Abstract
Photodynamic therapy (PDT) uses light activatable molecules that after illumination produce reactive oxygen species and unwanted tissue destruction. PDT has dual selectivity due to control of light delivery and to some extent selective photosensitizer (PS) accumulation in tumors or other diseased tissue, additional targeted selectivity of PS for disease is necessary. The delivery of drugs to selected lesions can be enhanced by the preparation of targeted macromolecular conjugates that employ cell type specific targeting by ligand-receptor recognition. Macrophages and monocytes express a scavenger-receptor that is a high-capacity route for delivering molecules into endocytic compartments in a cell-type specific manner. We have shown that by attaching PS to scavenger-receptor ligands it is possible to get three logs of selective cell killing in macrophages while leaving non-macrophage cells unharmed. The capability to selectively kill macrophages has applications in treating cancer and in the detection and therapy of vulnerable atherosclerotic plaque and possibly for autoimmune disease and some infections.
Keywords: macrophages, photosensitizer conjugate, cancer, inflammation, atherosclerosis
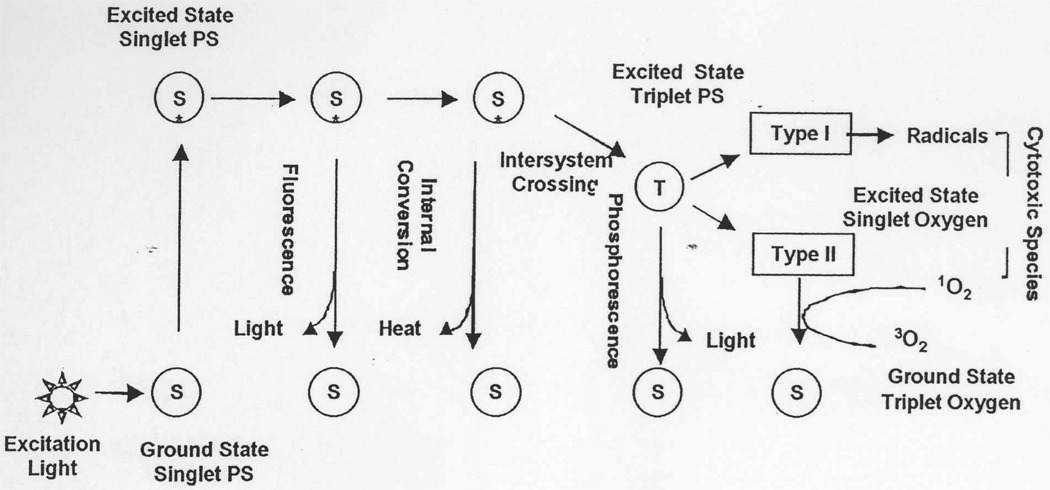
Photodynamic therapy (PDT) is relatively new approach for treatment of various diseases (1). It has received regulatory approval for several applications, including the therapy of cancers and age-related macular degeneration (2). PDT uses certain harmless substances know as photosensitizers (PS) that after illumination with appropriate light in the presence of oxygen generate cytotoxic species and provoke tissue destruction (3). The mechanism by which illumination of the PS with light can lead to cytotoxic reactive oxygen species (ROS) is schematically illustrated in Fig 1. Light of the correct wavelength is absorbed by the ground state PS and this energy raises the electrons in the molecular orbitals of the PS to the excited singlet state, which, if it does not lose energy as light (by fluorescence) or as heat (by internal conversion), may undergo intersystem crossing to the slightly lower energy triplet state. If this PS triplet state has a sufficiently long lifetime, and a sufficiently high energy level, it may undergo further reaction to produce reactive and cytotoxic species. These species can arise from the PS triplet by two distinct mechanisms. The first mechanism (known as Type I process) involves electron transfer reactions which produce PS radicals or radical ions, which usually react quickly with other molecules such as oxygen or biological substrates to produce a mixture of reactive and cytotoxic molecules known as reactive oxygen species. These consist of the very reactive hydroxyl radical, less reactive alkylperoxy radicals, superoxide and hydrogen peroxide. The second mechanism (known as Type II process) involves the transfer of energy from the PS triplet state to ground state oxygen (which is also a triplet) producing the excited state singlet oxygen, which is a reactive oxidant. Both singlet oxygen and Type I radicals (or superoxide) can oxidize important biomolecules such as lipids (unsaturated fatty acids), proteins (histidine, tryptophan and cysteine residues), and nucleic acids (guanosine residues). Since both Type I and II processes depend on the PS triplet state, it is clear that those PS with a high triplet yield, and a long triplet lifetime will be most active in PDT (4). There is a considerable debate in the literature on the relative contributions of Type I and Type II processes to the PDT mediated destruction of tumors observed both in experimental animal models and clinically in patients (3, 5).
Fig. 1.
Photophysical/photochemical mechanisms underlying PDT. Light is absorbed by the ground state PS that then moves to the first singlet excited state, from where it can lose energy by fluorescence or internal conversion and return to the ground state. Alternatively the excited singlet state PS can undergo intersystem crossing to the excited triplet state, which in addition to losing energy by phosphorescence, can transfer its energy to the ground state of molecular oxygen, which is also a triplet. This results in the PS returning to the ground singlet state, and the oxygen rising to the excited singlet state (Type II). Alternatively the triplet PS may undergo reactions with substrates leading to free radicals (Type I). Both these pathways lead to highly cytotoxic species by reacting with proteins, nucleic acids and lipids in cells.
The majority of PS used both clinically and experimentally are derived from the tetrapyrrole aromatic nucleus found in many naturally occurring pigments such as heme and chlorophyll. They can be divided into several structural groups including porphyrins, chlorines, phthalocyanines, purpurins, and texaphyrins. These compounds generally have low levels of dark toxicity and are stable in both humans and experimental animals. ROS, on the other hand, have short lifetimes and are very unstable and therefore the targeted delivery of PS is necessary to ensure that ROS are created where they are desired to act and to minimize the side effects and harm to healthy tissues.
PS have relatively high absorption bands in the red wavelengths of visible light (600–750 nm); this light can penetrate for about 1–3 mm deep in the tissue. Increased penetration depth can be achieved at longer wavelengths (700–850 nm) and therefore many efforts are made to develop of PS absorbing at such wavelength. The absorption of light by the PS itself can limit light penetration into tissue. Many PS are prone to photo-destruction during light exposure in the process called “ photobleaching” (6). Moreover light delivery can be complicated by the optical absorption (due to endogenous tissue chromophores, mainly hemoglobin) and by optical scattering within the tissue. Both of these parameters differ from tissue to tissue. For example liver exhibits especially poor light penetration due to its high hemoglobin and cytochrome content, and brain tissue has a high degree of light scattering.
Light delivery is either by a focused spot using a lens or a fiber optic, or by a cylindrical or spherical diffusing fiber tip for interstitial application (where the fiber tip is physically inserted into the tissue). Similar cylindrical diffusing fibers can be used for narrow tubular structures such as the bronchus, bowel or arteries, or by a diffusing fiber encased in an inflatable balloon for body cavities such as the bladder or uterus.
Although these techniques increase the selectivity for diseased tissue, multifocal disease may require additional PS targeting strategies needed. Selectivity can be achieved by many ways that are specific for different applications. One of the approaches for targeting PS to tumors, tissues or cells is by using covalent conjugates (or non-covalent complexes) between PS and macromolecular carriers with targeting properties (7). Such delivery vehicles have the advantage that the PS can be chosen on its optical and photophysical properties, without relying on the molecular structure of the PS to provide the targeting effect. Macromolecular targeting may rely on two facets of molecular structure. Firstly, features of the macromolecules such as size, charge, hydrophobicity and biodegradability may be manipulated to increase accumulation or retention in the tumor (8). Secondly, the macromolecular conjugate may be designed to recognize antigens, receptors or other cell type specific structures present on cancer cells or other cells (9).
In this report we will discuss different applications of macromolecular-PS conjugates with cells of the monocyte-macrophage lineage that are distinct from traditional tumor-targeting techniques.
Macrophage-targeted PDT
Macrophages are among the most versatile mammalian cells and are found in every tissue in the body (10). In addition to performing a number of diverse and beneficial functions macrophages are involved in the pathogenesis of many human diseases. They play a pivotal role both in the development of atherosclerosis (12), and also in the vulnerability of the atherosclerotic plaques to rupture (13), an occurrence that frequently precipitates heart attacks (14). Macrophages are involved in the pathology arising from many infectious and inflammatory diseases and have been implicated in the progression of some cancers (15). They therefore represent a prime target for therapeutic intervention. However, only the macrophages that are localized in the diseased tissue are undesirable, while the majority of monocyte-macrophages in the circulation and in healthy tissues are valuable and productive cells. Hence, although it is possible in principle to administer selective macrophage toxins that would kill all cells of the monocyte-macrophage lineage, this would be unnecessarily severe and uncertain (16). A method of killing only those macrophages in a certain anatomical location affected by disease would be desirable. Macrophage-targeted PDT might allow this goal to be accomplished. This approach involves covalently attaching the PS to a macromolecule that is specifically recognized by cell surface receptors expressed on macrophages, and by only illuminating the diseased tissue resulting in localized and specific destruction of macrophages (17). Class A scavenger receptor (SRA) is an appropriate target for macrophage-specific PDT (17). Its expression is largely confined to mature macrophages (18). Some of the natural function of SRA include: phagocytosis of bacteria and other pathogens, phagocytosis of apoptotic cells, phagocytosis of senescent red blood cells, endocytosis of oxidized low density lipoprotein and advanced glycation endoproduct-modified proteins, and calcium independent adhesion (19). The SRA is a multidomain trimeric transmembrane protein and has a high capacity to internalize ligands, and therefore investigators have explored the possibility of preparing covalent conjugates between SRA ligands and various drugs to produce macrophage targeted therapy (17, 20). The precise structural motifs that lead to SRA recognition of ligands are complex and incompletely understood and their function has been referred to as “molecular flypaper” (21). After initial binding, the ligands are rapidly internalized and are routed to lysosomes for degradation by proteases and other lysosomal enzymes. When PS are joined to ligands of the scavenger receptor cellular accumulation is enhanced because the SRA is high capacity and leads to efficient degradation of endocytosed molecules in lysosomes thus releasing the possibly more photoactive free PS.
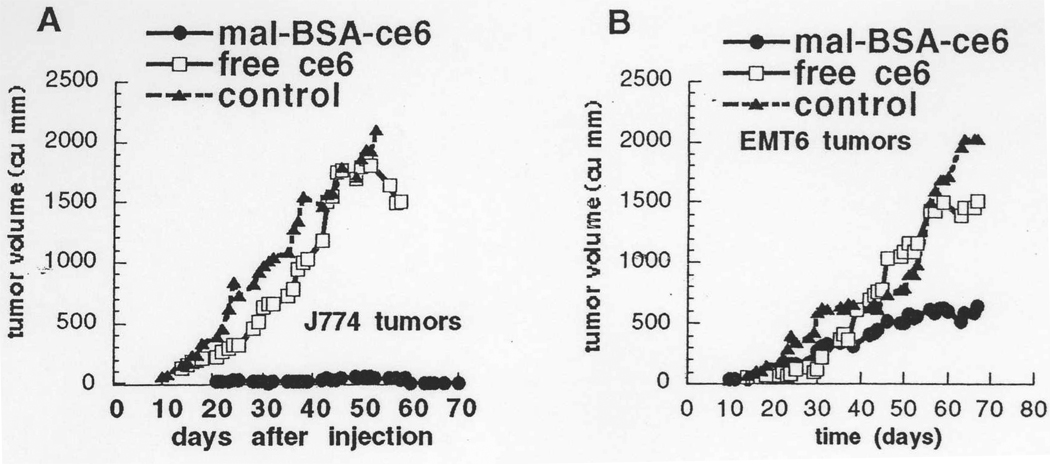
Our molecular construct designed to target the SRA is bovine serum albumin to which three molecules of the PS chlorin(e6) have been covalently attached via amide bond formation between the carboxyl groups on the tetrapyrrole and the epsilon amino groups of lysine residues in the protein (17). To further increase the affinity of this molecule to macrophage SRA we further modify the protein amino groups by maleylation to give BSA-ce6-mal. The high degree of selectivity of this SRA-targeted PS conjugates for macrophages is shown in Fig 2A and 2B. We studied three macrophage murine cell lines (J774, RAW264.7 and P388D1) as models for primary macrophages. Macrophages were cultured on plastic dishes in RPMI medium with 10% fetal calf serum for 24 hours. A mammary sarcoma cell line (EMT6) was used as an example of a non-macrophage cell type. PS uptake by cells was measured by flow cytometry after incubation with 2-mM ce6 equivalent of BSA-ce6-mal. As can be seen in Fig 2A the dye was taken up in a concentration dependent manner by the three macrophage cell lines and this uptake was between 5 and 9 times higher than the non-SRA expressing cell EMT6. Consequent light-dose dependent phototoxicity towards macrophages was seen after illumination with 600-nm light, while EMT6 cells were spared (Fig 2B). It should be noted however that primary macrophages isolated form bone marrow or peripheral blood may behave differently.
Fig. 2.
Cells were incubated with conjugate (5 µM for J774 cells, 2 µM for other cells) for 24 hours and uptake was determined by flow cytometry (Fig 2A); or cells were illuminated with 660-nm light at a fluence rate of 50 mW/cm2 and survival fractions were calculated with respect to non-illuminated cells by the MTT assay 24 hours later (Fig 2B).
Macrophage-targeted PDT: application to cancer
Macrophages have a dual role in cancer. Some of them fight cancer while others can help the tumor to grow and metastasize (22). The latter can happen in four distinct ways.
Firstly, Tumor associated macrophages (TAM) can secrete paracrine growth factors (TNF-α (23), EGF (24), IL-1, and IL-6) for tumor cells. Secondly, TAMs produce a wide range of angiogenic molecules including both chemoattractant molecules for endothelial cells and factors that stimulate their growth (25). These factors include VEGF, thymidine phosphorylase, IL-1α, TNF-α, and IL-8. Thirdly, TAMs could be important in the production of extracellular matrix-degrading enzymes (stromelysin-3, gelatinase A, collagenase-3, and metalloelastase) that facilitate tumor invasion and metastatic spread (26). Fourthly, TAMs produce factors that have been shown to deactivate various components of the immune system (27). The consequences of the relationship between tumors and their TAMs are that a tumor with a high number of TAMs generally has an increased tumor growth rate, and more local spread and distant metastasis. For the above reasons TAMs have been proposed to be a “target for cancer therapy” (28) and the ability to specifically target PS to macrophages is of great interest.
In PDT studies it has been shown that the macrophage content of different tumors correlates well with the localization of PS (29) and that macrophages can accumulate several times more PS than tumor cells (30). The therapeutic tumor response to PDT can be dramatically potentiated by treatment with a macrophage activating factor (31) or a colony-stimulating factor (32).
BSA-ce6-mal conjugates developed in our laboratory were used to demonstrate the principle that targeting TAMs with PDT can have beneficial effects on the tumors (33). Two distinct types of tumors in syngeneic Balb/c mice were employed. In tumors grown from the J774 cell line all malignant cells were macrophages, while tumors grown from EMT6 cells contained a relatively high percentage of TAMs attracted into the tumor from the host. Responses to PDT mediated by BSA-ce6-mal and by free ce6 were investigated for both tumor kinds. A very significant inhibition of tumor growth after conjugate-mediated PDT was observed in both cases Figs 3A and 3B. While the tumoricidal effect was perhaps greater for J774 tumors, the overall therapeutic outcome was greater for EMT-6 tumors. This is because J774 tumors are highly metastatic and mice die from metastases even though the primary tumor is eradicated. Free ce6 at the same dose had virtually no tumoricidal effect but gave a slight growth delay. These results show that the concept of targeting TAMs with receptor-mediated PS delivery and spatial control of light delivery has a significant tumoricidal effect and in the case of EMT-6 tumors this may be due to removal of growth and angiogenesis stimuli provided by TAMs.
Fig. 3.
In vivo tumoricidal effects of macrophage-targeted PDT compared to non-targeted free ce6. A) subcutaneous J774 tumors were grown in Balb/c mice and 14 days later injected IV with BSA-ce6-mal or free ce6 (2 mg ce6 equivalent/kg) and 24 hours later illuminated with 200 J/cm2 660 nm light. B) Subcutaneous EMT-6 tumors were grown in Balb/c mice and treated exactly as described for J774 tumors in Fig 3A.
Macrophage-targeted PDT and atherosclerosis
Atherosclerosis is no longer considered as a simple fat buildup leading to progressive narrowing of the arterial wall, but is now believed to be an active cellular process (34). Atherosclerotic plaque is a collection of different migrated, proliferated, and infiltrated cells (mainly macrophages, T-lymphocytes and smooth muscle cells) along with immune-triggering agents, such as oxidized LDL, infectious agents, heat shock proteins and other to-be-discovered factors. This process may begin in any area where the endothelial layer is injured. Growth of atherosclerotic plaques can lead to two pathological processes. First of all, progressive luminal narrowing eventually can lead to blood flow obstruction and angina. Secondly, vulnerable plaque (35) rupture induced by macrophage-derived metalloproteinases and enhanced by cyclic mechanical stress of the luminal blood stream (36) can cause occlusive thrombosis, the underlying cause for the majority of sudden cardiac death (14).
Although Paris Constantinides reported plaque rupture 40 some years ago (37), the phenomenon did not come to the focus of attention in cardiovascular medicine until the mid-1980s. Several autopsy series (14) suggested plaque rupture superimposed by occlusive thrombosis as the underlying mechanism for the majority of sudden cardiac deaths, particularly in young men. Attention turned to the angiographically non-significant lesions that are excessively inflamed and characterized by large necrotic lipid cores thin fibrous caps, dense macrophage infiltration and paucity of smooth muscle cells. Muller (35) named these plaques “vulnerable to rupture”. Shah reported (36) that the matrix metalloproteinases released from monocyte-derived macrophages could soften the plaque cap and promote them to rupture upon cyclic mechanical stress of the luminal blood stream. Atherosclerotic plaque has been proposed as suitable for PDT (38) and the presence of macrophages has been advocated as a reason for the preferential accumulation of traditional PS in plaque (39). The use of macrophage targeted PS conjugates could give much higher selectivity for early plaque (17, 40) and/or vulnerable plaque that is particularly prone to rupture causing fatal cardiac events (41).
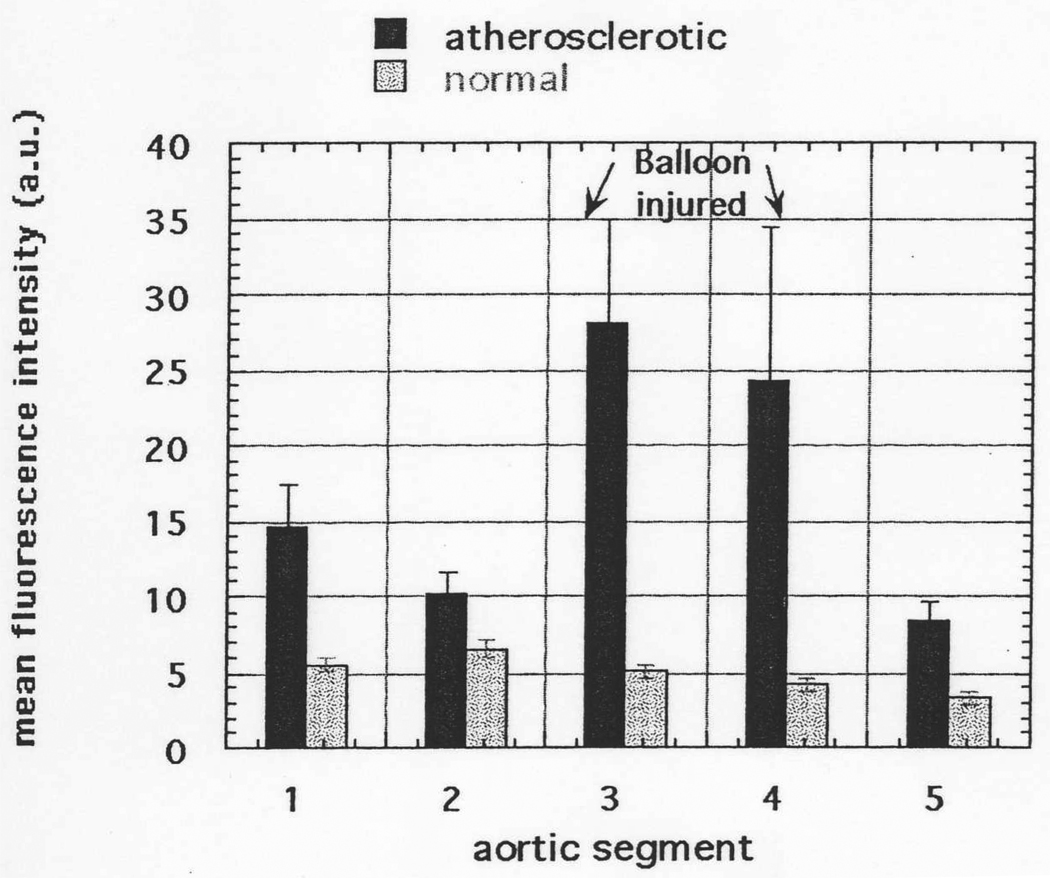
We tested this hypothesis in the Constantinides rabbit model of atherosclerosis (37). This involves a balloon injury of the aorta followed by 12 weeks of an atherogenic diet and produces highly inflamed atherosclerotic plaques in the injured regions of the aorta. The same SRA targeted conjugate described above (BSA-ce6-mal) was injected in the ear vein and 24 hours later rabbits were sacrificed and their aortas examined for fluorescence localized to the intimal surface of the arterial segments. The data presented in Fig 4 show a significant fluorescent signal from the intimal surface in all aortic sections from atherosclerotic rabbits compared to the corresponding sections of aorta from normal rabbits injected with conjugate. The fluorescence signal was particularly enhanced in the sections from the balloon-injured areas (3 and 4) that develop the most plaque after feeding with the atherogenic diet. The section numbering is l=thoracic aorta, 2=upper abdominal aorta below diaphragm, 3=mid abdominal aorta, 4=lower abdominal aorta, 5=pelvic aorta just above bifurcation. At least 6 separate measurements were taken from each artery segment. By the nature of the balloon injury sections 3 and 4 generally sustain a more severe endothelial injury than other sections and hence develop more severe atherosclerosis. The mean signal from aortic section 3 from atherosclerotic rabbits was greater than that from the corresponding sections from control conjugate injected rabbits (p<0.0005), and the signal from atherosclerotic section 4 was greater than control section 4 (p<0.005). While not exhibiting the frank plaque seen in sections 3 and 4, sections 1, 2, and 5 from atherosclerotic rabbits are nevertheless diseased tissue, and exhibit significantly increased intimal fluorescence compared to control rabbits.
Fig. 4.
Intimal fluorescence signal from different sections of aortas from atherosclerotic and normal rabbits. Values are means of 4–8 spectra obtained from sections taken from aortas from 6 atherosclerotic and 6 normal rabbits and bars are SEM.
We have carried out preliminary experiments on intravascular PDT using BSA-ce6-mal in this model. Twenty-four hours after an IV injection of 2 mg/kg of BSA-ce6-mal the rabbits were anesthetized and a 200 mm outer diameter 3-cm diffusing tip fiber was introduced into the femoral artery and advanced to the mid abdominal aorta. Two hundred J of 660-nm light per cm of diffusing tip length was delivered through flowing blood and the rabbits were sacrificed 8 days later. Examination of the aortic segments by histology and immunohistochemistry showed a significant reduction by 69% in the number of macrophages present in the illuminated sections compared to the number in non-illuminated sections (Fig 5). It remains to be seen for how long this reduction in macrophages is maintained, and if other markers of inflammation and vulnerability are also reduced.
Fig. 5.
Trichrome (left) and reverse DAPI (right) staining of aortic sections (upper is non-illuminated and lower is illuminated) taken from a rabbit injected with BSA-ce6-mal and receiving intravascular light 24 hours later.
Other possible applications of macrophage-targeted PDT
Macrophages are immune cells that play a pivotal role in the detection and elimination of pathogenic microorganisms. Macrophages possess a variety of surface receptors devoted to the recognition of non-self by discriminating between host and pathogen-derived structures. Recognition of foreign microorganisms by the macrophage ultimately results in phagocytosis. This process involves the engulfment of pathogens within phagosomes that rapidly develop into microbiocidal organelles and cause destruction of microorganisms by lysosomal enzymes, toxic reactive oxygen and nitrogen intermediates, and/or nutrient deprivational mechanisms. However numerous pathogens, such as species of Toxoplasma, Leishmania, Mycobacterium, Salmonella, Francisella, Legionella, Brucella and Yersinia pestis, parasitize macrophages (42, 43), utilizing them as a host cell for their growth, replication, and/or maintenance of their life cycles, sometimes with disastrous effects (44, 45). These infected macrophages therefore are a prime target for therapy and macrophage-targeted PDT may have a role to play especially when the infected macrophages are present in a localized granuloma.
CONCLUSION
The preparation and use of covalent conjugates between PS and macromolecular targeting vehicles has the potential to dramatically improve the selectivity and efficacy of PDT. Although in many cases the process of forming covalent conjugates between PS and macromolecules can significantly reduce the quantum yield of phototoxic species, this reduction may be compensated by increased selectivity and reduced collateral damage to non-target tissue. The use of PS conjugates as sensitizers to carry out PDT means that the actual dye molecule can be chosen on its photochemical, photophysical properties, allowing the targeting to be carried out by the macromolecule. Macrophages are an example of a cell type that is involved in many diseases and where a specific strategy to photoeradicate them may have therapeutic benefits in diseases as important as cancer, heart attack and infections.
ACKNOWLEDGMENTS
Research in the authors’ laboratory is supported by the US National Institutes of Health (R01-CA/AI838801 and R01-AI050875 to MRH). We are grateful to Florencia Anatelli for a critical reading of the manuscript.
REFERENCES
- 1.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002;20:3. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 3.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B. 1997;39:1. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 4.Ando T, Irie K, Koshimizu K, Takemura T, Nishino H, Iwashima A, Takeda N, Nakajima S, Sakata I. Photocytotoxicity of water-soluble metalloporphyrin derivatives. Photochem. Photobiol. 1993;57:629. doi: 10.1111/j.1751-1097.1993.tb02928.x. [DOI] [PubMed] [Google Scholar]
- 5.Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002;75:382. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Georgakoudi I, Nichols MG, Foster TH. The mechanism of Photofrin photobleaching and its consequences for photodynamic dosimetry. Photochem. Photobiol. 1997;65:135. doi: 10.1111/j.1751-1097.1997.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasan T. Photosensitizer delivery mediated by macromolecular carrier systems. In: Henderson BW, Dougherty TJ, editors. Photodynamic therapy: Basic principles and clinical applications. New York: Marcel Dekker; 1992. p. 187. [Google Scholar]
- 8.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89:307. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rihova B. Targeting of drugs to cell surface receptors. Crit. Rev. Biotechnol. 1997;17:149. doi: 10.3109/07388559709146611. [DOI] [PubMed] [Google Scholar]
- 10.Winston BW, Krein PM, Mowat C, Huang Y. Cytokine-induced macrophage differentiation: a tale of 2 genes. Clin. Invest. Med. 1999;22:236. [PubMed] [Google Scholar]
- 11.DiPietro L. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233. [PubMed] [Google Scholar]
- 12.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Ann. Rev. Med. 1992;43:219. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 13.Casscells W, Hathorn B, David M, Krabach T, Vaughn WK, McAllister HA, Bearman G, Willerson JT. Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet. 1996;347:1447. doi: 10.1016/s0140-6736(96)91684-0. [DOI] [PubMed] [Google Scholar]
- 14.Falk E. Why do plaques rupture? Circulation. 1992;86:III30. [PubMed] [Google Scholar]
- 15.Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab. Invest. 1994;71:5. [PubMed] [Google Scholar]
- 16.Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Meth. 1989;124:1. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin MR, Miller JL, Ortel B. Scavenger-receptor targeted photodynamic therapy. Photochem. Phothobiol. 2000;72:533. doi: 10.1562/0031-8655(2000)072<0533:srtpt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Yamada N. Modification of macrophage function and effects on atherosclerosis. Curr. Opin. Lipidol. 1996;7:320. doi: 10.1097/00041433-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol. Life Sci. 1998;54:628. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay A, Mukhopadhyay B, Basu SK. Circumvention of multidrug resistance in neoplastic cells through scavenger receptor mediated drug delivery. FEBS Lett. 1995;376:95. doi: 10.1016/0014-5793(95)01250-6. [DOI] [PubMed] [Google Scholar]
- 21.Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem. Sci. 1992;17:141. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Boyer CM, Whitaker RS, Berchuck A, Wiener JR, Weinberg JB, Bast RC., Jr Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993;53:1939. [PubMed] [Google Scholar]
- 24.O’Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993;342:148. doi: 10.1016/0140-6736(93)91348-p. [DOI] [PubMed] [Google Scholar]
- 25.Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor beta-1. Ann. Surg. Oncol. 1998;5:271. doi: 10.1007/BF02303785. [DOI] [PubMed] [Google Scholar]
- 26.Yoshino H, Endo Y, Watanabe Y, Sasaki T. Significance of plasminogen activator inhibitor 2 as a prognostic marker in primary lung cancer: association of decreased plasminogen activator inhibitor 2 with lymph node metastasis. Br. J. Cancer. 1998;78:833. doi: 10.1038/bjc.1998.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 1999;65:2. doi: 10.1002/jlb.65.1.2. [DOI] [PubMed] [Google Scholar]
- 28.Wahl LM, Kleinman HK. Tumor-associated macrophages as targets for cancer therapy. J. Natl. Cancer Inst. 1998;90:1583. doi: 10.1093/jnci/90.21.1583. [DOI] [PubMed] [Google Scholar]
- 29.Korbelik M, Krosl G. Photofrin accumulation in malignant and host cell populations of various tumours. Br. J. Cancer. 1996;73:506. doi: 10.1038/bjc.1996.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korbelik M, Krosl G. Photofrin accumulation in malignant and host cell populations of a murine fibrosarcoma. Photochem. Photobiol. 1995;62:162. doi: 10.1111/j.1751-1097.1995.tb05253.x. [DOI] [PubMed] [Google Scholar]
- 31.Korbelik M, Naraparaju VR, Yamamoto N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer. 1997;75:202. doi: 10.1038/bjc.1997.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996;56:3281. [PubMed] [Google Scholar]
- 33.Hamblin MR, O’Donnell DA, Huzaira M, Zahra T. Scavenger receptor-targeted photodynamic therapy of J774 tumors in mice: tumor response and concomitant immunity. Proc. S.P.I.E. 2002;4617:1. [Google Scholar]
- 34.Forrester JS. Prevention of plaque rupture: a new paradigm of therapy. Ann. Intern. Med. 2002;137:823. doi: 10.7326/0003-4819-137-10-200211190-00012. [DOI] [PubMed] [Google Scholar]
- 35.Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J. Am. Coll. Cardiol. 1994;23:809. doi: 10.1016/0735-1097(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 36.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565. [PubMed] [Google Scholar]
- 37.Constantinides P, Chakravati RN. Rabbit arterial thrombosis production by systemic procedures. Arch. Pathol. 1961;72:197. [PubMed] [Google Scholar]
- 38.Rockson SG, Lorenz DP, Cheong WF, Woodburn KW. Photoangioplasty: An emerging clinical cardiovascular role for photodynamic therapy. Circulation. 2000;102:591. doi: 10.1161/01.cir.102.5.591. [DOI] [PubMed] [Google Scholar]
- 39.Hamblin MR, Newman EL. On the mechanism of the tumour-localising effect in photodynamic therapy. J. Photochem. Photobiol B. 1994;23:3. doi: 10.1016/s1011-1344(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 40.Hamblin MR, Tawakol A, Castano AP, Gad F, Zahra T, Ahmadi A, Stern J, Ortel B, Chirico S, Shirazi A, Syed S, Muller JE. Macrophage-targeted photodynamic detection of vulnerable atherosclerotic plaque. Proc. SPIE. 2003;4949:466. [Google Scholar]
- 41.Plutzky J. Atherosclerotic plaque rupture: emerging insights and opportunities. Am. J. Cardiol. 1999;84:15J. doi: 10.1016/s0002-9149(99)00352-5. [DOI] [PubMed] [Google Scholar]
- 42.Amer AO, Swanson MS. A phagosome of one’s own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 2002;5:56. doi: 10.1016/s1369-5274(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 43.Stafford JL, Neumann NF, Belosevic M. Macrophage-mediated innate host defense against protozoan parasites. Crit. Rev. Microbiol. 2002;28:187. doi: 10.1080/1040-840291046731. [DOI] [PubMed] [Google Scholar]
- 44.Ismail N, Olano JP, Feng HM, Walker DH. Current status of immune mechanisms of killing of intracellular microorganisms. FEMS Microbiol. Lett. 2002;207:111. doi: 10.1111/j.1574-6968.2002.tb11038.x. [DOI] [PubMed] [Google Scholar]
- 45.Sansonetti P. Host-pathogen interactions: the seduction of molecular cross talk. Gut. 2002;50 S3:III2. doi: 10.1136/gut.50.suppl_3.iii2. [DOI] [PMC free article] [PubMed] [Google Scholar]