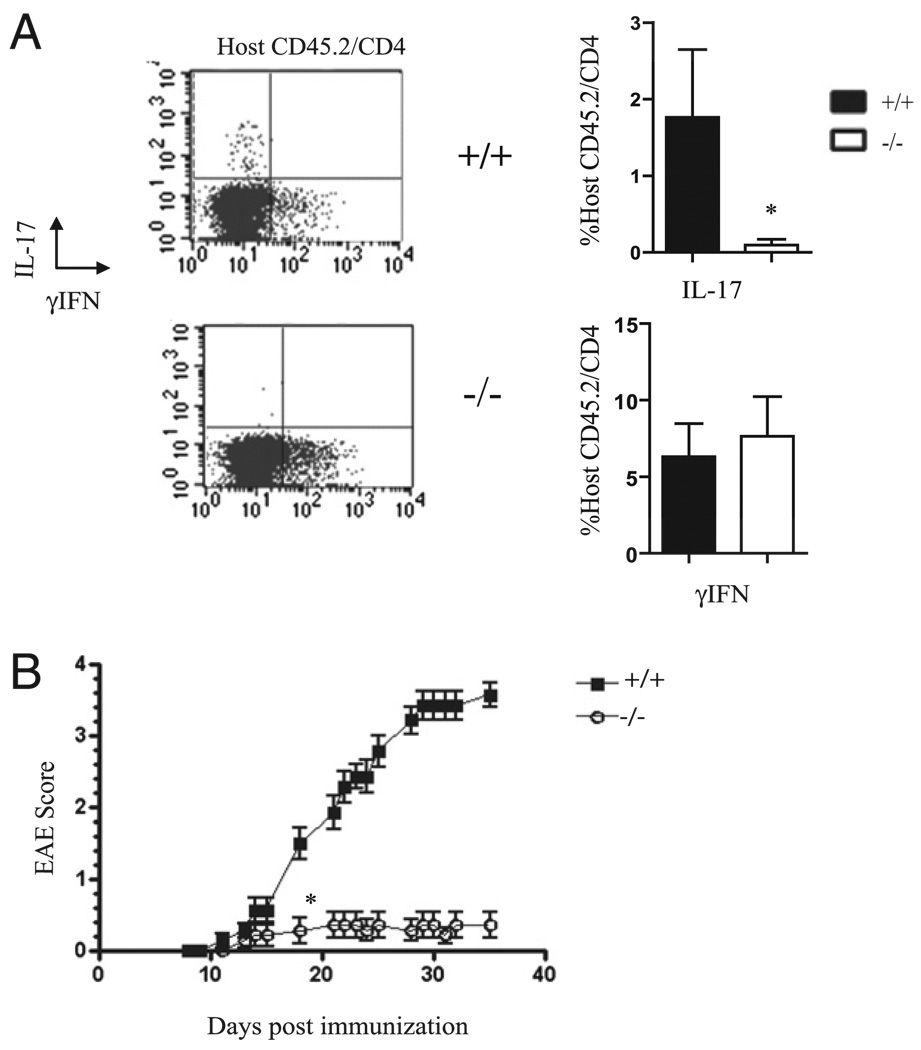
We next sought to assess whether in vivo induction of IL-17 required KLF4. We thus conducted studies in which we evaluated the differentiation of Th17 cells in vivo and the subsequent development of EAE. MOG-reactive pathogenic T cells were generated in wt CD45.1+ mice so that they could be differentiated from host CD45.2+ T cells. KLF4−/− and control wt host mice were generated as described in Materials and Methods. Host mice were injected with MOG-reactive CD45.1+ T cells. After 10 d, four to five mice from each group were euthanized and their T cells analyzed, and an additional 10 mice/group were followed for EAE behavioral analysis. Similar to the in vitro results, KLF4−/− (CD45.2+) T cells did not effectively polarize into Th17 cells following in vivo immunization but were as effective as wt cells in developing into Th1 cells (Fig. 2A).
FIGURE 2.
KLF4 is required for Th17 polarization in vivo and subsequent induction of EAE. CD45.1+ mice were injected with MOG35–55, then spleens and lymph nodes were harvested, cultured, and injected into chimeric recipients containing CD45.2+ hematopoietic cells (either wt or KO, as shown). Ten days posttransfer, spleens were harvested from five mice/group, whereas an additional 10 mice were followed for EAE progression. Shown in A is a representative FACS plot of a spleen and a summary of the data on the percentages of host T cells expressing IL-17 and IFN-γ (statistical difference marked with *). Data from three different experiments are compiled in the graphs shown. Shown in B is one representative of two behavioral studies conducted after adoptive transfer (p < 0.05 starting at *).
The relative contributions of host and donor T cells in this setting have been investigated, and a previous study demonstrated the importance of host T cell production of IL-17 in the pathogenesis of EAE after adoptive transfer of encephalitogenic Th1-polarized T cells (18). Consistent with this finding, our results revealed that mice deficient in KLF4 (and host production of IL-17) were also resistant to the development of EAE (Fig. 2B).
KLF4 occupies the IL-17 promoter and does not affect RORγt expression
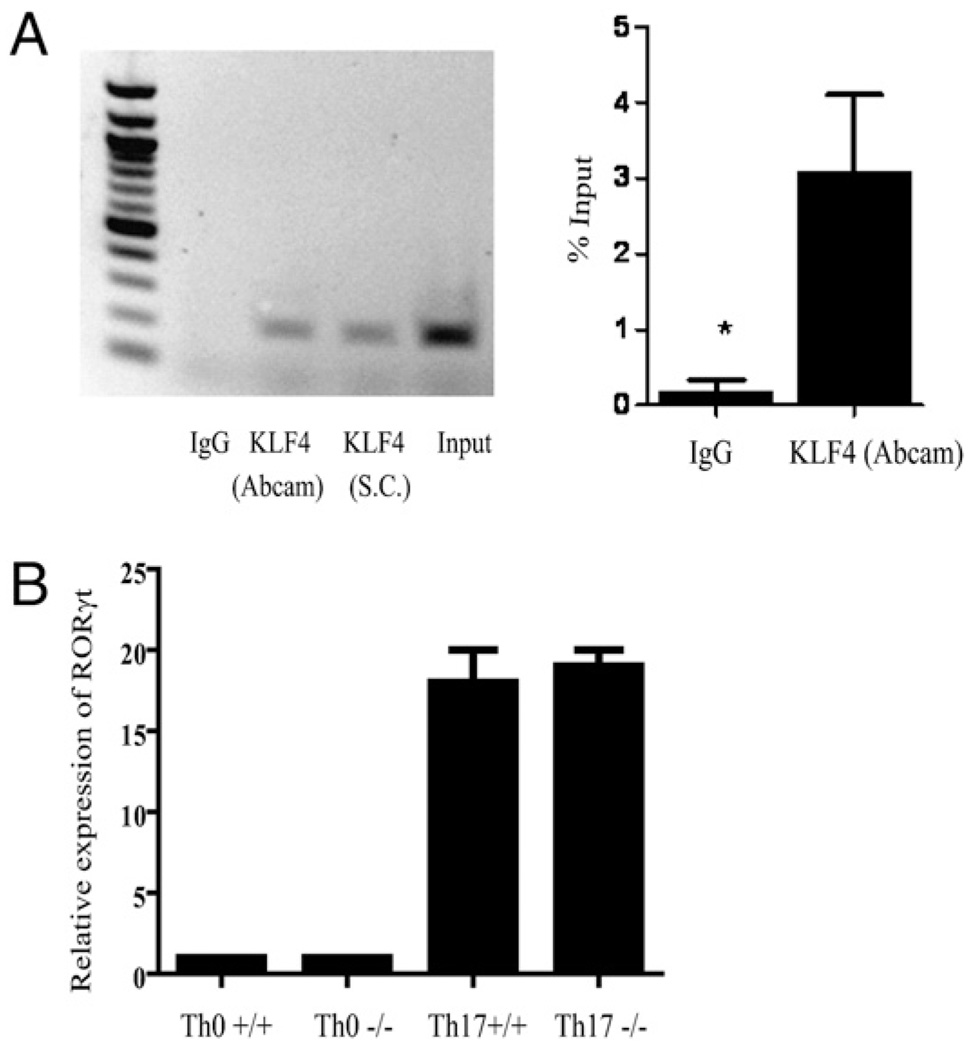
Transcription of IL-17 is known to be regulated by RORγt. However, other transcription factors could also play an essential role in regulating this gene. We next sought to determine the relationship between KLF4 and the subsequent activation of IL-17. The results of the in vitro polarization using anti-CD3/anti-CD28 stimulation showed an intrinsic defect in the ability of the KLF4-deficient T cells to secrete IL-17. We thus assessed whether the loss of IL-17 production was due to a direct or indirect effect by conducting a ChIP analysis. For precipitation of KLF4, we evaluated two different commercially available Abs against KLF4 for comparison and then used quantitative PCR to amplify binding sites within the IL-17 promoter. As Fig. 3A shows, following stimulation of wt CD4+ T cells under Th17-polarizing conditions, KLF4 specifically binds to the IL-17 promoter. This binding is not observed when immunoprecipitation is performed with an isotype control Ab. Moreover, the IL-17 promoter contains two known KLF4 consensus sites located approximately −1097 to −1083 bp upstream of the transcription start site, and this is the region of the promoter that was amplified. Although it does not eliminate the possibility that KLF4 can regulate additional members of the IL-17 differentiation pathway, this finding suggests a direct requirement of KLF4 for the induction of transcription of IL-17 and explains the observed decrease in IL-17 production in the absence of KLF4. In both these studies and subsequent Western analyses (data not shown), we found the Ab obtained from Abcam to precipitate more efficiently and thus used results from that comparison for the quantitative analyses.
FIGURE 3.
KLF4 occupies the IL-17 promoter and does not affect RORγt expression. Wild-type spleen cells were harvested, and CD4+ T cells were isolated and then polarized toward IL-17. Four days later, cells were processed and subjected to ChIP analysis. A shows the results of the PCR analysis; the left panel shows an agarose gel of the PCR products as labeled, and in the right panel is an analysis of the quantitative PCR compared with the positive input. The experiment was repeated twice with similar results. B shows the relative expression levels of RORγt from unpolarized (Th0) cells and polarized (Th17) cells from either wt or KLF4−/− CD4+ cells, as described in Fig. 1. Shown in the graph are the combined results of three independent experiments. No significant difference was observed between the wt and KLF4−/− mice.
To assess whether the effect of KLF4 on IL-17 production was independent of RORγt, we quantified its expression in wt and KLF4-deficient T cells that were subjected to Th17-polarizing conditions (taken from the cells shown in the FACS plot from Fig. 1). As Fig. 3B shows, wt and KLF4−/− T cells upregulated expression of RORγt under these conditions similarly. This finding indicates that KLF4 provides a separate and additional function in the production of IL-17. One possibility consistent with these results might be that RORγt is necessary for the initiation of IL-17 production, whereas KLF4 is required as a cotranscription factor to complete transcription, but additional studies would be necessary to confirm the exact molecular mechanism.
In summary, our results suggest a role for KLF4 in the differentiation of Th17 cells. This finding provides, to our knowledge, new information regarding the mechanism by which T cells polarize and may have implications for the development of new targeted therapies against pathways regulated by this transcription factor.
Acknowledgments
We thank Mario Skarica, Jonathan Powell, and Chris Gamper for assistance and expertise.
This work was supported by National Institutes of Health Grant RO-1 NS041435 (to P.A.C.)
Abbreviations used in this paper
- ChIP
chromatin immunoprecipitation
- KLF
Kruppel-like factor
- MOG
myelin oligodendrocyte glycoprotein
- ROR
retinoic acid-related orphan receptor
- Treg
regulatory T cell
- wt
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 3.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J. Biol. Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J. Biol. Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 7.Drissen R, von Lindern M, Kolbus A, Driegen S, Steinlein P, Beug H, Grosveld F, Philipsen S. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 2005;25:5205–5214. doi: 10.1128/MCB.25.12.5205-5214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc—dependent pathway. Nat. Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 11.Racke MK, Scott DE, Quigley L, Gray GS, Abe R, June CH, Perrin PJ. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J. Clin. Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skarica M, Wang T, McCadden E, Kardian D, Calabresi PA, Small D, Whartenby KA. Signal transduction inhibition of APCs diminishes th17 and Th1 responses in experimental autoimmune encephalomyelitis. J. Immunol. 2009;182:4192–4199. doi: 10.4049/jimmunol.0803631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alder JK, Georgantas RW, III, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J. Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J. Biol. Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 18.Lees JR, Iwakura Y, Russell JH. Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J. Immunol. 2008;180:8066–8072. doi: 10.4049/jimmunol.180.12.8066. [DOI] [PubMed] [Google Scholar]