Abstract
Background
Helicobacter pylori colonizes the mucus layer of the human stomach and may cause peptic ulcer and adenocarcinoma. Novel antimicrobial approaches are sought due to the occurrence of antibiotic resistance and consequent treatment failure. We report here that H. pylori is susceptible to inactivation by blue light.
Study Design/Materials and Methods
A controlled, prospective, blinded, trial of endoscopically delivered blue light to eradicate H. pylori in regions of the gastric antrum, in 10 patients between the ages of 21 and 80 who tested positive for H. pylori. Light (405 nm) (40 J/cm2) was delivered to a 1-cm diameter spot in the gastric antrum via optical fiber passed through the endoscope and weighed biopsies were taken from treated and control spots and colonies quantitatively cultured.
Results
Blue light killed 5 logs of bacteria in vitro. The mean reduction in H. pylori colonies per gram tissue between treated and control spots was 91% (7.4 ± 4.8 × 106 vs. 8.1 ± 1.9 × 107, two-tailed P < 0.0001). Some patients had reductions approaching 99%. No differences were observed on histological examination of light-treated and control gastric tissue.
Conclusion
Blue light phototherapy may represent a novel approach to eradication of H. pylori, particularly, in patients who have failed standard antibiotic treatment.
Keywords: porphyrin, photodynamic therapy, phototherapy, gastric biopsy, diode laser, endoscopy, gastric ulcer
INTRODUCTION
Helicobacter pylori is a Gram-negative microaerophilic bacterium, which selectively colonizes the mucus layer of the human stomach and duodenum; the organism is a major cause of chronic gastritis, gastric ulcer, duodenal ulcer, gastric lymphoma, and gastric adenocarcinoma [1]. H. pylori is a common pathogen with adult prevalence rates of approximately 80% in developing countries, and 20%–50% in industrialized nations [2]. Current treatment of H. pylori requires a combination of multiple antibiotics and proton pump inhibitors (PPI), administered over a 7–14 day period. The favored regimens employ metronidazole in combination with a PPI, tetracycline, and bismuth, or clarithromycin in combination with a PPI and amoxicillin [3,4]. These antibiotic regimens are complex, are often associated with unpleasant side effects and unsatisfactory patient compliance, and have eradication rates that vary between 72% and 95% [5]. Acquired resistance of H. pylori to both metronidazole and clarithromycin is well-documented; clarithromycin resistance in the United States has been identified in 8%–12% of isolates, and metronidazole resistance can be found in 13%–39% of isolates [5]. The impact of resistant isolates on treatment can be significant since resistant strains have a lower cure rate than susceptible strains. For the metronidazole-based regimens, resistant H. pylori strains can result in an excess failure rate of 20% compared to non-resistant strains; the overall treatment failure rate for clarithromycin resistant strains can be as high as 77% [6]. The increasing occurrence of resistance necessitates a search for alternative treatment modalities to eradicate H. pylori.
Many Gram-negative and Gram-positive bacteria, mycoplasma, fungi, and viruses have been shown to be sensitive to the combination of photosensitizing dyes and different wavelengths of light [7]. This combination known as photodynamic therapy (PDT) has been clinically approved for various malignant, premalignant, and ophthalmologic conditions [8]. PDT involves absorption of light by the dye leading to formation of a long-lived excited triplet state of the dye molecule that can transfer energy to molecular oxygen, thus forming the reactive singlet oxygen [9]. This oxidizing species can destroy proteins, lipids, and nucleic acids causing cell death and tissue necrosis. PDT is being investigated as a treatment for infectious disease including H. pylori [7,10]. The Gram-positive bacterium that causes acne, Propionibacterium acnes, is killed by both blue and red lights in the absence of dyes [11,12]; recently, the FDA approved a high-intensity narrow-band blue light therapy for the treatment of acne vulgaris [13]. Sensitive autofluorescence spectroscopy demonstrates that P. acnes appears to naturally synthesize the fluorescent photosensitizer protoporphyrin IX (PPIX) which mediates the killing effect of visible light [14,15]. Other bacteria including Actinomyces odontolyticus and Porphyromonas gingivalis, organisms that cause periodontitis, also naturally synthesize or accumulate PPIX and coproporphyrin (CP), and are also killed by red light [12].
A few studies have documented the susceptibility of H. pylori to PDT. There are two reports [16,17] of PDT killing H. pylori in vitro using porphyrins, phthalocyanines phenothiazinium dyes as photosensitizers. Millson et al. [18] exposed the gastric epithelium removed from ferrets infected with H. mustelae to phenothiazinium dyes and red light, and eradicated the bacteria. A preliminary clinical trial was carried out in 13 patients using oral 5-aminolevulinic acid that acts as a metabolic precursor of PPIX (20 mg/kg) and, 45 minutes later, a zone of gastric antrum was illuminated through an endoscope with a blue laser (410 nm, 50 J/cm2) [19]. They demonstrated greater reduction of H. pylori in biopsies from illuminated areas compared to control zones.
We demonstrate in this report that H. pylori (ATCC 43504) is killed in vitro by exposure to modest levels of blue light. Our hypothesis is that, like P. acnes, H. pylori naturally accumulates significant quantities of PPIX and CP, and that specific wavelengths, intensities, and time exposures of visible light lead to a photodynamic inactivation of the bacterium. The objective of this study was to test the hypothesis that blue light, at the proper wavelengths and fluences, could be employed as a therapeutic method to inactivate and eradicate H. pylori, a porphyrin-containing bacterium, without the use of external photosensitizers. This was carried out by a controlled, prospective, blinded therapeutic trial of endoscopically delivered blue light to a region of the mucosa in the gastric antrum in chronically infected symptomatic patients.
PATIENTS AND METHODS
In Vitro Experiments
H. pylori ATCC 43504 was routinely grown in liquid medium consisting of Brucella broth supplemented with 10% fetal bovine serum, and an antibiotic mixture of 10 µg/ml vancomycin, 5 µg /ml trimethoprim, 6 µg /ml nalidixic acid, and 5 µg /ml amphotericin B [20]. The solid medium consisted of the former ingredients with 1.5% agar. One milliliter of frozen stock (medium described above plus 20% glycerol) was added to 10-ml medium in a 25-ml Ehrlenmyer flask and incubated in a microaerophilic atmosphere (BBL GasPak with CampyPak Plus, Fisher Scientific, Hampton, NH) and rotation at 200 rpm at 37°C. After 96 hours, the optical density at 650-nm was generally between 2 and 3, and the bacteria were spun down and resuspended at the same density in phosphate buffered saline (PBS). Two hundred microliters of bacterial suspension was exposed to light from a laser light source (manufactured by Seedling Enterprises, LLC, Boston, MA) that combined multiple blue light-emitting diodes, capable of delivering up to 200 mW/cm2 of 405 ± 2 nm blue light through a flexible optical fiber. The spot size was 2 cm2 and the irradiance was 100 mW/cm2. Aliquots (2 × 10 µl) of the bacterial suspension were withdrawn at intervals of 40, 80, 160, and 320 seconds when 4, 8, 16, and 32 J/cm2 had been delivered, respectively. Aliquots were also withdrawn at the corresponding times from a non-illuminated well that was just exposed to laboratory air. The aliquots were serially diluted in PBS and horizontally streaked on square agar plates according to the method of Jett et al. [21]. The plates were incubated at 37°C in microaerophilic gas jars for 5–7 days until colonies were easily countable. Survival fractions were calculated with reference to non-illuminated bacteria at time zero.
Study Design
The study protocol and consent form were approved by the Abbott-Northwestern Institutional Review Board (Minneapolis, MN). The study was designed as a controlled, prospective, blinded, therapeutic trial of endoscopically delivered visible (blue) light to eradicate H. pylori in regions of the gastric antrum, in chronically infected symptomatic patients. Eligible patients were adults of either gender, between the ages of 21 and 80, seen in the outpatient clinic for symptoms of dyspepsia or suspected peptic ulcer disease. To qualify for the study, patients needed to test positive for H. pylori by either breath test (Meretek Diagnostics, Lafayette, CO) or stool antigen test (Specialty Laboratories, Santa Monica, CA), and should not have received oral or intravenous antibiotics, Pepto-Bismol, or proton-pump blocking agents for at least 3 months prior to enrollment. At the time of enrollment, the patients underwent a brief standard medical history and physical examination. All the patients underwent an informed consent process and had the study protocol including the light treatment procedure explained to them. Additional exclusion criteria included pregnancy, history of gastric or duodenal carcinoma, history of previous gastric or duodenal surgery, or history of a bleeding disorder or anti-coagulant use that would prevent biopsy.
Procedure
Subjects who fulfilled the recruitment criteria, i.e., appropriate symptoms, positive H. pylori status by noninvasive testing, and the ability to undergo recommended endoscopy, were recruited. Study subjects underwent a standard diagnostic endoscopy (Olympus GIF-160, Melville, NY) using topical analgesia (Hurricaine Spray, Beutlich, Inc., Waukegan, IL) and moderate anesthesia (Fentanyl, Versed). All patients received glucagon to inhibit gastric motility. During endoscopy, any abnormalities were noted and dealt with in the usual manner. Following the diagnostic portion of the procedure, two adjacent areas, approximately 1 cm2, in the pre-pyloric antrum, were marked using radiofrequency ball-tip cautery. These areas, randomly chosen, lay side-by-side typically along the lesser curve aspect of the antrum, and were within 5 cm of the pylorus. Sites were chosen that allowed optimal en-face light exposure. A pre-exposure set of two routine tissue biopsies were obtained from one of the specifically marked locations in the antrum of the stomach, using standard tissue biopsy forceps (Radial Jaw, CR Bard, Murray Hill, NJ). These biopsies served as matched preexposure controls, and were serially cultured for H. pylori as described below. The adjacent site was then exposed to blue light (405 ± 2 nm) from the diode laser described above via a flexible optical fiber (Rare Earth Medical, West Yarmouth, MA) used in standard PDT, which was passed through the biopsy channel of the endoscope. When properly positioned en-face to the gastric mucosa, the fiber emitted a collimated beam, visible as a circular spot of blue light, of variable diameter depending on the distance that it was positioned from the gastric wall. To ensure consistent light energy delivery, a consistent spot size, and a consistent distance from the fiber to the gastric mucosa in the same patient and between patients, a video template method was employed. A template in the shape of a circular target was traced on a thin transparent sheet of plastic and taped to the endoscopy video monitor screen. The endoscope and fiber were withdrawn from the gastric mucosa until the illumination spot completely filled the circular template; this corresponded to an approximate 1 cm2 spot size on the gastric wall. When this occurred, the tip of the fiber was approximately 3 cm from the gastric mucosa. This method also helped ensure that the endoscopically marked site was completely exposed to light without any missed areas. In this manner, the marked treatment areas were exposed to 405-nm light, using a power setting of 150 mW/cm2, for 4.5 minutes, for a total blue light energy exposure of 40.5 J/cm2. Following the light treatment, two standard biopsies were taken from the light-exposed area, and quantitatively cultured. Four biopsies were necessary to consistently remove the mucosa in the 1 cm2 treated area. Biopsies from the control sites and every light-exposed site were sent for histological assessment via light microscopy. The pathologist was blinded as to the location of the tissue samples.
Biopsy Analysis
Biopsy samples from the control sites, and from the light-exposed treatment sites, were coded by number, and then placed in Brucella broth containing 10% fetal calf serum and 20% glycerol, and were snap frozen at −60°C for transport to the Wellman Laboratories in Boston, MA. Each tissue sample (weighing from 2–20 mg) was thawed, blotted dry of liquid, weighed, and homogenized individually (using a PowerGen 35, Fisher Scientific, Pittsburgh, PA) in 2 ml of Brucella broth containing 10% fetal calf serum together with the antibiotic mixture described above. The tissue homogenates were serially diluted in PBS and colonies grown and counted as described above. Results were expressed as colony forming units (CFU) per gram of biopsy tissue. The microbiology lab personnel were blinded to the endoscopic results and sample identities.
Histology
Four different patients each had a biopsy from a control spot and from a light-treated spot fixed in buffered formalin, embedded in paraffin and processed for standard hematoxylin and eosin staining.
Statistical Analysis
Statistical analysis was performed using the two-tailed unpaired Student’s t-test.
RESULTS
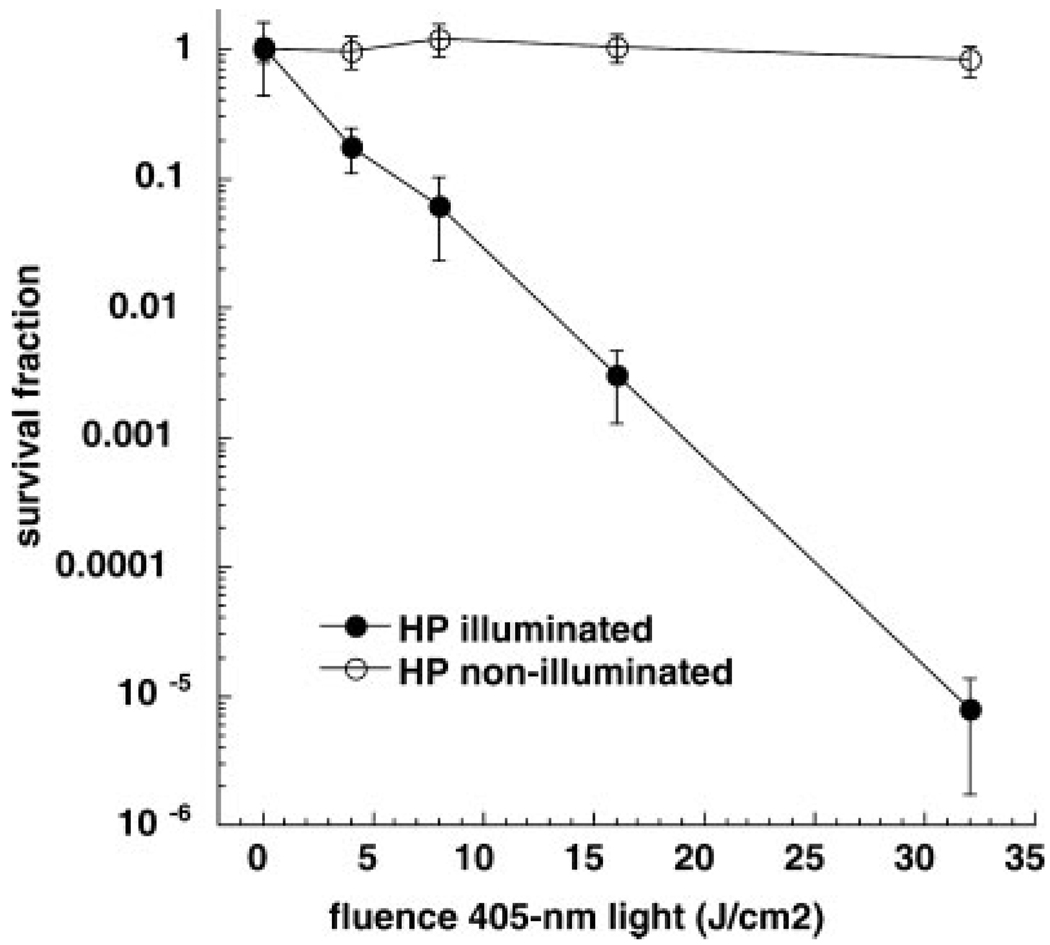
Figure 1 shows that H. pylori is killed in a light dose dependent manner in vitro after blue light illumination. Five minutes of light delivery (representing 32 J/cm2 energy density) was sufficient time to reduce the bacterial viability by 99.999% or 5 logs of cell killing. Control non-illuminated bacteria exposed to laboratory air for the same time were not affected. This encouraging result, prompted us to design a clinical trial and suggested an initial light dose for in vivo illumination within the stomach.
Fig. 1.
In vitro killing of H. pylori. Suspensions of bacteria in phosphate buffered saline (PBS) were illuminated or not with 405-nm light. Aliquots were withdrawn from both illuminated and non-illuminated wells at calculated times after incremental light doses had been delivered and serial dilutions and colony counting allowed survival fractions to be calculated. Values are means of three independent experiments and bars are SEM.
Table 1 shows the demographic characteristics and endoscopic findings of the study of patients. Ten patients were initially enrolled, however, one patient inadvertently received H. pylori treatment with 10 days of Helidac (Prometheus Labs, San Diego, CA) prior to the study endoscopy. This was not discovered until after the study endoscopy when the patient’s control biopsies failed to grow H. pylori. This patient’s (patient 7) samples were eliminated from the study. Nine patients completed the study and had samples available for analysis. Of the nine patients, there were six men and three women; all the patients had endoscopically visible gastropathy consistent with chronic H. pylori infection (edema, erythema, nodularity, or the classic H. pylori “chicken skin” appearance), and two of the nine patients had active peptic ulcer disease. Most of the patients had two separate sites treated with blue light and multiple biopsies were taken from illuminated and from control sites (see Table 1). The endoscopist maintained the spot of light, as consistently as possible, in position for 4.5 minutes, and then biopsied the treated area. Small, superficial RF marking sites helped to maintain the position of light in a consistent area. At times, the light would transiently shift off target via patient movement, gastric peristalsis, and belching, but these were held to a minimum; no patient received more than 4.5 minutes of light exposure.
TABLE 1.
Demographic Characteristics, Endoscopic Findings, and Number of Cultures From Biopsies of the Study of Patients
| Patient | Demographics | Control spots (no. of cultures) |
Light spot 1 (no. of cultures) |
Light spot 2 (no. of cultures) |
|---|---|---|---|---|
| 1 | 44 yo, male | 3 | 2 | 3 |
| 2 | 26 yo, male | 3 | 2 | |
| 3 | 42 yo, female | 9 | 2 | 3 |
| 4 | 27 yo, male | 3 | 2 | 2 |
| 5 | 63 yo, male | 2 | 3 | 3 |
| 6 | 46 yo, female | 2 | 3 | 3 |
| 7 | 44 yo, male | No colonies | ||
| 8 | 25 yo, male | 6 | 3 | 3 |
| 9 | 75 yo, female, gastric ulcer | 5 | 3 | 3 |
| 10 | 69 yo, male, gastric ulcer | 4 | 4 | 3 |
| Total | 37 | 24 | 23 |
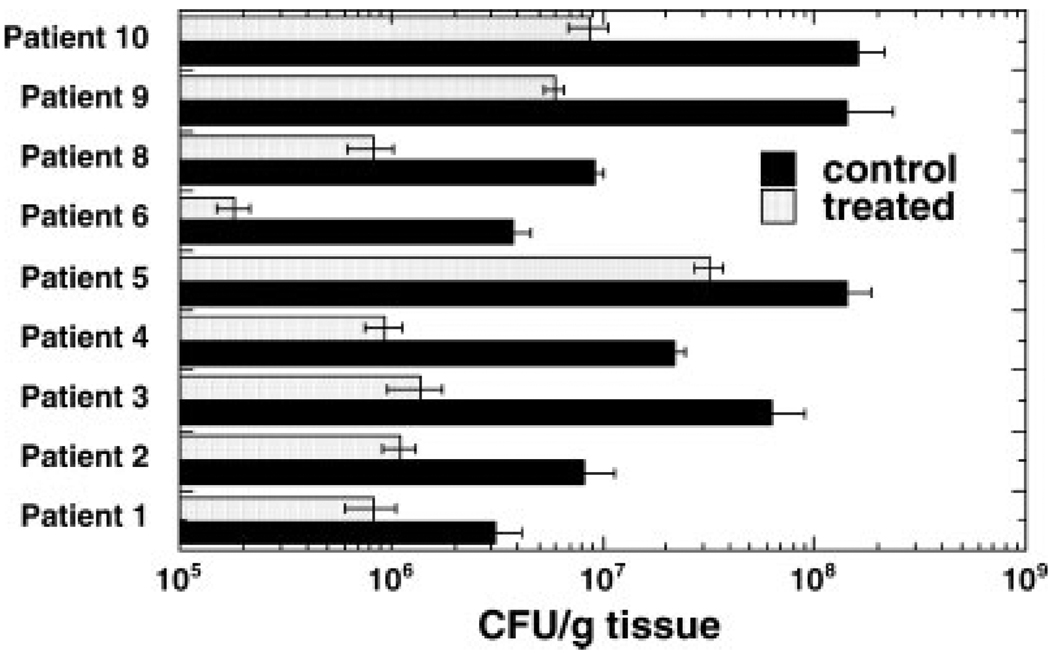
Figure 2 compares the number of CFU between each individual patient’s control site and subsequent treatment site. CFUs were graphed per gram of tissue plated to account for variability in the size of the biopsy samples. The control samples contained 106–108 organisms per gram of tissue at baseline. In all nine treated subjects, the illuminated samples showed a significant bacterial kill as compared to the control samples. Eradication of at least 90% of the illuminated bacteria was accomplished in seven of the nine patients (1 log reduction in CFUs). In some of the samples, at least 99% of the bacteria were killed (2 logs reduction).
Fig. 2.
Mean colony forming units (CFU) per gram tissue (± SEM) from control and illuminated spots from each patient. Values are means of all cultures (see Table 1).
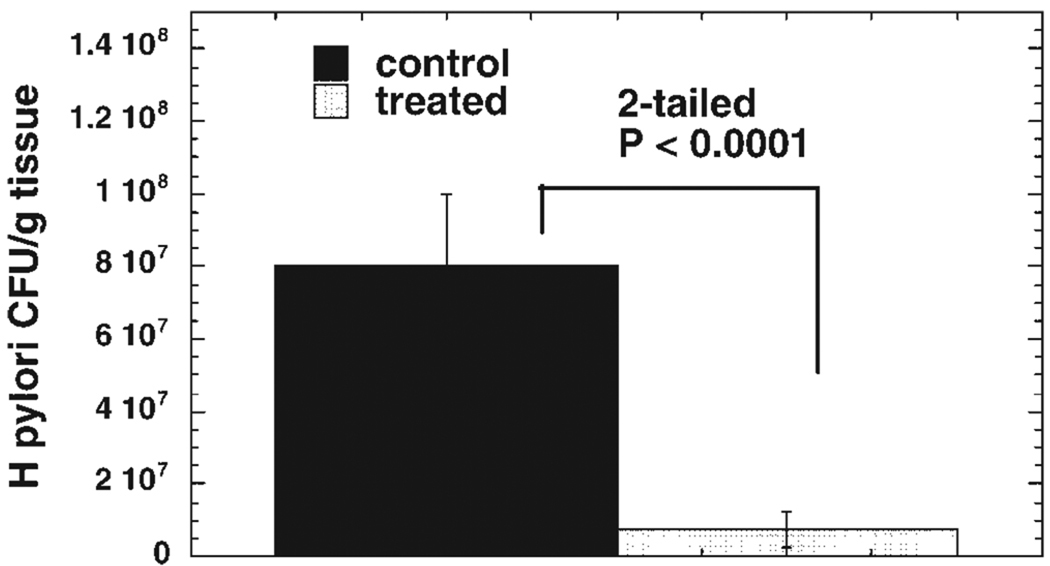
Figure 3 shows the CFU comparison when all the control and treatment samples are grouped together. There is a significant reduction (91%, P < 0.0001) in surviving bacteria as determined by a two-tailed Student’s t-test. There were no discernible cellular abnormalities (excess inflammatory infiltrate, pyknotic nuclei) as assessed by light microscopy, in the tissue obtained from the treated areas after light exposure compared to the control biopsies. Histologically, the treated cells appeared indistinguishable from the control cells (data not shown).
Fig. 3.
Mean CFU per gram tissue (± SEM) from all cultures from all nine patients. P value determined by two-tailed unpaired Student’s t-test.
DISCUSSION
This was a small pilot protocol aimed at studying the effect of a low dose of blue light, on the viability of H. pylori in vitro and in human subjects. The study demonstrates the feasibility of using blue light to inactivate or kill H. pylori, in that the organism can be killed with one short visible light treatment, and that blue light appears to be able to penetrate the gastric mucus layer to a depth necessary to affect bacterial viability. It is unclear from the current study whether blue light can penetrate to the level of the gastric glands and kill H. pylori that is adherent to crypts in the gastric epithelium, or whether the phototoxic effect is limited to “free-swimming” organisms in the mucus layer. Previous animal studies investigated the effective penetration depth (where the intensity is reduced to 37% of that at the surface) of various wavelengths of light into bovine muscle, and found the depth for 400-nm light to be 2 mm [22]. Even taking into account the possible differences in tissue optical properties (absorption and scattering) between muscle and gastric mucosa, this penetration should be adequate to reach the deep gastric epithelial glands [23].
We believe that the blue light therapy killed H. pylori or rendered it permanently non-viable, as opposed to placing the organism in a dormant state temporarily unable to replicate. The culture plates were maintained for several days and should have demonstrated viable organisms within this period of time. The study design did not allow us to obtain before and after counts of H. pylori from the illuminated areas, because red blood from initial biopsies may have impacted the ability of blue light to penetrate the mucosa. Since the areas for study were randomly selected and the control and illuminated sites were immediately adjacent, and multiple biopsies taken within each control and study site were cultured, we believe that we eliminated the possibility that the control sites would by chance contain more bacteria than the treated sites.
The dose of 40 J/cm2 blue light in this study was chosen in light of the excellent response of in vitro bacteria to 32 J/cm2. In an optically clear medium (PBS), this fluence completely eradicated H. pylori producing up to 5 logs of kill in a light-dose dependent manner. Also, the amount of light delivered by our device in this study falls below the threshold limit value for exposure of the eye or the skin published by the American Conference of Governmental Industrial Hygienists [24]. In addition, this dose of light energy and intensity has been demonstrated to be safe, as it falls within the range equivalent to light exposure from endoscopy [25]. Nishioka [25] has previously demonstrated that in the typical viewing mode, the maximum irradiance obtained from a standard endoscope was 1.6 W/cm2; in transillumination mode, the maximum irradiance was 8.0 W/cm2. Blue light comprises only a small fraction (<5%) of the white light emanating from a typical endoscope; the dose of blue light used in our study was 100 times greater than what could be obtained from a standard endoscope, but still within a safe range. At the relatively low power and energy levels used in this study, the blue light source generated no appreciable heat, and would not damage gastric tissue even if used in transillumination mode.
Exactly, why H. pylori is killed by blue light at the current fluences is not known. At the dose of light used the killing effect was not due to heat. We postulate that the mechanism is similar to the destruction of bacterial cells using photodynamic therapy and exogenous porphyrin photosensitizers in other circumstances [7]. H. pylori synthesizes and accumulates the naturally occurring porphyrins PPIX and CP. When these endogenous bacterial porphyrins are then exposed to light of the proper wavelength, they achieve an excited singlet state that can undergo a transition to a long-lived triplet state, and can then pass energy to ground state oxygen molecules in the vicinity, creating reactive singlet oxygen and other reactive oxygen species, which result in cell death via disruption of organelles and chromosomal genetic material [9,26]. This mechanism of cell death is well accepted for photodynamic therapy using exogenous porphyrins, such as hematoporphyrin derivative, and red light in the treatment of gastrointestinal tumors [10].
The optimal dose of blue light needed to eradicate H. pylori in vivo is also not known. It is unclear whether the 1–2 logs reduction in bacterial counts achieved in this study would be adequate to allow a vigorous host immune response to eradicate the remaining organisms, or whether higher doses of blue light capable of achieving complete sterilization is necessary. It is also unclear what magnitude of log reduction is achieved with current antibiotic therapy. Recent advances in technology (blue light-emitting diodes each giving 5 W) could allow higher doses of blue light to illuminate the entire stomach without missing significant regions. If the penetration of the light into the gastric glands is adequate to kill adherent organisms, one can see the potential use of blue light therapy, without exogenous photosensitizers, in the treatment of de-novo or antibiotic resistant H. pylori. One key advantage of this approach would be the probable lack of acquired bacterial resistance, since phototherapy kills organisms by cellular disruption [27]. It would need to be studied to what extent the surviving bacteria regrow in the days and weeks following therapy. This question could be answered if the entire stomach was illuminated and the patients could be serially monitored by the urea breath test. If bacterial regrowth was found to occur, then it might be possible to use blue light therapy to produce a rapid decline of bacterial numbers, and to follow it with antibiotics to prevent recurrence of the infection. The results of this preliminary study suggest that further investigation into this technique is warranted.
Acknowledgments
Contract grant sponsor: Seedling Enterprises LLC; Contract grant sponsor: LumeRx, Inc.; Contract grant sponsor: US NIH (to MRH); Contract grant number: R01AI050879.
Footnotes
M.R.H. has disclosed a potential financial conflict of interest with this study.
REFERENCES
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 3.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE study. Gastroenterology. 2002;123:1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 4.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: A prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 5.Megraud F, Marshall BJ. How to treat Helicobacter pylori. First-line, second-line, and future therapies. Gastroenterol Clin North Am. 2000;29:759–773. doi: 10.1016/s0889-8553(05)70145-x. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ, Hennessy TW, Bensler JM, Bruden DL, Parkinson AJ, Morris JM, Reasonover AL, Hurlburt DA, Bruce MG, Sacco F, Butler JC. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139:463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 9.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 10.Bown SG, Millson CE. Photodynamic therapy in gastroenterology. Gut. 1997;41:5–7. doi: 10.1136/gut.41.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 12.Konig K, Teschke M, Sigusch B, Glockmann E, Eick S, Pfister W. Red light kills bacteria via photodynamic action. Cell Mol Biol (Noisy-le-grand) 2000;46:1297–1303. [PubMed] [Google Scholar]
- 13.Elman M, Slatkine M, Harth Y. The effective treatment of acne vulgaris by a high-intensity, narrow band 405–420 nm light source. J Cosmet Laser Ther. 2003;5:111–117. [PubMed] [Google Scholar]
- 14.Melo TB, Johnsson M. In vivo porphyrin fluorescence for Propionibacterium acnes. A characterization of the fluorescing pigments. Dermatologica. 1982;164:167–174. [PubMed] [Google Scholar]
- 15.Romiti R, Schaller M, Jacob K, Plewig G. High-performance liquid chromatography analysis of porphyrins in Propionibacterium acnes. Arch Dermatol Res. 2000;292:320–322. doi: 10.1007/s004030000122. [DOI] [PubMed] [Google Scholar]
- 16.Bedwell J, Holton J, Vaira D, MacRobert AJ, Bown SG. In vitro killing of Helicobacter pylori with photodynamic therapy (letter) Lancet. 1990;335:1287. doi: 10.1016/0140-6736(90)91361-d. [DOI] [PubMed] [Google Scholar]
- 17.Millson CE, Wilson M, Macrobert AJ, Bedwell J, Bown SG. The killing of Helicobacter pylori by low-power laser light in the presence of a photosensitiser. J Med Microbiol. 1996;44:245–252. doi: 10.1099/00222615-44-4-245. [DOI] [PubMed] [Google Scholar]
- 18.Millson CE, Wilson M, MacRobert AJ, Bown SG. Ex vivo treatment of gastric Helicobacter infection by photodynamic therapy. J Photochem Photobiol B. 1996;32:59–65. doi: 10.1016/1011-1344(95)07190-3. [DOI] [PubMed] [Google Scholar]
- 19.Wilder-Smith CH, Wilder-Smith P, Grosjean P, van den Bergh H, Woodtli A, Monnier P, Dorta G, Meister F, Wagnieres G. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: Preliminary human studies. Lasers Surg Med. 2002;31:18–22. doi: 10.1002/lsm.10066. [DOI] [PubMed] [Google Scholar]
- 20.Piccolomini R, Di Bonaventura G, Festi D, Catamo G, Laterza F, Neri M. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens. J Clin Microbiol. 1997;35:1541–1544. doi: 10.1128/jcm.35.6.1541-1544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty TJ, Marcus SL. Photodynamic therapy. Eur J Cancer. 1992;28A:1734–1742. doi: 10.1016/0959-8049(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 23.Uribe A, Gundersen HJ. Three-dimensional estimation of the glandular volume, and of the number and volume of epithelial cells in two glands from the antral mucosa of five healthy volunteers. APMIS. 1997;105:571–574. doi: 10.1111/j.1699-0463.1997.tb05055.x. [DOI] [PubMed] [Google Scholar]
- 24.Okuno T, Saito H, Ojima J. Evaluation of blue-light hazards from various light sources. Dev Ophthalmol. 2002;35:104–112. doi: 10.1159/000060814. [DOI] [PubMed] [Google Scholar]
- 25.Nishioka NS, Schomacker KT. Mucosal exposure to light during routine endoscopy. Gastrointest Endosc. 1999;49:456–461. doi: 10.1016/s0016-5107(99)70042-8. [DOI] [PubMed] [Google Scholar]
- 26.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]