Abstract
1,2-Distigmasterylhemisuccinoyl-sn-glycero-3-phosphocholine (DSHemsPC) is a new lipid in which two molecules of stigmasterol (an inexpensive plant sterol) are covalently linked via a succinic acid to glycerophosphocholine. Since amphotericin B (AmB) interacts with sterols, we postulated that DSHemsPC could be used in AmB liposome formulations. Thirty-two DSHemsPC-AmB formulations were prepared using various mole ratios of DSHemsPC, phosphatidylcholine and phosphatidylglycerol at different pH. Most formulations had physical properties similar to AmBisome™: a particle diameter of about 100 nm, a monomodal distribution and a negative zeta potential. The red blood cell potassium release (RBCPR) IC50s for formulations spanned a range, with some being comparable to or greater than the IC50 observed using AmBisome™. A number of formulations had superior in vitro antifungal activity compared to AmBisome™. against all of the tested pathogenic yeasts and molds. The IC50s of formulations against L. major promastigotes and amastigotes for certain formulations were comparable with AmBisome™ and Fungizone™. Most formulations had maximum tolerated intravenous doses (MTD) of less than 10 mg/kg. However the formulation consisting of DSHemsPC/DMPC/DMPG/AmB mole ratio 1.25/5.0/1.5/1.0 (prepared at pH 5.5) had excellent colloidal properties, a high IC50 for RBCPR, antifungal and antileishmanial activity similar to AmBisome™ and an MTD of 60 mg/kg. The characteristics of this DSHemsPC/DMPC/DMPG/AmB formulation make it suitable for further investigation to treat AmB-responsive pathogens.
Keywords: Amphotericin B, Cholesterol, Distigmasteryl-Modified Phospholipid, Drug Carrier
1. Introduction
Amphotericin B (AmB), a polyene antibiotic produced from the natural fermentation of Streptomyces nodosus (Ellis, 2002), is one of the more effective drugs for the treatment of invasive fungal infections and visceral leishmaniasis (Groll and Walsh, 2002; Croft and Coombs, 2003). AmB is insoluble in aqueous media and the traditional formulation of AmB is provided as a mixed micelle with deoxycholate as a surfactant (Fungizone™). The severe toxic adverse effects, including the acute infusion related toxicities and the chronic nephrotoxicity, limit the clinical use of the traditional micelle AmB dosage form (Deray, 2002). In order to reduce the adverse effects and increase the therapeutic index of AmB, three different lipid-based formulations of AmB have been developed and commercialized in United States and Europe. Amphotec™ (Three Rivers Pharmaceuticals, LLC, Cranberry Township, PA) is an AmB stable disk-like colloidal dispersion with a diameter of approximately 100 nm and a thickness of <10 nm in which AmB is complexed with cholesteryl sulfate in a molar ratio of 1:1 (Guo et al., 1991). Abelcet™ (Enzon Pharmaceuticals, Inc., Bridgewater, NJ) is an AmB lipid complex with a micron sized ribbon-like structure and a final particle diameter of 1–6 μM in which AmB is complexed with dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) in a molar ratio of 3:10:7 (Janoff et al., 1998). AmBisome™ (Gilead Sciences, Inc., Foster City, CA) is a unilamellar liposomal formulation of AmB with particle size diameter of <100 nm and is composed of hydrogenated soy phosphatidylcholine (HSPC), cholesterol, disteroylphosphatidylglycerol (DSPG) and AmB in a molar ratio of 2:1:0.8:0.4 (Adler-Moore and Proffitt, 2002). These commercial lipid-based formulations reduce the toxicity of AmB to varying degrees and have different pharmacokinetic profiles (Andes et al., 2006; Bellmann et al., 2009; Walsh et al., 1999; Herbrecht et al., 2003; Wingard et al., 2000).
Among these formulations, AmBisome™ has significantly lower toxicity compared to the other formulations (Bellmann et al., 2009; Boswell et al., 1998; Walsh et al., 1999; Moen et al., 2009; Leenders et al., 1997; Dupont, 2002; Herbrecht et al., 2003; Wingard et al., 2000). The reason for the lower toxicity of AmB in AmBisome™ is that AmB is very tightly integrated within the liposomal membrane bilayer through 1) electrostatic interaction between the positive charge of the mycosamine group in AmB and the negative charge on the DSPG, 2) a favorable chain-packing arrangement between the AmB and the aliphatic acyl chains and 3) the hydrophobic interaction between AmB and cholesterol in the liposome bilayer. Due to these characteristics, AmB in AmBisome™ is firmly associated with the liposome bilayer and is not readily released while it is in blood circulation (Adler-Moore and Proffitt, 2008). Furthermore, AmBisome™ due its small diameter and rigid bilayer (phase transition temperature of approximately 55 °C) has a long circulation time in the bloodstream which promotes its distribution into sites of inflammation (Adler-Moore and Proffitt, 2002; Walsh et al., 1999).
Liposomes containing a high percentage of cholesterol (up to 50%) are generally more stable and less leaky than those without cholesterol (Torchilin, 2005). However, when some types of liposomes composed of free cholesterol and phospholipids are placed in a biological milieu, free cholesterol rapidly transfers from the liposome into the biomembranes and serum lipoproteins (Fahr et al., 2005; Kan et al., 1992; Phillips et al., 2003). This transfer of free cholesterol from the liposome bilayer results in a decrease in liposome stability and the loss of the encapsulated contents.
We have designed and synthesized a family of chimeric sterol-modified glycerophospholipids (SML) in which either the sn-1 or sn-2 position or both are covalently attached to cholesterol and the remaining position contains an aliphatic chain (Huang and Szoka, 2008; Huang et al., 2009). SMLs form liposomes by themselves and in mixtures with diacylglycerophospholipids. SMLs stabilize the liposome bilayer but are not released from the liposomes in the biological milieu of serum at 37 °C (Huang and Szoka, 2008; Huang et al., 2009). Hence SMLs can be used in place of the current phospholipids to improve liposomal drug delivery for drugs whose premature release is not desired (Huang and Szoka, 2008; Huang et al., 2009).
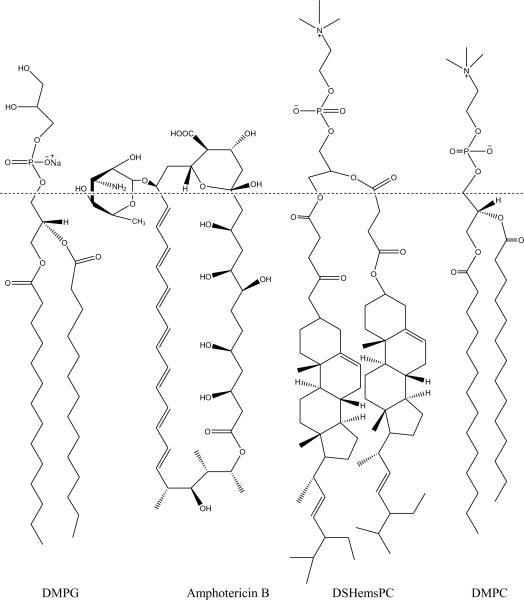
One of the mechanisms of stabilization of AmB in the AmBisome™ formulation is through the hydrophobic interactions between AmB and cholesterol in the liposome bilayer (Jensen et al., 1999; Ellis, 2002; Guo et al., 1991). Thus, we hypothesized that SMLs could provide an alternative; possibly improved liposomal formulations of AmB because cholesterol would be further anchored into the liposome bilayer via the SMLs. 1, 2-Distigmasterylhemisuccinoyl-sn-glycero-3-phosphocholine (DSHemsPC) is a new lipid in which two molecules of stigmasterol are covalently linked via a succinic acid to glycerophosphocholine. DSHemsPC is prepared from an inexpensive plant sterol that provides liposomes with excellent stability in biological fluids because stigmasterol in DSHemsPC does not transfer from the liposome into lipoproteins and biomembranes (Fig. 1) (Huang et al., 2009). Stigmasterol is an unsaturated plant sterol that is chemically similar to animal sterol (cholesterol) (Mora et al., 1999; Sriti et al., 2009). Since one of the main drawbacks in using AmBisome™ is its considerable cost, using stigmasterol instead of cholesterol might decrease overall cost of the AmB preparation.
Fig. 1.
Structure of DSHemsPC, DMPC, DMPG and Amphotericin B.
Herein we describe the preparation of thirty-two different DSHemsPC-AmB formulations, their colloidal, red blood cell potassium release properties, in vitro antifungal, and antileishmanial activity. In order to compare directly the acute toxicity of the DSHemsPC-AmB formulations, maximum tolerated dose (MTD) was also determined in BALB/c mice after intravenous administration of the formulations for selected formulations.
2. Materials and methods
2.1. Materials
The DSHemsPC was synthesized, purified and its structure confirmed as described previously by Huang et al (2009). The phospholipids used in this study were consisted of hydrogenated soy phosphatidylcholine (HSPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dimyristoyl-sn-Glycero-3-[Phospho-rac-(1-glycerol)] (Sodium Salt) (DMPG), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-[Phospho-rac-(1-glycerol)] (Sodium Salt) (DPPG), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-[Phospho-rac-(1-glycerol)] (Sodium Salt) (DSPG), Cholesterol (Chol.) which were purchased from Avanti Polar Lipids (Birmingham, USA). AmB, HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], disodium succinate hexahydrate (DSSH) and MOPS were purchased from Sigma (St. Louis, MO). Fungizone™ (Bristol-Myers Squibb Company, Princeton, NJ) and AmBisome™ (Gilead Sciences, Inc., Foster City, CA) were purchased and reconstituted according to the manufacturer's package insert instructions. Chloroform and methanol were purchased from Merck (Germany). Alamar Blue was purchased from Biosource (International, Inc., USA).
All other chemicals were of reagent grade and were used as received.
2.2. Animals
Female BALB/c mice, 6–8 weeks old, were obtained from the Pasteur Institute (Tehran, Iran). The mice were housed in a standard environment at a constant temperature of 25°C under a 12-h light/dark cycle with free access to food and drinking water.
2.3. Parasite propagation and maintenance
The virulence of Leishmania major strain MRHO/IR/75/ER was maintained by passing in BALB/c mice. The amastigotes were isolated from the spleen of an infected mouse and were cultured on NNN (Novy-MacNeal-Nicolle) medium and subcultured in RPMI 1640 (Sigma) containing 10%V/V heat-inactivated fetal calf serum (FCS), 2mM glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin sulfate (RPMI-FCS) at 25 ± 1°C.
2.4. Macrophage culture conditions
J774 A.1 mouse macrophage cell lines were purchased from Pasteur Institute of Iran (Tehran, Iran) and maintained in DMEM containing 10% FBS at 37°C in 5% CO2 atmosphere.
2.5. Liposome preparation
DSHemsPC-liposomes intercalated with AmB (DSHemsPC-AmB liposomes) were prepared by hydration of a thin lipid film followed by sonication (Szoka et al., 1987; Tremblay et al., 1984). Briefly, the lipid components were weighed and dissolved in chloroform. AmB was dissolved in methanol at 0.2 mg/mL. The lipid and AmB solutions were mixed in a round-bottom flask at the desired amount according to the molar ratio for each formulation presented in Table 1 and 2. A thin-lipid film was formed by removing the solvent on a rotary evaporator under reduced pressure. Liposomes were prepared by rehydrating the lipid film with HEPES buffer (10 mM, pH 7.4) or succinate-buffered solution with 9% sucrose (pH 5.5 or 6.5) followed by mixing on a vortex mixer for 10–15 min, sonication at 65°C for 60 min in a bath type sonicator (Laboratory Supplies Company Inc., Hicksville, NY) under argon. The final total lipid concentration of all formulations was adjusted to 70 mM.
Table 1.
Physical and potassium release properties of liposomal AmB formulations prepared from DSHemsPC using HEPES buffer pH 7.2
| AmB Formulation | Molar ratio | Zeta average size (nm) ± SD | Distribution | Polydispersity ± SD | Zeta potential (mv) ± SD | IC50 for potassium release (μg/ml, Lower and Upper 95% limit) |
|---|---|---|---|---|---|---|
| F1-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/0.5/1.0 | 100.6±0.2 | Monomodal | 0.209±0.04 | −36.1±7.8 | 6.19 (1.31–29.24) |
| F2-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/1.0/1.0 | 112.8±0.6 | Bimodal | 0.171±0.04 | −44.5±5.97 | 1.79 (0.21–15.5) |
| F3-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/1.5/1.0 | 127.3±0.9 | Bimodal | 0.173±0.03 | −50.5±7.96 | 0.95 (0.27–3.4) |
| F4-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 115.2±0.3 | Monomodal | 0.209±0.02 | −54.1± 6.27 | 3.7 (0.11–0.12) |
| F5-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/0.5/1.0 | 141.6±2.3 | Bimodal | 0.183± 0.03 | −41.8±9.4 | 335 (24–4495) |
| F6-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/1.0/1.0 | 343.2±21.0 | Bimodal | 0.456±0.12 | −45.7± 7.72 | 9279 (3333–25836) |
| F7-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/1.5/1.0 | 122.6±0.7 | Monomodal | 0.205±0.03 | −60.5±11.3 | 55 (0.93–3243) |
| F8-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/2.0/1.0 | 117.9±2.3 | Monomodal | 0.209±0.14 | −60.7±10.7 | 171 (12–2450) |
| F9-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/0.5/1.0 | 103.2±0.6 | Monomodal | 0.246±0.03 | −53.6±5.82 | 28590 (5233–156215) |
| F10-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/1.0/1.0 | 107.8±19.8 | Monomodal | 0.212±0.02 | −62.2±5.15 | 3105329 (151955–63460000) |
| F11-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/1.5/1.0 | 110.2±2.1 | Monomodal | 0.292±0.01 | −68.9±5.04 | 585515 (42–822038) |
| F12-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/2.0/1.0 | 76.89±0.2 | Monomodal | 0.254±0.01 | −69.1 ±5.92 | 67571 (2069–2207288) |
| F13-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/0.5/1.0 | 149.2±4.8 | Bimodal | 0.393±0.12 | −25.6±4.08 | 110 (38–313) |
| F14-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/1.0/1.0 | 134.2±1.4 | Bimodal | 0.268±0.12 | −45.1± 8.8 | 33 (8.7–124) |
| F15-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/1.5/1.0 | 115.8±0.6 | Bimodal | 0.178±0.04 | −46.2±4.63 | 1.59(0.018–140) |
| F16-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 78.11±0.6 | Monomodal | 0.198±0.03 | −56.9±8.92 | 35 (0.93–1285) |
| F17-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/0.5/1.0 | 108.4±0.8 | Monomodal | 0.226±0.05 | −29.1±4.35 | 2.71 (0.26–28.3) |
| F18-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/1.0/1.0 | 112.1±2.6 | Monomodal | 0.292±0.0 | −46.9±11.6 | 3.7 (0.54–25) |
| F19-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/1.5/1.0 | 90.91±1.3 | Monomodal | 0.176±0.26 | −61.4±9.78 | 8.4 (2.3–31) |
| F20-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/2.0/1.0 | 99.08±0.7 | Monomodal | 0.152±0.16 | −53.6±6.88 | 2.9 (0.27–31) |
| AmBisome™ | 120.3±1.5 | Monomodal | 0.2±0.2 | −54.5±1.1 | 529.97 (326.6–1101.7) | |
| Fungizone™ | 0.76 (0.41–1.4) |
The IC50 of F5–F13 and AmBisome™ was significantly (P < 0.001) higher than Fungizone™. The IC50 among F1– F4, F14–F20 and Fungizone™ was not significantly different (P > 0.05). The IC50 of AmBisome™ was significantly (P < 0.001) greater than F5, F7, F8, and F13. The IC50 of F9–F12 was significantly (P < 0.001) greater than AmBisome™.
Table 2.
Physical and potassium release properties of liposomal AmB formulations prepared from DSHemsPC using DSSH buffer pH 6.5
| AmB Formulation | Molar ratio | Zeta average size (nm) ± SD | Distribution | Polydispersity ± SD | Zeta potential (mv) ± SD | IC50 for potassium release (μg/ml, Lower and Upper 95% limit) |
|---|---|---|---|---|---|---|
| F21-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 143.3±1.0 | Bimodal | 0.254±0.01 | −33.4±4.8 | 81(22–310) |
| F22-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/2.0/1.0 | 99.36±2.5 | Bimodal | 0.21±0.13 | −40.9±9.6 | 22360 (1500–33700) |
| F23-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/1.5/1.0 | 121.1±4.5 | Monomodal | 0.183±0.01 | −42.2±5.5 | 6000 (290–125510) |
| F24-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/2.0/1.0 | 78.22±0.4 | Monomodal | 0.23±0.02 | −24.9± 11.5 | 1970 (465–8340) |
| F25-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 97.07±0.7 | Monomodal | 0.187±0.03 | −31±7.1 | 55 (8–355) |
| F26-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/1.5/1.0 | 169.3±0.4 | Monomodal | 0.288±0.04 | −37.5±6.1 | 119 (34–420) |
| AmBisome™ | 120.3±1.5 | Monomodal | 0.2±0.2 | −54.5±1.1 | 529.97 (326.6–1101.7) | |
| Fungizone™ | 0.76 (0.41–1.4) |
The IC50 of F21– F24, F26 and AmBisome™ was significantly (P < 0.01) higher than Fungizone™. The IC50 among F25 and Fungizone™ was not significantly different (P > 0.05). The IC50 of AmBisome™ was significantly (P < 0.001) greater than F21, F25, and F26. The IC50 of F22, F23, and F24 was significantly (P < 0.05–0.001) greater than AmBisome™.
2.6. Liposome characterization
The particle diameter of each sample was measured in triplicate using Dynamic Light Scattering Instrument (Nano-ZS; Malvern, UK). The zeta potential of liposomes was determined on the same machine using the zeta potential mode as the average of 20 measurements.
2.7. Drug analysis for DSHemsPC-AmB liposome formulations
The amphotericin B concentration of the final formulation was measured by diluting the sample 1/1000 in methanol, measuring the absorbance at 406 nm, and comparing the absorption to a standard curve prepared from solid amphotericin B diluted in methanol (Tremblay et al., 1984). The standard curve was linear up to 6 micrograms amphotericin B per mL methanol. The intra-and inter day variation for AmB was performed and there was no significant difference between day-to-day analysis. The validation results were established for three repeats per concentration and 5 concentrations (Tremblay et al., 1984).
2.8. Analysis of potassium release from red blood cells
Whole blood was collected in tube containing heparin as anti-coagulant from rabbit and stored at 2–8 °C for up to 48h. Just prior to use, the blood cells washed three times in a buffer consisting of 147 mM NaCl, 3 mM KCl and 10 mM dibasic sodium phosphate (Farhoudi-Moghaddam et al., 1990), by centrifugation at 3000 × g for 12 min at 4 °C. The DSHemsPC-AmB formulations were diluted with dextrose water 5% to adjust different concentrations of AmB from 4 mg/ml to 0.0001 mg/ml. In eppendorf tubes 50 μl of each concentrations of DSHemsPC-AmB formulations, blank (HEPES 10 mM, pH 7.2), positive control (1% TritonX-100 in water) and negative control (dextrose water 5%) were mixed with 450 μl of washed blood as triplicates. The tubes were gently rotated to mix the samples and incubated at 37 °C for 3 hours, mixing the samples every 30 min. After incubation, the eppendorf tubes were centrifuged at 1000 × g for 10 min at 4 °C. The supernatant was evaluated for [k+] using flame atomic absorption (Seac, SP20, Radim Company, Italy) (Jensen et al., 1999). The IC50 for potassium release (the concentration of AmB required to cause a 50% release of potassium from red blood cells of each DSHemsPC-AmB formulation (based on AmB concentration), AmBisome and Fungizone were calculated using CalcuSyn Software, Version 2.1 (Biosoft, Cambridge, UK).
2.9. Antifungal in vitro assay to determine drug MICs
A microtiter dilution assay was used to determine the MICs for the yeasts Candida albicans (PTCC 5027) and Candida glabrata (PTCC 5297), and the molds Aspergillus fumigatus (PTCC 5009), Aspergillus terreus (PTCC 5021), and Aspergillus flavus (PTCC 5006). The yeast cells were prepared by daily subculturing in Sabouraud's dextrose broth for 2 days, pelleting, and rinsing twice with 0.01 M phosphate-buffered saline (PBS), pH 7.2. The final pellets were resuspended in PBS, cells were counted with a hemocytometer, and blastospore suspensions were adjusted with RPMI-MOPS to give 6 × 104 blastospores/ml. The Aspergillus species were cultured on plates with inhibitory mold agar, available as a premixed powder (BBL Microbiology Systems), at 35°C for 9 to 10 days. Conidia were dislodged from the hyphal mats by dispersal in 0.9% saline with 0.05% Tween 80 (Sigma, St. Louis, MO) and stored at 4°C. The conidial count for each species was determined with a hemocytometer, and the conidial suspension was adjusted to 6 × 104 conidia/ml of RPMI-MOPS. The viability of the blastospores or conidia was assessed by plating 200 μl of a given suspension onto inhibitory mold agar plates, followed by incubation at 35°C for 24 to 48 h. A series of twofold dilutions of each AmB formulation (0.156 to 80 μg/ml) in RPMI-MOPS were prepared, and 100-μl aliquots of each drug dilution were dispensed into triplicate wells of a 96-well flat-bottom microtiter plate. Final AmB concentrations in the wells ranged from 0.078 to 40 μg/ml. Aliquots (100 μl/well) of each test organism were then dispensed into the appropriate wells. Alamar blue (20 μl/well) was added to all wells, and the plate was incubated at 35°C for 48 h. Negative control wells contained 100 μl of RPMI-MOPS and 100 μl of the drug at 80 μg/ml; positive control wells were made up of 100 μl of RPMI-MOPS and 100 μl of the test organism. The MIC was defined as the lowest concentration of the drug preventing the development of a red color (Olson et al., 2006).
2.10. In vitro cytotoxicity assay using promastigotes
Measurement of Alamar blue reduction was used to determine the effects of the formulations on the viability of Leishmania promastigotes. The effects of the formulations on the viability of Leishmania promastigotes were assessed by monitoring the absorbance of Alamar blue at two wavelengths after a 48-h culture period in the presence of the formulations. Parasites were harvested at stationary phase of culture, and 2.5 × 106 promastigotes were added to each well of 96-well flat-bottom plates containing different concentrations of the DSHemsPC-AmB formulations; triplicate wells were used for each concentration, Alamar blue (20 μl/well) was added to all wells, and the plate was incubated at 25°C for 48 h. Negative control wells contained 100 μl of RPMI-MOPS and 100 μl of the drug at 80 μg/ml; positive control wells were made up of 100 μl of RPMI-MOPS and 100 μl of the test organism. The relative absorbance was correlated to the number of promastigotes per well by calculating percent of reduction when the samples were read at 570nm & 600nm. The 50% effective dose (ED50) for each formulation was calculated by the CalcuSyn software Version 2.1 (Biosoft, Cambridge, UK) (Dutta et al., 2005; Yardley and Croft, 2000; Chou and Talalay, 1984).
2.11. In vitro cytotoxicity assay using amastigotes
Cells of the J774 A.1 mouse macrophage cell line were dispensed at a concentration of 50,000 macrophages/ well into eight-well Lab-Tek (Nunc) chamber slides and maintained at 37°C in 5% CO2 for 24 h to allow attachment of the cells. The cells were then infected with L. major promastigotes at a ratio of five promastigotes per macrophage and incubated at 37°C in 5% CO2 for 24 h to allow internalization of the parasites in the cells. The excess amount of promastigotes was removed by gently washing the cells with PBS three times, and the infected cells were incubated for an additional 24 h to allow the establishment of the infection. The cells were then exposed to different concentrations of DSHemsPC-AmB formulations in triplicate for 2 days. The experiment was terminated by methanol fixation of the slides. The slides were then stained with Giemsa and evaluated microscopically to calculate the percentage of infected cells. The ED50 for each formulation was calculated by the CalcuSyn software Version 2.1 (Biosoft, Cambridge, UK) (Yardley and Croft, 2000; Dutta et al., 2005; Chou and Talalay, 1984; Jaafari et al., 2009).
2.12. Maximum tolerated dose of determination of DSHemsPC-AmB formulations in healthy BALB/c mice
To determine the maximum tolerated dose (MTD), healthy female BALB/c mice were injected via the tail vein with the DSHemsPC-AmB formulations diluted in 5% dextrose. The dose was adjusted for each animal on the basis of body weight. The mice were administered employing rising doses of selected DSHemsPC-AmB formulations at concentrations of 10, 20, 40, and 60 mg/kg AmB. Deaths occurring within 1 h after dosing were considered immediate deaths. The DSHemsPC-AmB formulations, which killed mice at concentrations of 10 mg/kg AmB, were injected at lower doses until mice were survived. Mice that survived for 96 h invariably lived until sacrifice at 30 days (Reed and Muench, 1938; Walsh et al., 2001; Lee et al., 2006).
2.13. Statistical Analysis
The one-way ANOVA statistical test was used to assess the significance of the differences among the various groups. In the case of a significant F value, multiple comparison Tukey test was used to compare the means of different treatment groups. Results with p < 0.05 were considered to be statistically significant.
3. Results
3.1. Liposome characterization
DSHemsPC-AmB liposomes were prepared by thin lipid film hydration followed by sonication. Thirty-two different formulations were prepared using DSHemsPC, phosphatidylcholine (PC) and phosphatidylglycerol (PG) with different aliphatic chain lengths (14, 16 and 18) at different molar ratios and various pH (7.4, 6.5 and 5.5). The zeta average size, size distribution and polydispersity index (PDI) of the DSHemsPC-AmB formulations are shown in Table 1, 2, and 3. A monomodal distribution indicates presence of only one peak in the particle size distribution of vesicle population. A lower PDI for a formulation presents a narrower particle size distribution for the vesicle population.
Table 3.
Physical and potassium release properties of liposomal AmB formulations prepared from DSHemsPC using DSSH buffer pH 5.5
| AmB Formulation | Molar ratio | Zeta average size (nm) ± SD | Distribution | Polydispersity ± SD | Zeta potential (mv) ± SD | IC50 for potassium release (μg/ml, Lower and Upper 95% limit) |
|---|---|---|---|---|---|---|
| F27-DSHemsPC/DSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 84.7±0.8 | Monomodal | 0.198±0.03 | −23.3± 6.07 | 130 (16.7–990) |
| F28-DSHemsPC/DPPC/DPPG/AmB | 1.25/5.0/2.0/1.0 | 202.4±20 | Monomodal | 0.5±0.03 | −14.8±4.16 | 14710(599–361070) |
| F29-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/1.5/1.0 | 111.6±1.0 | Monomodal | 0.21±0.02 | −25.3±9.05 | 1342000(307000–58729000) |
| F30-DSHemsPC/DMPC/DMPG/AmB | 1.25/5.0/2.0/1.0 | 112.8±3.2 | Monomodal | 0.207±0.01 | −25.3±13 | 114510(29560–443580) |
| F31-DSHemsPC/HSPC/DSPG/AmB | 1.25/5.0/2.0/1.0 | 99.53±2.8 | Monomodal | 0.237±0.03 | −21.3±7.97 | 50 (6.6–380) |
| F32-DSHemsPC/HSPC/DSPG/AmB | 1.75/5.0/1.5/1.0 | 130.1±2.0 | Monomodal | 0.384±0.01 | −24±5.13 | 217 (56–840) |
| F33-DSHemsPC/DMPC/DMPG | 1.25/5.0/1.5 | 76.98±0.39 | Monomodal | 0.21±0.056 | −42.7±0.5 | Inactive |
| AmBisome™ | 120.3±1.5 | Monomodal | 0.2±0.2 | −54.5±1.1 | 529.97(326.6–1101.7) | |
| Fungizone™ | 0.76 (0.41–1.4) |
The IC50 of F27– F30, F32 and AmBisome™ was significantly (P < 0.01) higher than Fungizone™. The IC50 among F31 and Fungizone™ was not significantly different (P > 0.05). The IC50 of AmBisome™ was significantly (P < 0.001) greater than F27, and F32. The IC50 of F29 was significantly (P < 0.001) greater than AmBisome™.
The colloidal properties of DSHemsPC-AmB liposomes prepared in HEPES buffer pH 7.4 are in Table 1. The starting point for the selection of molar ratio for each aliphatic chain length was the formulation of AmBisome™ (Cholesterol/HSPC/DSPG/AmB in a molar ratio of 2.5/5/2.0/1.0). Indeed formulations F4, F8, F12 and F16 in Table 1 have the same molar ratio of lipids as in AmBisome™ (each mole of DSHemsPC provides two mole of stigmasterol, the plant sterol). In the remaining formulations (F1–F3, F5–F7, F9–F11, F13–F15), the molar ratio of DSHemsPC, PC and AmB was kept constant and only the molar ratio of PG was altered. In formulations F17–F20 the molar ratio of DSHemsPC was increased from 1.25 to 1.75 to evaluate the effect of higher molar ratio of stigmasterol in the formulations.
Most formulations had physical properties like AmBisome™, zeta average diameter of about 100 nm (except F6), a monomodal distribution (except F2, F3, F5, F6 and F13–15) and polydispersity index of around 0.2 (Table 1). The zeta potential of all the formulations was negative due to the presence of PG and the zeta potential increased as the molar ratio of PGs in the formulations increased. Among the formulations, F4, F8, F9– F12, F16, F19, and F20 have a better colloidal stability than the others. During storage at 4°C these liposomal suspensions were completely clear and showed no evidence for sedimentation.
One of the mechanisms of stabilization of AmB in the liposomal bilayer is through electrostatic interaction between the positive charge of the mycosamine group in AmB and the negative charge on the PG (Jensen et al., 1999; Ellis, 2002; Guo, 1991). Therefore, we hypothesized that decreasing the pH of formulations would protonate the amino group in amphotericin B. This would increase the electrostatic interaction between the amphotericin B and phosphatidylglycerol. Based on their colloidal properties, potassium release and MTD results; F4, F8, F11, F12, F16 and F19 formulations were selected and prepared in DSSH buffer pH 6.5 (Table 2) and pH 5.5 (Table 3). Most of these formulations (F21–26 and F27–F32) had physical properties similar to AmBisome™ at pH 5.5, zeta average diameter of about 100 nm (except F28), a monomodal distribution (except F21 and F22) and polydispersity index of around 0.2.
A non-drug containing liposome was prepared using DSHemsPC, DMPC and DMPG with the same molar ratio as F29 (F33, Table 3). This formulation had one of the smallest diameters and PDI among all the formulations. The diameter of F33 was significantly less than F29 and AmBisome™ which indicates that incorporation of AmB in this formulation increases the particle diameter.
3.2. In vitro toxicity of DSHemsPC-AmB formulations as measured by potassium release from red blood cells
The red blood cell potassium release (RBCPR) assay was used to assess the membrane toxicity of the DSHemsPC-AmB formulations (Table 1, 2, and 3). A higher RBCPR IC50 value indicates lower toxicity of the formulation for the RBCs. Fungizone™ was the most toxic (RBCPR IC50 0.76μg/ml) which is due to the loose attachment of AmB to the deoxycholate in the micelle form. Furthermore, deoxycholate itself is a surface-active agent that destabilizes bilayers. The RBCPR IC50 for AmBisome™ was 529.97μg/ml. The IC50s of F5–F13, F21–24, F26–30, F32 and AmBisome™ were significantly (P < 0.001) greater than Fungizone™. The IC50s among F1- F4, F14–20, F25, and F31 and Fungizone™ were not significantly different (P > 0.05). The IC50 of AmBisome™ was significantly (P < 0.001) greater than F5, F7, F8, F13, F21–27 and F32. However the IC50 of F29 was significantly (P < 0.001) greater than AmBisome™.
Certain formulations (F5–F7 and F13) had a relatively high IC50 due to their large particle diameter and multilamellar structure, but other formulations (F9–F12 and F29) had IC50 much greater than AmBisome™ but also good vesicle colloidal characteristics. The control liposome (F33) did not release potassium from red blood cells hence had no RBCPR activity.
3.3. In vitro antifungal activity of DSHemsPC-AmB formulations
The in vitro cytotoxic activities of the formulations against a variety of pathogenic yeasts and molds were determined (Table 4). The MICs of all the DSHemsPC-AmB formulations for C. albicans (except F21–F32), C. glabrata (except F27, F28, F30 and F31), and A. fumigatus (except F1–F6, F9, F10, F13, F14, F17, F18, F21, F22, F24, F30 and F31) were similar to or less than the MICs of the AmBisome™. The MICs of all the formulations for A. flavus (except F9, F22, F26, F28 and F32), and A. terrus (except F21–F26 and F28–F32) were more than MICs of the AmBisome™. The control liposome (F33) has no cytoxic activity in all the tested yeasts and molds.
Table 4.
Minimum inhibitory concentrations (MIC, μg/ml) of liposomal AmB formulations prepared from DSHemsPC against selected fungal and mold pathogens
| AmB formulations | C. albicans | C.glabrata | A.fumigatus | A.terreus | A.flavus |
|---|---|---|---|---|---|
| F1-DSHemsPC/DSPC/DSPG/AmB | 0.07 | 0.28 | 0.14 | 9.1 | 4.55 |
| F2-DSHemsPC/DSPC/DSPG/AmB | 0.036 | 0.28 | 0.14 | 9.1 | 4.55 |
| F3-DSHemsPC/DSPC/DSPG/AmB | 0.07 | 0.28 | 0.14 | 9.1 | 4.55 |
| F4-DSHemsPC/DSPC/DSPG/AmB | 0.0089 | 0.28 | 0.14 | 9.1 | 2.3 |
| F5-DSHemsPC/DPPC/DPPG/AmB | 0.018 | 0.28 | 0.14 | 9.1 | 4.55 |
| F6-DSHemsPC/DPPC/DPPG/AmB | 0.018 | 0.28 | 0.14 | 9.1 | 4.55 |
| F7-DSHemsPC/DPPC/DPPG/AmB | 0.036 | 0.14 | 0.07 | 9.1 | 4.55 |
| F8-DSHemsPC/DPPC/DPPG/AmB | 0.036 | 0.14 | 0.07 | 4.55 | 4.55 |
| F9-DSHemsPC/DMPC/DMPG/AmB | 0.036 | 0.14 | 0.14 | 4.55 | 0.28 |
| F10-DSHemsPC/DMPC/DMPG/AmB | 0.07 | 0.28 | 0.14 | 4.55 | 4.55 |
| F11-DSHemsPC/DMPC/DMPG/AmB | 0.036 | 0.28 | 0.07 | 4.55 | 2.3 |
| F12-DSHemsPC/DMPC/DMPG/AmB | 0.036 | 0.28 | 0.07 | 4.55 | 2.3 |
| F13-DSHemsPC/HSPC/DSPG/AmB | 0.07 | 0.28 | 0.14 | 9.1 | 2.3 |
| F14-DSHemsPC/HSPC/DSPG/AmB | 0.07 | 0.28 | 0.14 | 9.1 | 4.55 |
| F15-DSHemsPC/HSPC/DSPG/AmB | 0.036 | 0.28 | 0.07 | 9.1 | 2.3 |
| F16-DSHemsPC/HSPC/DSPG/AmB | 0.07 | 0.28 | 0.07 | 4.55 | 1.14 |
| F17-DSHemsPC/HSPC/DSPG/AmB | 0.036 | 0.14 | 0.14 | 9.1 | 4.55 |
| F18-DSHemsPC/HSPC/DSPG/AmB | 0.07 | 0.14 | 0.14 | 4.55 | 2.3 |
| F19-DSHemsPC/HSPC/DSPG/AmB | 0.036 | 0.14 | 0.07 | 4.55 | 2.3 |
| F20-DSHemsPC/HSPC/DSPG/AmB | 0.036 | 0.14 | 0.07 | 4.55 | 1.14 |
|
| |||||
| F21-DSHemsPC/DSPC/DSPG/AmB | 0.28 | 0.14 | 0.14 | 2.3 | 1.14 |
| F22-DSHemsPC/DPPC/DPPG/AmB | 0.57 | 0.14 | 0.14 | 2.3 | 0.57 |
| F23-DSHemsPC/DMPC/DMPG/AmB | 4.51 | 0.07 | 0.07 | 1.14 | 1.14 |
| F24-DSHemsPC/DMPC/DMPG/AmB | 4.51 | 0.14 | 0.14 | 1.14 | 1.14 |
| F25-DSHemsPC/HSPC/DSPG/AmB | 2.3 | 0.14 | 0.07 | 0.57 | 1.14 |
| F26-DSHemsPC/HSPC/DSPG/AmB | 0.14 | 0.14 | 0.07 | 1.14 | 0.57 |
|
| |||||
| F27-DSHemsPC/DSPC/DSPG/AmB | 0.57 | 0.57 | 0.14 | 4.55 | 1.14 |
| F28-DSHemsPC/DPPC/DPPG/AmB | 0.14 | 0.57 | 0.14 | 2.3 | 0.28 |
| F29-DSHemsPC/DMPC/DMPG/AmB | 0.14 | 0.28 | 0.07 | 0.57 | 2.3 |
| F30-DSHemsPC/DMPC/DMPG/AmB | 0.28 | 1.14 | 0.28 | 0.57 | 2.3 |
| F31-DSHemsPC/HSPC/DSPG/AmB | 0.28 | 0.57 | 0.28 | 1.14 | 2.3 |
| F32-DSHemsPC/HSPC/DSPG/AmB | 0.57 | 0.14 | 0.07 | 1.14 | 0.57 |
| F33-DSHemsPC/DMPC/DMPG | Inactive | Inactive | Inactive | Inactive | Inactive |
| AmBisome™ | 0.07 | 0.28 | 0.07 | 2.3 | 0.57 |
| Fungizone™ | 0.07 | 0.57 | 0.28 | 1.14 | 0.57 |
3.4. Effects of DSHemsPC-AmB formulations on L. major promastigotes in vitro
The ED50s of most of DSHemsPC-AmB formulations against L.major promastigotes were almost the same as AmBisome™ (Table 5). Fungizone™ had the lowest ED50. The ED50s of F1–F8, F13–F20, F22, F23 and F31 were significantly (P < 0.001) greater than AmBisome™ and Fungizone™. There were no significant (P > 0.05) differences in the ED50s of F21, F24–F30, F32 and AmBisome™; and also among F24, F27 and Fungizone™. The control liposome (F33) was inactive against L.major promastigotes.
Table 5.
In vitro activities of liposomal AmB formulations prepared from DSHemsPC against L. major promastigotes and amastigotes
| ED50 for L. major (μg/ml, Lower and Upper 95% limit) |
||
|---|---|---|
| AmB formulations | Promastigotes | Amastigote |
| F1-DSHemsPC/DSPC/DSPG/AmB | 8(1.7–38.3) | 5.55(2.5–12.5) |
| F2-DSHemsPC/DSPC/DSPG/AmB | 6.8(1–46) | 5.046(2.3–11.2) |
| F3-DSHemsPC/DSPC/DSPG/AmB | 11.3(6.4–19.9) | 5.64(4.4–7.3) |
| F4-DSHemsPC/DSPC/DSPG/AmB | 6.51(1.3–31.9) | 4.03(2.1–7.9) |
| F5-DSHemsPC/DPPC/DPPG/AmB | 12.4(1.5–103.3) | 9.8(4.5–21.6) |
| F6-DSHemsPC/DPPC/DPPG/AmB | 19.6(3.1–127) | 9.6(5.4–17.1) |
| F7-DSHemsPC/DPPC/DPPG/AmB | 7.7(1.1–54) | 6.59(4.9–8.8) |
| F8-DSHemsPC/DPPC/DPPG/AmB | 7.9(2.9–21.1) | 6.47(4–10.5) |
| F9-DSHemsPC/DMPC/DMPG/AmB | 3.6(0.4–33.2) | 2.97(2.3–3.9) |
| F10-DSHemsPC/DMPC/DMPG/AmB | 4.8(2.2–10.4) | 4.28(3.2–5.8) |
| F11-DSHemsPC/DMPC/DMPG/AmB | 3.1(0.8–12.3) | 2.8(2.3–3.6) |
| F12-DSHemsPC/DMPC/DMPG/AmB | 4.9(0.6–40.3) | 2.14(1.7–2.7) |
| F13-DSHemsPC/HSPC/DSPG/AmB | 7.4(1–56.2) | 4.6(3.4–6.2) |
| F14-DSHemsPC/HSPC/DSPG/AmB | 8.5(1.8–40.1) | 4.85(3.2–7.5) |
| F15-DSHemsPC/HSPC/DSPG/AmB | 11.8(2.1–66.5) | 11.1(6.1–20.2) |
| F16-DSHemsPC/HSPC/DSPG/AmB | 8.9(2.1–37.5) | 6.3(4.3–9.3) |
| F17-DSHemsPC/HSPC/DSPG/AmB | 15.6(5.1–47.0) | 14.88(7.8–28.3) |
| F18-DSHemsPC/HSPC/DSPG/AmB | 12.8(2.7–60.8) | 9.5(7.4–12.3) |
| F19-DSHemsPC/HSPC/DSPG/AmB | 6.8(3.1–14.5) | 4.15(3.1–5.6) |
| F20-DSHemsPC/HSPC/DSPG/AmB | 6.5(2.65–16) | 4.1(2.8–6.1) |
|
| ||
| F21-DSHemsPC/DSPC/DSPG/AmB | 1.2 (0.8–2) | 0.185(0.13–0.3) |
| F22-DSHemsPC/DPPC/DPPG/AmB | 5.6 (4.9–7.7) | 0.732(0.5–1.2) |
| F23-DSHemsPC/DMPC/DMPG/AmB | 3.5 (2.1–6.8) | 0.76(0.5–1.1) |
| F24-DSHemsPC/DMPC/DMPG/AmB | 0.875 (0.7–2.1) | 0.24(0.058–1.08) |
| F25-DSHemsPC/HSPC/DSPG/AmB | 1.3 (0.7–2.1) | 0.21(0.1–0.34) |
| F26-DSHemsPC/HSPC/DSPG/AmB | 1.3(0.7–2.1) | 0.16(0.1–0.3) |
|
| ||
| F27-DSHemsPC/DSPC/DSPG/AmB | 1.3 (0.7–2.1) | 0.163(0.1–0.3) |
| F28-DSHemsPC/DPPC/DPPG/AmB | 1.8 (1.4–2.8) | 0.218(0.1–0.4) |
| F29-DSHemsPC/DMPC/DMPG/AmB | 1.4(0.7–4.9) | 0.14(0.1–0.2) |
| F30-DSHemsPC/DMPC/DMPG/AmB | 1.5 (0.7–2.8) | 0.168(0.1–0.3) |
| F31-DSHemsPC/HSPC/DSPG/AmB | 4.2(3.5–5.6) | 0.56(0.4–0.7) |
| F32-DSHemsPC/HSPC/DSPG/AmB | 2(1.4–3.5) | 0.208(0.2–0.3) |
| F33-DSHemsPC/DMPC/DMPG | Inactive | Inactive |
| AmBisome ™ | 1.4 (0.8–2.7) | 0.86(0.6–1.2) |
| Fungizone™ | 0.24 (0.1–0.9) | 0.108(0.16–0.7) |
Promastigotes assay: The ED50 of F1–F8, F13–F20, F22, F23, and F31 was significantly (P < 0.001) greater than AmBisome™ and Fungizone™. The ED50 of F21, F24–F30, and F32 was similar (P > 0.05) to AmBisome™. The ED50 of F24 and F27 was similar (P > 0.05) to Fungizone™.
Amastigote assay: The ED50 of F5–F8, and F15–F18 was significantly (P < 0.01) greater than AmBisome™ and Fungizone™. There were no significant (P > 0.05) differences in the activities of the F1–F4, F9–F14, F19, F20, F22 and F23 formulations against L. major amastigotes compare to AmBisome™. The ED50 of F5–F8, and F15–F18 was significantly (P < 0.001) greater than AmBisome™ and Fungizone™. The ED50 of F21, F24–F30, and F32 was similar (P > 0.05) to Fungizone™.
3.5. Cytotoxicity of DSHemsPC-AmB formulations on L. major amastigotes in vitro
The ED50s of DSHemsPC-AmB formulations against intracellular amastigotes were compared with Fungizone™ and AmBisome™ (Table 5). Fungizone™ had the lowest ED50. There were no significant (P > 0.05) differences in the activities of the F1–F4, F9–F14, F19, F20, F22 and F23 formulations against L. major amastigotes compared to AmBisome™. The ED50s of F5–F8, and F15–F18 was significantly (P < 0.001) greater than AmBisome™ and Fungizone™. The ED50s of F21, F24–F30 and F32 was similar (P > 0.05) to Fungizone™. The control liposome (F33) was inactive against L.major amastigotes.
3.6. Maximum tolerated dose of DSHemsPC-AmB formulations in healthy BALB/c mice
To compare the in vivo toxicities of DSHemsPC-AmB formulations with AmBisome™ and Fungizone™, formulations were selected based upon their vesicle colloidal characteristics and RBCPR IC50 for the determination of maximum tolerated dose in mice (Table 6).
Table 6.
Maximum tolerated dose (MTD, mg/kg) following intravenous injection of liposomal AmB formulations prepared from DSHemsPC in BALB/c mice
| AmB Formulation | No. of animals | Maximum tolerated dose (mg/kg) |
|---|---|---|
| F4-DSHemsPC/DSPC/DSPG/AmB | 3 | 12.5 |
| F8-DSHemsPC/DPPC/DPPG/AmB | 1 | 2.5 |
| F11-DSHemsPC/DMPC/DMPG/AmB | 3 | 10 |
| F12-DSHemsPC/DMPC/DMPG/AmB | 3 | 5 |
| F15-DSHemsPC/HSPC/DSPG/AmB | 1 | 2.5 |
| F16-DSHemsPC/HSPC/DSPG/AmB | 1 | 2.5 |
| F19-DSHemsPC/HSPC/DSPG/AmB | 3 | 20 |
| F20-DSHemsPC/HSPC/DSPG/AmB | 1 | 2.5 |
|
| ||
| F21-DSHemsPC/DSPC/DSPG/AmB | 1 | 5 |
| F22-DSHemsPC/DPPC/DPPG/AmB | 1 | <2.5 |
| F23-DSHemsPC/DMPC/DMPG/AmB | 3 | 20 |
| F24-DSHemsPC/DMPC/DMPG/AmB | 1 | 5 |
| F25-DSHemsPC/HSPC/DSPG/AmB | 1 | 5 |
| F26-DSHemsPC/HSPC/DSPG/AmB | 1 | 5 |
| F27-DSHemsPC/DSPC/DSPG/AmB | 3 | 10 |
| F28-DSHemsPC/DPPC/DPPG/AmB | 1 | 5 |
| F29-DSHemsPC/DMPC/DMPG/AmB | 4 | 60 |
| F30-DSHemsPC/DMPC/DMPG/AmB | 3 | 10 |
| F31-DSHemsPC/HSPC/DSPG/AmB | 3 | 10 |
| F32-DSHemsPC/HSPC/DSPG/AmB | 1 | 7.5 |
| AmBisome™ | 2 | >140 |
| Fungizone™ | 1 | 2 |
There were no deaths observed in the mice administered AmBisome™ at 140 mg/kg and MTD for Fungizone™ was 2 mg/kg which is comparable with the published results (Takemoto et al., 2004)
The MTD of F8, F15, F16 and F20 was almost the same as Fungizone™ (Table 6); however, the MTD of F4, F11 and F19 were greater than Fungizone™. When these formulations were prepared in DSSH buffer pH 6.5 and 5.5 only the MTD of F11 increased dramatically from 10 mg/kg to 20 mg/kg at pH 6.5 (F23) and to 60 mg/kg at pH 5.5 (F29). F29 formulation was also tried at 70 and 75 mg/kg, the mice injected with 70 mg/kg survived; however, the mice injected with 75 mg/kg died after 15 min.
4. Discussion
One of the main problems impeding the widespread use of AmBisome™ is its considerable cost. A contributing factor to its high cost is the use of cholesterol that is purified from animal sources. The animal source of cholesterol raises the concern of viral or prion contamination (http://avantilipids.com/index.php?view=items&cid=3&id=4&option=com_quickf aq&Itemid=385) and makes the cost of pyrogen-free injectable grade cholesterol very high. Recently new phospholipids termed sterol-modified lipids have been synthesized in which the sterol is covalently attached to the glycerol backbone (Huang and Szoka, 2008; Huang et al., 2009). Liposomes prepared from SML have greater bilayer stability and the sterol in a SML liposomes does not exchange (Huang and Szoka, 2008; Huang et al., 2009). In the synthesis of DSHemsPC, stigmasterol a plant sterol is used. Using stigmasterol, instead of cholesterol, might decrease the overall cost of the AmB preparation. Therefore, we used DSHemsPC liposomes to formulate AmB and evaluated if the SML liposomes provided a more stable AmB preparation with lower toxicity and better efficacy than currently available formulations (Fig. 1).
We systematically altered the mole ratios of DSHemsPC, phosphatidylcholine and phosphatidylglycerol with different aliphatic chain lengths (14, 16, 18 and mixed chain lengths HSPC) to prepare the liposomal formulation of AmB. HSPC provides a mixture of C16 and C18 aliphatic chain length (Matsumori et al., 2002) and HSPC is used in the formulation of many of the available commercial liposomal formulations (e.g. AmBisome™ and Doxil™) and has a very good safety profile (Torchilin, 2005). The starting point for the selection of molar ratio for each aliphatic chain length and HSPC was the formulation of AmBisome™ which is composed of Cholesterol/HSPC/DSPG/AmB in a molar ratio of 2.5/5/2.0/1.0 ((Adler-Moore and Proffitt, 2002; Dupont, 2002). The lipid molar ratios of F4, F8, F12 and F16 formulations in Table 1 is the same as molar ratio of lipids in AmBisome™ (each mole of DSHemsPC provides two mole of stigmasterol, the plant sterol). In the other formulations (F1–F3, F5–F7, F9–F11, F13–F15), the molar ratio of DSHemsPC, PC and AmB was kept constant and only the molar ratio of PGs was decreased to determine the effect of different molar ratio of negative charge in the formulations. In formulations F17–20 the molar ratio of DSHemsPC was increased from 1.25 to 1.75 to evaluate the effect of higher molar ratio of stigmasterol in the formulations. Most of the formulations had colloidal properties like AmBisome™ (Table 1).
One of the mechanisms of stabilization of AmB in the liposomal bilayer is through electrostatic interaction between the positive charge of the mycosamine group in AmB and the negative charge on the PG (Jensen et al., 1999; Ellis, 2002; Guo et al., 1991). Therefore, we hypothesized decreasing the pH of formulations would strengthen this electrostatic interaction and result to a better formulation of AmB. Based on their physical properties, potassium release properties and MTD results; F4, F8, F11, F12, F16 and F19 formulations were selected and prepared in DSSH buffer pH 6.5 (Table 2) and pH 5.5 (Table 3). Most of these formulations had colloidal properties like AmBisome™.
The particle diameter of control empty liposome (F33) was less than F29 and most of the other DSHemsPC-AmB formulations. The reason behind this could be the absence of AmB in liposome bilayer in this formulation. AmB intercalates in the liposome bilayer and it is usually hard to decrease the size of AmB-liposomes.
There are a few methods for the determination of toxicity of AmB formulations. Animal lethality test in mice and in vitro incubations of formulations with red blood cells are the most commonly used methods (Jensen et al., 1999; Espada et al., 2008; Szoka et al., 1987). The red blood cell tests are based on the effect of AmB increases the leakage of intracellular constituents such as potassium or hemoglobin from RBC (Butler and Cotlove, 1971). In this study the potassium release assay was used to assess the toxicity of formulations since potassium release has a good correlation with the animal lethality test (Jensen et al., 1999).
Among commercially available AmB formulations, Fungizone™ is the most toxic and AmBisome™ is the least toxic (Espada et al., 2008; Larabi et al., 2004). The RBCPR studies herein also showed that Fungizone™ was much more toxic than AmBisome™ and the results obtained in this study for these two AmB commercial products was almost the same as previously reported (Jensen et al., 1999). Among the DSHemsPC-AmB formulations prepared in HEPES buffer pH 7.4, F9- F12 formulations, which also had good vesicle properties (near 100 nm with a monomodal distribution and a low polydispersity index), showed higher IC50 for RBCPR compared to AmBisome™ (Table 1). The higher IC50 indicates less potassium leakage, hence lower toxicity of AmB for RBCs. The lower toxicity may indicate higher affinity of AmB for the lipid bilayer in the DSHemsPC-AmB formulations than for the lipid bilayer of AmBisome™. F5, F6 and F13 had also high IC50 for RBCPR but in these formulations the lesser toxicity is probably due to the multilamellar structures of formulations. Since AmB in the MLV structure has to pass several aqueous phases and bilayers of liposomes to reach the RBCs for its membrane disturbing activity and the drug is not readily available to interact with RBCs, the RBCPR activity of Formulation F5, F6 and F13 is low.
For F4, F8, F11, F12, F16 and F19, when the pH of formulations decreased to 6.5 and 5.5 the IC50 for RBCPR increased (Table 2 and 3). The reason for higher RBCPR IC50 and lower toxicity of formulations could be the higher affinity of AmB for PGs in liposome bilayer at the lower pH.
The results of in vitro antifungal activity of DSHemsPC-AmB formulations showed that most of formulations have comparable antifungal efficacy compared to AmBisome™ and Fungizone™ (Table 4). The leishmanicidal activities of the DSHemsPC-AmB formulations were tested against both the extracellular promastigote and the intracellular amastigote forms of the parasite. The ED50 of DSHemsPC-AmB formulations, AmBisome™ and Fungizone™ against promastigotes were greater than when tested versus the amastigotes. In general, intramacrophage amastigotes are more susceptible to AmB than promastigotes (Croft, 2001; Yardley and Croft, 1997). The ED50s of certain formulations (F9–F12) were comparable with the commercial liposomal AmB (AmBisome™). There were no significant (P > 0.05) differences in the activities of the F1–F4, F9–F14, F19, F20, F22 and F23 formulations against L. major amastigotes compared to AmBisome™. The ED50s of F5–F8, and F15–F18 were significantly (P < 0.001) greater than AmBisome™ and Fungizone™. The ED50 of F21, F24–F30, and F32 was similar (P > 0.05) to Fungizone™. The assays with promastigotes, amastigotes and antifungal also demonstrated that the processes used for the preparation of DSHemsPC formulation do not affect on the activity of the AmB.
In order to compare directly the in vivo toxicities of DSHemsPC-AmB formulations with AmBisome™ and Fungizone™, MTD was estimated in mice for formulations with favorable vesicle characteristics and RBCPR IC50 results (Table 6). After a single i.v. injection, the MTD observed for Fungizone™ was 2 mg/kg. Formulations F8, F15, F16 and F22 behaved in the MTD response like the micelle formulations of AmB and were as toxic as Fungizone™. Mice that received these formulations died in less than a minute. The reason for high toxicity of these formulations could either be due to the weak attachment of AmB to the liposomes bilayer with the consequence that AmB is released very fast when the formulation is in circulation. Alternatively the formulations might have aggregated when injected and resulted in hemostasis.
Interestingly the F11 formulation, which was prepared at pH 7.4 had an MTD of 10 mg/kg, when prepared in pH 6.5 (F23) the same formulation had an LD50 of 20 mg/kg and when prepared in pH 5.5 (F29), the MTD was increased to 60 mg/kg.
The decrease in toxicity of this composition prepared at lower pH was correlated with a decrease in the zeta potential of the formulations F11 = 69 mV, F23 = 42 mV and F29 = 25 mV(Table 1, 2, 3). Of the three different commercialized lipid-based formulations, Amphotec™ and Abelcet™ have LD50 about 30–32 mg/kg and AmBisome™ has an LD50 about 160 mg/kg (Jensen et al., 1999). Formulation F29 has less acute toxicity when compared to Amphotec™ and Abelcet™; however, its MTD is less than AmBisome™.
5. Conclusions
In summary, DSHemsPC provides a novel, stable matrix for solubilizing and delivering AmB in liposomes. The selection of an optimized liposome formulation requires characterization of the colloidal, in vitro and in vivo toxicity, antifungal and antileishmanial properties of the preparation. Minor changes in lipid composition can markedly alter some or all of these characteristics. Among the DSHemsPC formulations, the F29 formulation (DSHemsPC/DMPC/DMPG/AmB mole ratio 1.25/5.0/1.5/1.0, prepared at pH 5.5) had excellent colloidal properties, a high IC50 for RBCPR, antifungal and antileishmanial activity similar to AmBisome™ and an MTD of 60 mg/kg. F29 merits further investigation in murine models of fungal and leishmania infections to determine if F29 can provide a successful and economical formulation to treat such infections.
Acknowledgment
This project was supported by a grant from Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, IRAN and by NIH GM061851.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002;49(Suppl 1):21–30. doi: 10.1093/jac/49.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clin. Microbiol. Infect. 2008;14(Suppl 4):25–36. doi: 10.1111/j.1469-0691.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- Andes D, Safdar N, Marchillo K, Conklin R. Pharmacokineticpharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob. Agents Chemother. 2006;50:674–84. doi: 10.1128/AAC.50.2.674-684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann R, Cleary JD, Sterba J. Clinical roundtable monograph: safety and efficacy of lipid-based amphotericin B. Clin. Adv. Hematol. Oncol. 2009;7:1–8. [PubMed] [Google Scholar]
- Boswell GW, Buell D, Bekersky I. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 1998;38:583–592. doi: 10.1002/j.1552-4604.1998.tb04464.x. [DOI] [PubMed] [Google Scholar]
- Butler WT, Cotlove E. Increased permeability of human erythrocytes induced by amphotericin B. J. Infect. Dis. 1971;123:341–350. doi: 10.1093/infdis/123.4.341. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Croft SL. Monitoring drug resistance in leishmaniasis. Trop. Med. Int. Health. 2001;6:899–905. doi: 10.1046/j.1365-3156.2001.00754.x. [DOI] [PubMed] [Google Scholar]
- Croft SL, Coombs GH. Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs. Trends. Parasitol. 2003;19:502–8. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Deray G. Amphotericin B nephrotoxicity. J Antimicrob Chemother. 2002;49(Suppl 1):37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- Dupont B. Overview of the lipid formulations of amphotericin B. J Antimicrob. Chemother. 2002;49(Suppl):31–6. doi: 10.1093/jac/49.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 2005;54:119–22. doi: 10.1016/j.parint.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ellis D. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 2002;49(Suppl. S1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP, Torrado JJ. Effect of aggregation state on the toxicity of different amphotericin B preparations. Int. J. Pharmacol. 2008;361:64–69. doi: 10.1016/j.ijpharm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Fahr A, Van Hoogevest P, May S, Bergstrand N, ML SL. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery. Eur. J. Pharm. Sci. 2005;26:251–65. doi: 10.1016/j.ejps.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Farhoudi-Moghaddam AA, Katouli M, Jafari A, Bahavar MA, Parsi M, Malekzadeh F. Antimicrobial drug resistance and resistance factor transfer among clinical isolates of salmonellae in Iran. Scand. J. Infect. Dis. 1990;22:197–203. doi: 10.3109/00365549009037902. [DOI] [PubMed] [Google Scholar]
- Groll AH, Walsh TJ. Antifungal chemotherapy: advances and perspectives. Swiss Med. Wkly. 2002;132:303–311. doi: 10.4414/smw.2002.09729. [DOI] [PubMed] [Google Scholar]
- Guo LS, Fielding RM, Lasic DD, Hamilton RL, Mufson D. Novel antifungal drug delivery stable amphotericin B- cholesteryl sulfate discs. Int. J. Pharmacol. 1991;75:45–54. [Google Scholar]
- Herbrecht R, Natarajan-Ame S, Nivoix Y, Letscher-Bru V. The lipid formulations of amphotericin B. Expert. Opin. Pharmacother. 2003;4:1277–87. doi: 10.1517/14656566.4.8.1277. [DOI] [PubMed] [Google Scholar]
- Huang Z, Szoka FC. Sterol-modified phospholipids: cholesterol and phospholipid chimeras with improved biomembrane properties. J. Am. Chem. Soc. 2008;130:15702–15712. doi: 10.1021/ja8065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Jaafari MR, Szoka FC. Disterolphospholipids: nonexchangeable lipids and their application to liposomal drug delivery. Angew. Chem. Int. Ed. Engl. 2009;48:4146–9. doi: 10.1002/anie.200900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafari MR, Bavarsad N, Bazzaz BS, Samiei A, Soroush D, Ghorbani S, Heravi MM, Khamesipour A. Effect of topical liposomes containing paromomycin sulfate in the course of Leishmania major infection in susceptible BALB/c mice. Antimicrob. Agents. Chemother. 2009;53:2259–65. doi: 10.1128/AAC.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff AS, Boni LT, Popescu MC, Minchey SR, Cullis PR, Medden TD, Tearaschi T, Gruner SM, Shyamsunder E, Tate MW, Mendelsohn R, Bonner D. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc. Natl. Acad. Sci. USA. 1998;85:6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen GM, Skenes CR, Bunch TH, Weissman CA, Amirghahari N, Satorius A, Moynihan KL, Eley CGS. Determination of the relative toxicity of amphotericin B formulations: A red blood cell potassium release assay. Drug Delivery. 1999;6:81–88. [Google Scholar]
- Kan CC, Yan JS, Bittman R. Rates of Spontaneous Exchange of Synthetic Radiolabeled Sterols between Lipid Vesicles. Biochemistry. 1992;31:1866–1874. doi: 10.1021/bi00121a040. [DOI] [PubMed] [Google Scholar]
- Larabi M, Pages N, Pons F, Appel M, Gulik A, Schlatter J, Bouvet S, Barratt G. Study of the toxicity of a new lipid complex formulation of amphotericin B. J. Antimicrob. Chemother. 2004;53:81–8. doi: 10.1093/jac/dkh025. [DOI] [PubMed] [Google Scholar]
- Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA. 2006;103:16649–54. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, Borleffs JC, Allworth T, Kauffmann RH, Jones P, Kroon FP, Verbrugh HA, de Marie S. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. Aids. 1997;11:1463–71. doi: 10.1097/00002030-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Matsumori N, Yamaji N, Matsuoka S, Oishi T, Murata M. Amphotericin B covalent dimers forming sterol-dependent ion-permeable membrane channels. J. Am. Chem. Soc. 2002;124:4180–1. doi: 10.1021/ja012026b. [DOI] [PubMed] [Google Scholar]
- Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs Aging. 2009;69:361–92. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- Mora MP, Tourne-Peteilh C, Charveron M, Fabre B, Milon A, Muller I. Optimisation of plant sterols incorporation in human keratinocyte plasma membrane and modulation of membrane fluidity. Chemistry and Physics of Lipids. 1999;101:255–265. doi: 10.1016/s0009-3084(99)00067-5. [DOI] [PubMed] [Google Scholar]
- Olson JA, Adler-Moore JP, Schwartz J, Jensen GM, Proffitt RT. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 2006;50:2122–31. doi: 10.1128/AAC.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJ, Sudbery I, Ramsdale M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. USA. 2003;100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Sriti J, Talou T, Wannes WA, Cerny M, Marzouk B. Essential oil, fatty acid and sterol composition of Tunisian coriander fruit different parts. Journal of the Science of Food and Agriculture. 2009;89:1659–1664. [Google Scholar]
- Szoka FC, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu. Rev. Biophys. Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Szoka FC, Milholland D, Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposomeintercalated amphotericin B. Antimicrob. Agents Chemother. 1987;31:421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Yamamoto Y, Ueda Y, Sumita Y, Yoshida K, Niki Y. Comparative studies on the efficacy of AmBisome and Fungizone in a mouse model of disseminated aspergillosis. J. Antimicrob. Chemother. 2004;53:311–7. doi: 10.1093/jac/dkh055. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Tremblay C, Barza M, Fiore C, Szoka F. Efficacy of liposomeintercalated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob. Agents Chemother. 1984;26:170–3. doi: 10.1128/aac.26.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Holcenberg JS. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N. Engl. J. Med. 1999;340:764–71. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M, Barrett J, Anaissie EJ. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrobial agents and chemotherapy. 2001;45:3487–96. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin. Infect. Dis. 2000;31:1155–63. doi: 10.1086/317451. [DOI] [PubMed] [Google Scholar]
- Yardley V, Croft SL. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–6. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents. 2000;13:243–8. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]