Abstract
Resistance of vivax malaria to treatment with antifolates, such as pyrimethamine (Pyr), is spreading as mutations in the dihydrofolatereductase (dhfr) genes are selected and disseminated. We tested the antitumor drug methotrexate (MTX), a potent competitive inhibitor of dhfr, against 11 Plasmodium vivax isolates ex vivo, 10 of which had multiple dhfr mutations associated with Pyr resistance. Despite high-grade resistance to Pyr (median 50% inhibitory concentration [IC50], 13,345 nM), these parasites were all highly susceptible to MTX (median IC50, 2.6 nM). Given its potency against Pyr-resistant P. vivax, the antimalarial potential of MTX deserves further investigation.
In the early 1970s, 7 patients were successfully and safely treated for Plasmodium vivax with low doses of methotrexate (MTX) [1]. MTX, a potent antifolate, had then long been used to treat leukemia and severe psoriasis. However, these observations were not followed further. First, they coincided with reports of MTX hepatotoxicity in persons ingesting low doses (2.5 mg/kg) over prolonged periods (3 months to 6 years) [2], making the use of MTX for malaria prophylaxis or for repeated treatment of endemic residents untenable. Second, MTX offered no obvious advantages over other antifolates such as pyrimethamine (Pyr), which were then used effectively against malaria. Forty years on, widespread use of Pyr to treat malaria (principally in combination with sulphadoxine) has led to the selection and global dissemination of antifolate resistance, not only for P. falciparum but also for Plasmodium vivax [3–4]. Resistance to Pyr in P. falciparum and P. vivax is conferred by one or more mutations in the dihydrofolatereductase gene (dhfr) [5]. Interestingly, it was reported that MTX remains active against laboratory lines of Pyr-resistant P. falciparum that carry multiple dhfr mutations [6]. We wished to determine whether Pyr-resistant P. vivax isolates were also susceptible to MTX. Secondarily, we wished to investigate the effect of multiple mutations in another important antifolate resistance gene: P. vivax dihydropteroate synthase (dhps) on P. vivax MTX sensitivity.
METHODS
Isolate Collection and Culture
Twenty P. vivax isolates were collected from May to June 2009 from malaria patients attending the Shoklo Malaria Research Unit (SMRU) clinics, Mae Sot region of Tak Province in northwestern Thailand. These samples were collected from patients with no prior antimalarial therapy and with microscopically confirmed P. vivax (parasitemia range 3,000–10,000 parasites/μL). After written consent, blood samples were collected by venipuncture in 5-ml-volume lithium heparinized tubes, which were transported to the laboratory at SMRU within 5 hours of collection. In 14 isolates, the majority (>80%) of the parasites were at the mid-trophozoite stage (∼20 h after invasion), and no schizonts were detected. These isolates were chosen for drug susceptibility testing. After platelets and leukocytes removal, the susceptibility of these P. vivax isolates to MXT (Methotrexate hydrate MW 454.4; Sigma-Aldrich) or Pyr (Pyrimethamine Vetranal MW248.7; Sigma-Aldrich) was assessed as described elsewhere [7], with one important change. Folate-reduced media based on McCoy’s 5A (Biowest) were used: folic acid 0.01mg/mL and p-amino benzoic acid 0.0005 mg/L, as opposed to the regular McCoy’s 5A medium (Gibco) of 10 mg/L and 1 mg/L, respectively. To determine the effect of reduced-folate media on P. vivax growth and sensitivity to drugs, assays on the first 6 isolates were carried out in parallel using one or the other of McCoy’s 5A media types (Figure 1). Antifolate susceptibility testing using the other 5 isolates was performed with the folic acid–reduced media.
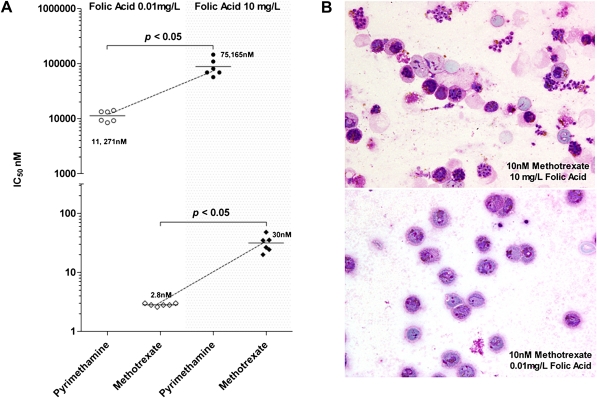
Figure 1.
A, The effect of folic acid concentration in the growth media (McCoy’s 5A), .01 mg/L (open symbols)vs. 10.0 mg/L (solid symbols), on the ex vivo susceptibility of 6 paired Thai Plasmodium vivax isolates to pyrimethamine and methotrexate (MTX). B, Photomicrographs of Giemsa-stained P. vivax thin films from a single isolate and grown in the presence of 10 nM MTX for 42h either in McCoy’s medium with normal, 10.0 mg/L (upper panel), or reduced, .01 mg/L (lower panel), concentrations of folic acid. At the higher folic acid concentration, P. vivax was protected from an otherwise inhibitory effect of MTX (lower panel), as demonstrated by observation of numerous asexual stages that developed into mature schizonts(upper panel).
Analysis
Dose response curves and 50% inhibitory concentration (IC50) values were calculated by fitting the data to a sigmoidal inhibitory E-max pharmacodynamic model using Winnonlin version 4.1 (Pharsight). Eleven of the 14 drug response curves were successfully modeled according to criteria published earlier [7]. The median IC50 values presented in Figure 1 were compared using a Wilcoxon matched-pairs test. Statistical analysis and graphics were carried out using GraphPad Prism 5 software (version 5).
Genotyping
In addition to confirmation of the parasite species by polymerase chain reaction [8], mutations present in the P. vivax dhfr and dhps genes (Pvdhfr and Pvdhps) for all the isolates were sought by amplification of the relevant fragments followed by DNA sequencing, as previously described [9].
Ethical Approval
The clinical samples examined in this study were collected under the following ethical guidelines in the approved protocols: OXTREC 027-025 (University of Oxford, Centre for Clinical Vaccinology and Tropical Medicine, United Kingdom) and MUTM 2008-215 from the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok.
RESULTS AND DISCUSSION
Of the 11 Thai P. vivax isolates, 10 were successfully genotyped for mutations in residues of Pvdhfr and Pvdhps associated with antifolate resistance (Table 1). Nine had quintuple mutations in Pvdhfr (residues F57I, S58R, T61M, S117T, D156N), and one had quadruple mutations. All carried a triple mutation in Pvdhps (residues A383G, K512M, A553G), and in 4 isolates an additional mutation S382A was detected. Ex vivo drug assays showed that all 11 isolates were highly resistant to inhibition by Pyr, with median IC50 = 13,345 nM (7036–15,287 nM [Table 1]), as predicted by the numerous mutations in Pvdhfr. By contrast, the 11 Pyr-resistant P. vivax isolated were highly susceptibility to MTX, with median IC50 = 2.6 nM(2.2–2.9 nM). Unsurprisingly, the presence of triple or quadruple mutations in Pvdhps did not affect the sensitivity of P. vivax to MTX.
Table 1.
The ex vivo sensitivity of 11 Thai Plasmodium vivax isolates to methotrexate (MTX) and pyrimethamine (Pyr) relative to mutations in dhps and dhfr
|
Plasmodium vivax gene locus |
Antimalarial |
||||||||||||
|
Pvdhpsb |
Pvdhfrb |
Average IC50nM |
|||||||||||
| Isolate | Speciesa | S382A | A383G | K512M | A553G | V585 | F57I | S58R | T61M | S117T | D156N | Pyr | MTX |
| WPP 5759 | Pv | A | G | M | G | V | I | R | M | T | N | 7,036 | 2.4 |
| WPP 3839 | Pv | A | G | K | G | V | I | R | M | T | N | 15,287 | 2.8 |
| WPP 3443 | Pv | S+A | G | K | G | V | I | R | M | T | N | 13,996 | 2.6 |
| WPP 6225 | Pv* | S+A | G | K | G | V | I | R | M | T | N | 14,837 | 2.6 |
| WPP 3597 | Pv | S | G | K | G | V | I | R | T | T | N | 10,691 | 2.2 |
| WPP 4206 | Pv | S | G | K | G | V | I | R | M | T | N | 12,412 | 2.6 |
| WPP 4767 | Pv | S | G | K | G | V | I | R | M | T | N | 12,259 | 2.9 |
| WPP 3649 | Pv | S | G | K | G | V | I | R | M | T | N | 13,345 | 2.2 |
| WPP 6100 | Pv | S | G | K | G | V | I | R | M | T | N | 14,085 | 2.6 |
| WPP 6385 | Pv* | S | G | K | G | V | I | R | M | T | N | 13,220 | 2.2 |
| WPP 3559 | Pv | S | G | K | G | V | ND | ND | ND | ND | ND | 15,287 | 2.6 |
NOTE. The mutant amino acid at each of the residues is boldface and italicized. ND = Not determined.
The presence of P. vivax (Pv) was confirmed by polymerase chain reaction; in 2 cases submicroscopic P. falciparum (*) was noted.
The position of the mutated residue is indicated, and the amino acid resulting from mutation is presented in boldface and italics.
The ex vivo assays used in this study were conducted using media with physiological folic acid levels (folic acid .01mg/ml). This contrasts with many studies where folate-enriched media (folic acid 10 mg/ml) were used, because it was thought that high levels of folic acid were needed to obtain the levels of P. vivax ex vivo maturation, which is necessary for meaningful dose response modeling [7]. In this study we used 6 of the isolates to establish that P. vivax maturation occurs efficiently in folate-depleted media and that under these conditions the IC50’s were 5- to 10-fold lower (Figure 1) than those obtained in parallel using folate-enriched media (1,000-fold more folate). These observations underline the importance of using folic acid–reduced media to conduct antifolate sensitivity testing.
Whereas it is clear that high doses of MTX (130–300 mg kg-1) taken over several weeks (with serum concentrations often >1,000 μM) to treat neoplastic disorders are associated with life-threatening adverse events [10], the lower doses of methotrexate (0.1–0.4 mg kg-1 in children and 7.5–30 mg kg-1 per adult) given once weekly for the management of rheumatoid arthritis, psoriasis, and juvenile idiopathic arthritis (including infants <one year old) have limited side effects [11–13]. Pharmacokinetic studies indicate that a daily dose of 5 mg in adults (0.05 mg kg-1) results in serum MTX concentrations between 250 and 500 nM [13]. These levels are sufficient to fully inhibit P. vivax (this study) and P. falciparum [14]. Thus, we propose that at low doses MTX or novel drugs with similar structures may have utility for the short-term treatment of malaria in children and in adults. Studies to explore the potential of MTX as an anti–P. falciparum agent (clinicaltrial.gov, NCT00791531) are underway, and it is hoped that similar investigations will eventually be conducted against P. vivax.
It should be noted that MTX-resistant P. falciparum could be relatively easily selected in culture [6], though the molecular basis of this resistance is yet to be determined. Therefore, it is important to ensure that any deployment of MTX for malaria treatment is done in the context of combination therapies. The P. vivax isolates that we collected in Thailand were more sensitive to MTX than to artesunate [15], a drug increasingly used in combination with piperaquine to treat chloroquine-resistant vivax malaria as second-line treatment. Further studies are needed to establish whether the pharmacokinetic properties of MTX make it an adequate partner to artemisinins. It would also be of interest to examine the potential synergy in a combination of methotrexate with a dhfr inhibitor (such as WR99210), which might be expected to reduce even further the in vivo concentration of methotrexate required. We suggest that in vivo studies are warranted to explore the potential of low doses of MTX in the treatment of P. vivax.
Funding
This work was supported by the Agency for Science Technology and Research (A*STAR, Singapore), Novartis Institute for Tropical Diseases (Singapore), and the Wellcome Trust (United Kingdom).
Acknowledgments
We thank all of the patients and staff of SMRU for their contribution to this study. SMRU is sponsored by the Wellcome Trust of Great Britain, as part of the Oxford Tropical Medicine Research Programme of Wellcome Trust–Mahidol University. MI is a Wellcome Trust intermediate fellow (Grant 080867/Z/06/Z) and is supported by the Thailand Research Fund and Commission on Higher Education. AN is supported by a Pfizer-Royal Society Award, United Kingdom; the EU Commission under Framework 6 as part of the AntiMal Integrated Project 018834; and the European and Developing Countries Clinical Trials Partnership. LR and GS are currently part of an official collaboration between the Singapore Immunology Network/Agency for Science Technology and Research and Institut National de la Sante et de la Recherche Medicale. Thanks go to Dr. Thierry Diagana (NITD) for his valuable scientific insight and support of this project.
MI, BR, RS, AN, FN, and LR conceived and designed the experiments/study. MI, BR, RS, KS, and SK performed the experiments. MI, BR, RS, MLL, GS, FN, and LR analyzed the data. MI, BR, AN, MLL, APP, GS, FN, and LR contributed reagents/materials/analysis tools. AN, APP, and FN provided clinical support/information and ethical oversight. MI, BR, RS, AN, MLL, KS, SK, APP, GS, FN, and LR wrote the manuscript.
References
- 1.Sheehy TW, Dempsey H. Methotrexate therapy for Plasmodium vivax malaria. JAMA. 1970;214:109–14. [PubMed] [Google Scholar]
- 2.Dahl MG, Gregory MM, Scheuer PJ. Methotrexate hepatotoxicity in psoriasis—comparison of different dose regimens. Br Med J. 1972;1:654–6. doi: 10.1136/bmj.1.5801.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imwong M, Pukrittakayamee S, Looareesuwan S, et al. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob Agents Chemother. 2001;45:3122–7. doi: 10.1128/AAC.45.11.3122-3127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rungsihirunrat K, Na-Bangchang K, Hawkins VN, Mungthin M, Sibley CH. Sensitivity to antifolates and genetic analysis of Plasmodium vivax isolates from Thailand. Am J Trop Med Hyg. 2007;76:1057–65. [PubMed] [Google Scholar]
- 5.McCutchan TF, Welsh JA, Dame JB, et al. Mechanism of pyrimethamine resistance in recent isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1984;26:656–9. doi: 10.1128/aac.26.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter RD, Bergmann B, Kansy M, Wiese M, Seydel JK. Pyrimethamine-resistant Plasmodium falciparum lack cross-resistance to methotrexate and 2,4-diamino-5-(substituted benzyl) pyrimidines. Parasitol Res. 1991;77:346–50. doi: 10.1007/BF00930913. [DOI] [PubMed] [Google Scholar]
- 7.Russell B, Chalfein F, Prasetyorini B, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52:1040–5. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol Med. 2002;72:189–203. doi: 10.1385/1-59259-271-6:189. [DOI] [PubMed] [Google Scholar]
- 9.Imwong M, Pukrittayakamee S, Cheng Q, et al. Limited polymorphism in the dihydropteroatesynthetase gene (dhps) of Plasmodium vivax isolates from Thailand. Antimicrob Agents Chemother. 2005;49:4393–5. doi: 10.1128/AAC.49.10.4393-4395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabner BA, Amrein P, Drucker B, et al. Antineoplastic agents. In: Brunton L, editor. The pharmacological basis of therapeutics. New York: McGraw-Hill; 2006. pp. 1315–465. [Google Scholar]
- 11.Swierkot J, Szechinski J. Methotrexate in rheumatoid arthritis. Pharmacol Rep. 2006;58:473–92. [PubMed] [Google Scholar]
- 12.Niehues T, Lankisch P. Recommendations for the use of methotrexate in juvenile idiopathic arthritis. Paediatr Drugs. 2006;8:347–56. doi: 10.2165/00148581-200608060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Chladek J, Grim J, Martinkova J, Simkova M, Vaneckova J. Low-dose methotrexate pharmacokinetics and pharmacodynamics in the therapy of severe psoriasis. Basic Clin Pharmacol Toxicol. 2005;96:247–8. doi: 10.1111/j.1742-7843.2005.pto960318.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiara SM, Okombo J, Masseno V, et al. In vitro activity of antifolatepolymorphism in dihydrofolatereductase of Plasmodium falciparum isolates from the Kenyan coast: emergence of parasites with Ile-164-Leu mutation. Antimicrob Agents Chemother. 2009;53:3793–8. doi: 10.1128/AAC.00308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwanarusk R, Chavchich M, Russell B, et al. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J Infect Dis. 2008;198:1558–64. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]