Abstract
Background. Infections with extended-spectrum β-lactamase–producing Escherichia coli (ESBL-EC) have developed resistance to current therapies. Therefore, the underlying mechanisms of in vivo and in vitro activity of C-terminal–amidated thanatin (A-thanatin) against clinical isolates of ESBL-EC were studied in an attempt to resolve this problem.
Methods. A-thanatin was synthesized to determine its minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and kill curve for ESBL-EC. The hemolytic toxicity, stability, and resistance induction of A-thanatin were determined. ESBL-EC–infected mice were used to determine the in vivo activity of A-thanatin. Scanning and transmission electron microscopy and fluorescence microscopy were used to study the underlying mechanism of A-thanatin.
Results. A-thanatin is highly effective against ESBL-EC in vitro, with MIC values ≤4 μg/mL. It has been confirmed that A-thanatin has little hemolysis and relative high stability in plasma. Excellent in vivo therapeutic effects were also observed in a septicemic animal model, with survival rates of 50.0%, 66.7%, and 91.7% in the low-dose, middle-dose, and high-dose groups, respectively. Membrane permeabilization may be a major biological action of A-thanatin.
Conclusions. Because the development of multidrug resistance limits the available therapeutic options, A-thanatin may provide a novel strategy for treating ESBL-EC infection and other infections due to multidrug-resistant bacteria.
Strains of bacteria that produce extended-spectrum β-lactamase (ESBL) have become a particular problem in recent decades, and the number of nosocomial and community-acquired infections caused by multidrug-resistant (MDR) Escherichia coli (MDR-EC) has increased worldwide [1, 2].
Recent epidemiological statistics indicate that the prevalence of bacterial isolates expressing the ESBL phenotype varies with the geographical region. The isolation rates are much higher in Portugal, Italy, Turkey, the United States, and Latin American countries (34%–60%) than they are in Sweden, Japan, and Singapore (3%–8%) [3]. Almost all ESBL producers are resistant to penicillins and cephalosporins, and many are even resistant to antibiotics that belong to other classes, such as ciprofloxacin (91.3%), trimethoprim (84.8%), gentamicin (30.4%), and amikacin (4.3%) [4]. Clinical studies involving patients with infection due to ESBL-producing E. coli (ESBL-EC), which is one of the most common ESBL-producing isolates, and bacteremia have demonstrated a mortality rate of 60.8%, which is significantly higher than the mortality rate for non–ESBL-producing E. coli (23.7%); this means that ESBL-associated infections have become resistant to current therapies [4].
Cationic antimicrobial peptides have recently received extensive attention because of their great potential for use in an entirely new approach to infection therapy [5, 6]. Thanatin (GSKKPVPIIYCNRRTGKCQRM, meaning an intramolecular disulfide bridge between the 2 cysteines) is a peptide isolated from the hemipteran insect Podisus maculiventris. Thanatin exhibits broad-spectrum activity, with rapid and strong bactericidal effects against gram-negative bacteria, gram-positive bacteria, and fungi and with superior effects against ESBL-EC and ESBL-producing Klebsiella pneumonia (ESBL-KP) [7], which are highly resistant to many traditional antibiotics. Because the hemolytic activities of naturally occurring antimicrobial peptides, such as melittin, severely limit their extensive application, thanatin exhibits little hemolysis [7], making it a potential agent with broad prospects for therapeutic exploitation.
Previous studies have confirmed that thanatin possesses lipopolysaccharide (LPS) binding and neutralizing activity in vitro, but little work has been performed to demonstrate the in vivo effectiveness of thanatin to reduce plasma endotoxin levels in animal models [8]. The bactericidal mechanisms of thanatin still remain controversial, and whether the biological activity of thanatin depends on membrane permeabilization is ambiguous [9, 10]. A previous study has found that thanatin is not a pore-forming peptide and does not target the cytoplasmic membrane [7]. However, antimicrobial peptides are generally initiated by disrupting the integrity of the cell membrane through interaction with the phospholipid component [11, 12], and the underlying mechanisms of thanatin need to be further studied.
The present study was undertaken to investigate the underlying mechanisms of thanatin's activity against clinical isolates of ESBL-EC. We synthesized C-terminal–amidated thanatin (A-thanatin), which has greater proteinase resistance than does native thanatin [7], and observed its activity on clinical isolates of ESBL-EC in vitro. Furthermore, BALB/c mice infected with ESBL-EC were used to evaluate the reliability and treatment effectiveness of A-thanatin in vivo. Moreover, morphologic methods and fluorescent techniques were used in this study to explore the mechanism of action of A-thanatin.
MATERIALS AND METHODS
Chemicals
All antibiotics used were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals and solvents were of analytical grade.
Organisms
The MDR clinical isolates methicillin-resistant Staphylococcus aureus (XJ75302), methicillin-resistant Staphylococcus epidermidis (XJ75284), ESBL-KP (XJ75297), MDR E. coli (MDR-EC) (XJ74283) and MDR-Pseudomonas aeruginosa (XJ75315) were obtained from the clinical laboratory of Xijing Hospital (Xi'an, China). ESBL-EC (HN10318), ESBL-EC (HN10322), ESBL-KP (HN10349), and ESBL-KP (HN10500) were obtained from the clinical laboratory of Henan Provincial People's Hospital (Zhengzhou, China). ESBL-EC (SX49657) and ESBL-EC (SX49660) were collected from the clinical laboratory of Shaanxi Provincial People's Hospital (Xi'an, China). ESBL-EC (JT11074) and MDR-EC (JT11092) were collected from the clinical laboratory of the Second Affiliated Hospital of Medical College of Xi'an Jiaotong University (Xi'an, China). E. coli (ATCC 25922), Staphylococcus aureus (ATCC 29213), and ESBL-EC (ATCC 35218) were used as reference from the Chinese National Center for Surveillance of Antimicrobial Resistance.
Peptide Synthesis
Thanatin (GSKKPVPIIYCNRRTGKCQRM), melittin (GIGAVLKVLTTGLPALISWIKRKRQQ), and K4-S4(1–16) [13, 14] (ALWKTLL KKVLKAAAK) were synthesized by the solid-phase method applying Fmoc (9-fluorenylmethyloxycarbonyl) active ester chemistry. K4-S4(1–16) is one of the short analogs of dermaseptin S4 (ALWMTLLKKVLKAAAKAALNAVLVGANA), with substitution of the methionine for lysine residue at position 4. The C-terminuses of the 3 peptides above were amidated and termed as A-thanatin, A-melittin, and K4-S4(1–16)a, respectively. The crude compounds were purified to >95% chromatographic homogeneity by reverse-phase high-performance liquid chromatography (HPLC), and the purified compounds were identified by mass spectrometer (MS). HPLC runs were performed on a C18 column with a linear gradient of acetonitrile in water (1% per min); both solvents contained 0.1% trifluoroacetic acid. The purified peptide in reduced form was taken up in oxidation buffer (1 mg per 10 mL) [7] and was purified by semipreparative reverse-phase HPLC. The purified compounds were confirmed by MS analysis.
Bacterial Susceptibility Testing and Growth Assay
The minimum inhibitory concentration (MIC) values were determined by a microdilution assay in sterilized 96-well polypropylene microtiter plates according to the broth microdilution guideline of Clinical and Laboratory Standards Institute (CLSI) [15].
The minimum bactericidal concentration (MBC) values were determined by plating 0.01-mL samples from the wells with no visible turbidity onto Mueller-Hinton agar plates. The least concentration showing no visible growth on agar subculture was taken as the MBC value. All experiments were performed independently 3 times, each time using duplicate samples.
Compounds were added to cell cultures containing ESBL-EC to a final concentration of 4, 12, or 24 μg/mL A-thanatin or 24 μg/mL ampicillin, with the addition of an equal volume of diluent (0.2% bovine serum albumin and 0.01% acetic acid) as a control. Aliquots of each culture were collected after 1 h, diluted, and inoculated on solid agar, and the plates were incubated for 24 h at 37°C.
To determine the kill curve for ESBL-EC, A-thanatin and ampicillin were added to ESBL-EC cultures to a final concentration of 24 μg/mL with the addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h and were diluted and inoculated on solid agar. The number of colony-forming units (CFU) was calculated from the number of colonies growing on the plates. Statistical data for each experiment were obtained from at least 2 independent assays performed in duplicate.
Hemolysis Assay
Hemolysis was evaluated by determining the hemoglobin released in 2% and 10% suspensions of fresh human erythrocytes [16, 17]. Suspensions of human red blood cells (hRBC) (100 μL) were added to a 96-well microtiter plate and incubated individually with A-thanatin or A-melittin at 8, 16, 32, 64, or 128 μg/mL for 1 h at 37°C. Hemolysis values of 0% and 100% were determined with a spectrophotometer in phosphate-buffered saline solution and 1% Triton X-100, respectively.
Stability in 50% Plasma
A-thanatin or K4-S4(1–16)a [18] were dissolved in dilution at the initial concentration of 64 times the MIC value, then diluted 2-fold in fresh human plasma and preincubated at 37°C for 0, 3, and 6 h. After incubation, each sample was subjected to serial 2-fold dilutions in Mueller-Hinton broth in 96-well plates and the MIC was determined for ESBL-EC as described for the antibacterial assay. Statistical data were obtained from at least 2 independent experiments performed in duplicate.
Induction of Resistance
After MIC values were determined for ESBL-EC as described above, MIC values were determined anew daily as follows [19]: for each compound tested, bacteria suspension was obtained from the one-half MIC well of the previous MIC assay, the concentration of which was adjusted to 0.5 Mcfarland Standard in Mueller-Hinton broth. Then, the suspension was diluted by the proportion of 1:100 to the concentration of 5 × 105 cells/mL in Mueller-Hinton broth. MIC was determined again as described for the initial MIC assay. The procedures described above were repeated for 15 days. The relative MIC value was calculated from the ratio of the MIC obtained for the fifteenth subculture to that obtained for the first-time exposure.
Therapeutic Effects of A-thanatin on Infected Animals
Male and female BALB/c mice 8–10 weeks of age and weighing 18–22 g were used in this study. The experimental and animal care procedures were approved by the animal care and use committee of Fourth Military Medical University. Infection was induced by the intraperitoneal administration of a mean (± standard deviation [SD]) of 3.4 ± 0.7 × 105 CFU ESBL-EC inoculum in 0.4 mL of Mueller-Hinton broth (with 5% mucin). After bacterial challenge, the mice were randomly administered a 20-mg/kg dose of ampicillin by intraperitoneal injection or 2.5, 5, or 10 mg/kg of A-thanatin at 1, 6, 20, and 28 h after infection. To assess bacterial clearance, 4 mice in each group were sacrificed, and bacterial counts were determined in the liver, spleen, lung, and kidney; these tissues were harvested aseptically from each animal, weighed, and homogenized in a sterile saline solution. Colony counts in the 4 tissues were expressed as CFU/g of tissue. The survival of 12 mice in each group was monitored every 6 h for 7 days after infection, and the cumulative percentage survival was determined.
Parts of the liver, spleen, lung, and kidney were harvested, washed with sterile saline, fixed in 10% neutral buffered formalin for 24 h, and then their morphologies were observed using hematoxylin and eosin staining.
Preparation of ESBL-EC Samples for Scanning and Transmission Electron Microscopy
Bacteria (1 × 108 CFU/mL) were cultured with A-thanatin (24 μg/mL) at 120 rpm for 30, 60, and 90 min, then harvested and washed. Some specimens were observed in a scanning electron microscope (Hitachi S-3400N) or a transmission electron microscope (JEM-2000EX; JOEL), and images were recorded.
Bacterial Membrane Permeabilization and Aggregation Assays
ESBL-EC (5 × 107 CFU/mL) was cultured with 4, 12, or 24 μg/mL A-thanatin or 24 μg/mL ampicillin at 120 rpm at 37°C for 90 min. Bacteria were harvested, washed, stained using SYTO 9 and propidium iodide (BacLight Bacterial Viability kit; Molecular Probes), and then examined by fluorescence microscopy.
Statistical Analysis
Results are expressed as mean values (±SD). Student's t test and analysis of variance were used to evaluate statistical significance, as appropriate. A probability value of P < .05 was considered indicative of statistical significance.
RESULTS
Bacterial Susceptibility Testing and Growth Assay
A-thanatin exerted potent bactericidal effects against almost all gram-negative bacteria tested, including clinically prevalent and challenging species, such as Escherichia and Klebsiella, with most MIC values ranging from 2 μg/mL through 4 μg/mL (Table 1). In contrast, A-thanatin exerted weak effects against gram-positive strains, with MIC values of at least 256 μg/mL for S. aureus (ATCC 29213) and methicillin-resistant S. aureus. The MIC values for oxacillin, ampicillin, ceftazidime, levofloxacin, and ciprofloxacin against 2 sensitive strains were all low (0.125–8 μg/mL) but were higher against resistant strains to varying degrees. Imipenem showed good antibacterial activity against all strains (<2 μg/mL). The MBC values of A-thanatin against E. coli, K. pneumonia, and P. aeruginosa were each double the MIC values (Table 1).
Table 1.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of C-terminal–Amidated Thanatin (A-thanatin) in Mueller-Hinton Broth Culture.
| Strain | MIC, μg/mL) |
MBC, fold increase | ||||||
| A-THA | OXA | AMP | CAZ | LVX | CIP | IPM | THA | |
| Staphylococcus aureus ATCC29213 | 256 | ≤.25 (S) | 1 (S) | 8 (S) | ≤.25(S) | ≤.25(S) | ≤.125 (S) | ND |
| MRSA XJ75302 | >256 | >256 (R) | 256 (R) | 256 (R) | 8 (R) | 16 (R) | 2 (S) | ND |
| MRSE XJ75284 | 16 | >256 (R) | 256 (R) | 256 (R) | 8 (R) | 8 (R) | 0.5 (S) | 2 |
| Escherichia coli ATCC25922 | 16 | >256 | 8 (S) | ≤.25 (S) | ≤.25 (S) | ≤.25 (S) | ≤.25 (S) | 2 |
| ESBL-EC ATCC35218 | 4 | >256 | >256 (R) | ≤.25 (S) | ≤.25 (S) | ≤.25 (S) | ≤.25 (S) | 2 |
| MDR-EC XJ74283 | 8 | >256 | >256 (R) | 8 (R) | 4 (I) | 8 (R) | ≤.25 (S) | 2 |
| JT11092 | 2 | >256 | 0.5 (S) | ≤.25 (S) | 8 (R) | 64 (R) | ≤.25 (S) | 2 |
| ESBL-EC HN10318 | 2 | >256 | 32 (R) | 64 (R) | 16 (R) | 32 (R) | ≤.25 (S) | 2 |
| HN10322 | 2 | >256 | 32 (R) | 64 (R) | 16 (R) | 32 (R) | ≤.25 (S) | 2 |
| SX49657 | 4 | >256 | 64 (R) | 32 (R) | 4 (I) | 4 (R) | ≤.25 (S) | 2 |
| SX49660 | 4 | >256 | 16 (I) | 16 (I) | 4 (I) | 8 (R) | ≤.25 (S) | 2 |
| JT11074 | 2 | >256 | 32 (R) | 128 (I) | 8 (R) | 8 (R) | ≤.25 (S) | 2 |
| ESBL-KP XJ75297 | 4 | >256 | >256 (R) | >256 (R) | 2 (S) | 2 (I) | 2 (S) | 2 |
| HN10349 | 2 | >256 | >256 (R) | >256 (R) | 1 (S) | 0.5 (S) | 2 (S) | 2 |
| HN10500 | 2 | >256 | >256 (R) | 256 (R) | 32 (R) | 64 (R) | 0.25 (S) | 2 |
| MDR-PA XJ75315 | 16 | >256 | >256 (R) | 64 (R) | 1 (S) | ≤.25 (S) | ≤.25 (S) | 2 |
NOTE. A-THA, A-thanatin; AMP, ampicillin; CAZ, ceftazidime; CIP, ciprofloxacin; EC, E. coli; ESBL, extended-spectrum β-lactamase; I, intermediate; IPM, imipenem; KP, Klebsiella pneumoniae; MRSA, methicillin-resistant S. aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; THA, thanatin; LVX, levofloxacin; ND, test not done; OXA, oxacillin; R, resistant; S, susceptible.
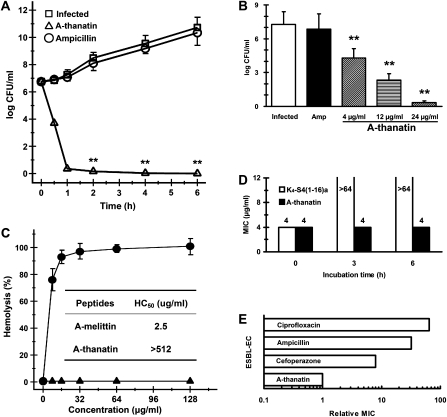
Studies of the kill curves showed that A-thanatin reduced the bacterial population from 106 to <50 CFU/mL within 1 h. Compared with the control group, ampicillin exerted no bactericidal effects against ESBL-EC at the same concentration (Figure 1A). A-thanatin at 4 and 12 μg/mL reduced the CFU of ESBL-EC by 103- and 105-fold, respectively (Figure 1B). The growth of ESBL-EC was close to zero (ie, <50 CFU/mL) in the group treated with 24 μg/mL A-thanatin, but it was not influenced by treatment with 24 μg/mL ampicillin.
Figure 1.
In vitro properties of C-terminal–amidated thanatin (A-thanatin). A, Kill curves of antimicrobial agents at 6 times the minimum inhibitory concentration (MIC). A-thanatin and ampicillin were added to cell cultures containing extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC) to a final concentration of 24 μg/mL, with addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h and were then diluted and inoculated on solid agar. The number of colony-forming units (CFUs) was calculated from the number of colonies growing on plates. *P < .05, **P < .01 versus control. B, Effects of antimicrobial peptides on the growth of bacteria colonies. Compounds were added to cell cultures containing ESBL-EC to a final concentration of 4, 12, or 24 μg/mL A-thanatin or 24 μg/mL ampicillin, with the addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 1 h, diluted, and inoculated on solid agar. The number of CFU was calculated from the number of colonies growing on plates. The data were shown as mean values ± standard deviations of 8 samples. **P < .01 versus control. C, Hemolysis of A-thanatin ( ) and A-melittin (
) and A-melittin ( ) determined on human red blood cells (2% hematocrit) after 1 h of incubation. Inset, median hemolytic concentration of A-thanatin and A-melittin after 3 h of incubation with 10% hematocrit. D, Antibacterial activity before and after preincubation of A-thanatin (black) in 50% human plasma. E, Emergence of resistance in ESBL-EC after 15 serial passages in the presence of antimicrobials. Relative MIC is the normalized ratio of MIC obtained for the fifteenth subculture to MIC obtained upon first exposure.
) determined on human red blood cells (2% hematocrit) after 1 h of incubation. Inset, median hemolytic concentration of A-thanatin and A-melittin after 3 h of incubation with 10% hematocrit. D, Antibacterial activity before and after preincubation of A-thanatin (black) in 50% human plasma. E, Emergence of resistance in ESBL-EC after 15 serial passages in the presence of antimicrobials. Relative MIC is the normalized ratio of MIC obtained for the fifteenth subculture to MIC obtained upon first exposure.
Hemolysis Assay
Incubation of 2% hRBC suspensions with A-thanatin did not yield the characteristic dose-response profile observed with A-melittin (Figure 1C). Similar behavior was observed in experiments using 10% hRBC suspensions (Figure 1C). A-thanatin at up to 512 μg/mL displayed very low hemolytic activity, which was 100 times higher than the MIC value for ESBL-EC. Moreover, the median hemolytic concentration (HC50) of A-thanatin was much higher than that of the positive control antimicrobial peptide A-melittin.
Stability in Plasma
Exposure to human plasma markedly inhibited the activity of K4-S4(1–16)a, but the MIC of A-thanatin remained unchanged after preincubation for 3 or 6 h in plasma (Figure 1D).
Induction of Resistance
After 15 successive subcultures, the relative MIC values of A-thanatin remained unchanged, indicating no induction of antibiotic resistance. However, MIC values of classical antibiotics, including cefoperazone, ampicillin, and ciprofloxacin, increased by 8- to 64-fold, reflecting the emergence of resistant strains (Figure 1E).
In vivo Antibacterial Activity
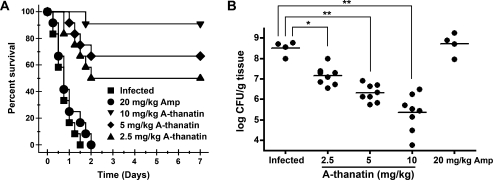
All infected mice in the infected group or in the 20 mg/kg ampicillin–treated group died within 2 days. The administration of each dose of A-thanatin (2.5, 5, and 10 mg/kg) significantly improved the animal survival rate (from 0% for the control group to 50.0%, 66.7%, and 91.7% for A-thanatin-treated groups) in a dose-dependent manner (Figure 2A). Survival was associated with reductions in the bacterial titers in the tissues of mice inoculated with ESBL-EC strains, from 3.2 × 108 CFU/mL to 1.4 × 107, 2.0 × 106, and 2.2 × 105 CFU/mL, respectively (Figure 2B). Treatment with ampicillin alone did not show any protective effects, either by decreasing the mortality rate of ESBL-EC–infected mice or altering the CFU counts in tissue samples from the septic mice.
Figure 2.
In vivo activities of C-terminal–amidated thanatin (A-thanatin). A, Survival of BALB/c mice (n = 12 mice per group) inoculated by intraperitoneal injection with extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC) (3.4 × 105 colony-forming units [CFUs]) and treated with 3 doses of A-thanatin at 2.5 mg/kg ( ), 5 mg/kg (□), and 10 mg/kg (
), 5 mg/kg (□), and 10 mg/kg ( ); a single dose of ampicillin at 20 mg/kg (
); a single dose of ampicillin at 20 mg/kg ( ); or diluent (
); or diluent ( , infected group) by intraperitoneal injection at 1, 6, 20, and 28 h after infection. B, Colonization of ESBL-EC inoculum in the liver, spleen, lung, and kidney cultures of A-thanatin– and Ampicillin-treated BALB/c mice (CFUs/gram of tissue). *P < .05, **P < .01 versus infected group.
, infected group) by intraperitoneal injection at 1, 6, 20, and 28 h after infection. B, Colonization of ESBL-EC inoculum in the liver, spleen, lung, and kidney cultures of A-thanatin– and Ampicillin-treated BALB/c mice (CFUs/gram of tissue). *P < .05, **P < .01 versus infected group.
Morphology of Lung, Liver, Spleen, and Kidney Tissue Sample
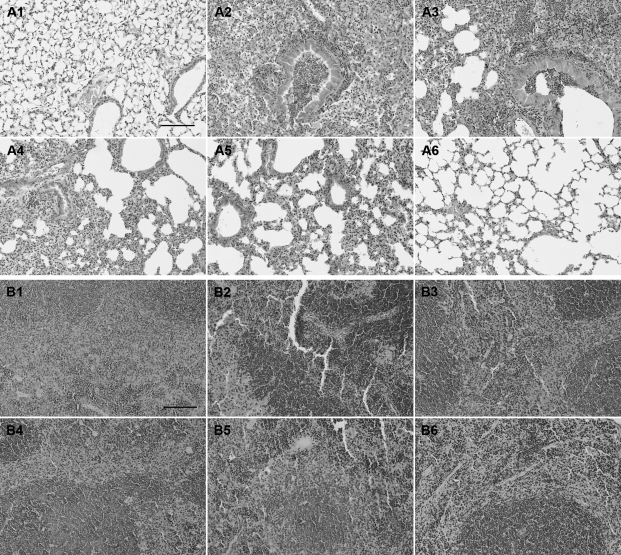
The morphological structure of lung tissue sample stained with hematoxylin and eosin was very similar in the infected and the ampicillin-treated groups (Figure 3A). In these groups, the normal alveolar structure disappeared, with necrosis and liquefaction being observed and a large number of neutrophils infiltrating and accumulating. Alveolar fusion, alveolar septum thickening, and neutrophil infiltration were gradually reduced in the groups treated with low, medium, and high doses of A-thanatin (Figure 3A). Hematoxylin and eosin staining of BALB/c mouse spleen tissue revealed obvious congestion in the spleen red pulp area in both the infected and the ampicillin-treated groups (Figure 3B); red pulp hyperemia gradually reduced in the low-, medium-, and high-dose A-thanatin-treated groups (Figure 3B).
Figure 3.
A, Morphology of lungs was examined with hematoxylin and eosin staining in BALB/c mice (original magnification, ×200). A1, Control; A2, lung infected with extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC); A3, lung infected with ESBL-EC and treated with 20 mg/kg ampcillin; A4, lung infected with ESBL-EC and treated with 2.5 mg/kg C-terminal–amidated thanatin (A-thanatin); A5, lung infected with ESBL-EC and treated with 5 mg/kg A-thanatin; A6, lung infected with ESBL-EC and treated with 10 mg/kg A-thanatin. B, Morphology of spleens was examined with hematoxylin and eosin staining in BALB/c mice (original magnification, ×200). B1, Control; B2, spleen infected with ESBL-EC; B3, spleen infected with ESBL-EC and treated with 20 mg/kg ampcillin; B4, spleen infected with ESBL-EC and treated with 2.5 mg/kg A-thanatin; B5, spleen infected with ESBL-EC and treated with 5 mg/kg A-thanatin; B6, spleen infected with ESBL-EC and treated with 10 mg/kg A-thanatin. Scale bars represent 500 μm.
There were large local necrotic liver abscesses in the control and ampicillin-treated groups. Only smaller abscesses were observed in the low- and medium-dose A-thanatin–treated groups, and no abscess was identified in the high-dose A-thanatin–treated group. No pathological changes were observed in the kidneys of infected animals.
Bacterial Membrane Permeabilization and Aggregation Assay
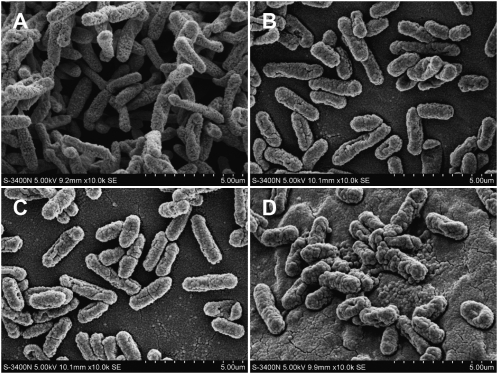
Scanning electron microscopy (SEM) indicated the rod-shaped form and relatively uniform length of ESBL-EC, with many uneven microvilli on the surface (Figure 4A). During A-thanatin treatment, the images showed dramatic morphologic changes in the ESBL-EC. The bacteria became swollen and translucent, the number of surface microvilli decreased, bacteria become shorter, and cracks or holes formed on their surfaces (Figure 4B–D).
Figure 4.
The morphology of extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC) was investigated by scanning electron microscopy at 0, 30, 60, and 90 min after treatment with 6 times the minimum inhibitory concentration of C-terminal–amidated thanatin (A-thanatin). A, Control; B, ESBL-EC treated with A-thanatin for 30 min; C, ESBL-EC treated with A-thanatin for 60 min; D, ESBL-EC treated with A-thanatin for 90 min.
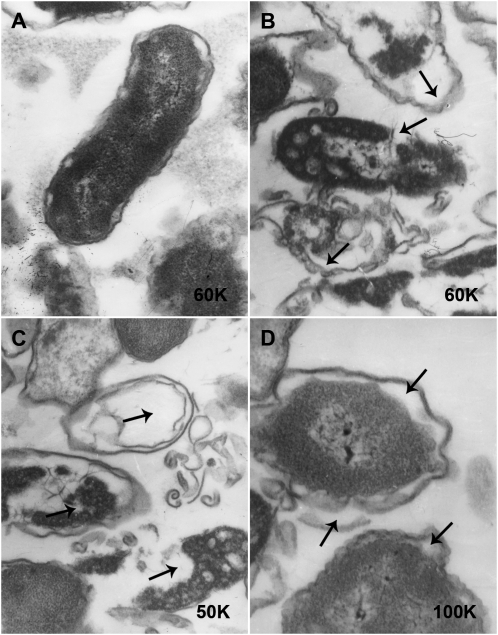
Transmission electron microscopy (TEM) demonstrated that the cell wall was seriously damaged, and flagella disappeared in the group treated with A-thanatin for 90 min (Figure 5B–D). The membrane integrity was seriously damaged, leading to the leakage of intracellular content (Figure 5B). The residual cell walls of E. coli displayed a bacterial “ghost” which appeared as a cavity indicating the absence of cytoplasmic contents and chromatin silk shift (Figure 5C). Edema became widespread, with noticeable gaps between the cell membrane and cell cytoplasm (Figure 5D).
Figure 5.
The morphology of extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC) was investigated by transmission electron microscopy after treatment with 6 times the minimum inhibitory concentration of C-terminal–amidated thanatin (A-thanatin) for 90 min. A, Control; B, C, and D, ESBL-EC treated with A-thanatin for 90 min.
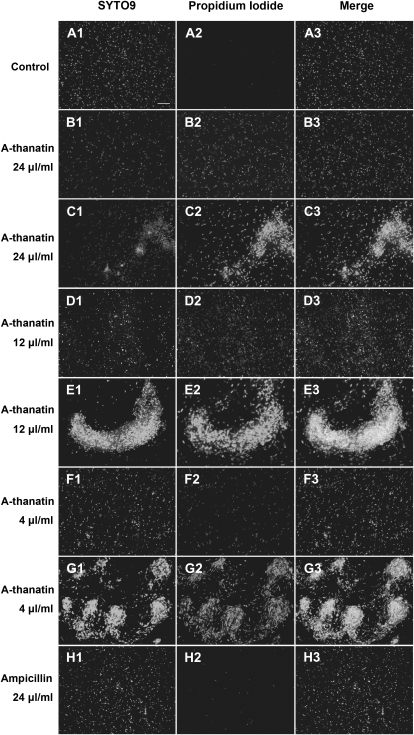
Membrane-permeant SYTO 9 green fluorescent nucleic acid stain has been shown to stain live and dead gram-positive and gram-negative bacteria with green fluorescence; membrane impermeant propidium iodide labels membrane-compromised bacteria with red fluorescence. When both dyes are present, propidium iodide penetrates bacteria with damaged membranes, causing a competitive decrease in the SYTO 9 stain fluorescence. In this case, propidium iodide emitting red fluoresce and SYTO 9 showing green fluoresce indicated dead and live bacteria, respectively. Under the microscope, almost no red fluorescence was observed in the control group (Figure 6A). Treatment with different concentrations of A-thanatin caused the number of fluorescence spots and intensity of fluorescence to gradually increase in a concentration-dependent manner. Almost no red fluorescence was observed in the ampicillin-treated group (Figure 6H). Adding 4, 12, or 24 μg/mL A-thanatin to ESBL-EC induced significant bacterial aggregation (Figure 6C, 6E, and 6G).
Figure 6.
C-terminal–amidated thanatin (A-thanatin)–mediated bacterial membrane permeabilization and aggregation (original magnification, ×400). Extended-spectrum β-lactamase (ESBL)–producing Escherichia coli (ESBL-EC) was incubated with A-thanatin or ampicillin for 90 min at 37°C. Bacteria were stained with the dyes SYTO 9 (which stains live bacteria) (A1–H1) and propidium iodide (which stains dead bacteria) (A2–H2) and were analyzed by fluorescence microscopy to assess viability and aggregation (A3–H3). A, Control; B, D, and F, 24 μg/mL, 12 μg/mL, and 4 μg/mL A-thanatin-mediated bacterial membrane permeabilization, respectively; C, E, and G, 24 μg/mL, 12 μg/mL, and 4 μg/mL A-thanatin–mediated bacterial aggregation, respectively; H, 24 μg/mL ampicillin-treated group. No bacterial aggregation was observed in the control or ampicillin-treated groups.
DISCUSSION
Outbreaks of infection due to ESBL-producing bacteria have become a global health issue in both hospitals and communities [20]. The ineffectiveness of commonly used antibacterial drugs makes it urgent to develop new strategies. One innovative approach is to use peptides as antibiotic agents, and antimicrobial peptides have been proposed as a new approach for treating infections mediated by MDR isolates.
A-thanatin displayed potent antibiotic activities, especially against gram-negative bacteria, to both susceptible and resistant strains. Similar results were obtained in the bacterial killing assay in vitro. Moreover, its MIC and MBC values were quite similar (differing by no more than 2-fold), which indicated that A-thanatin was a bactericidal agent.
Sepsis is a grave clinical syndrome resulting from a damaging host response to infection and is associated with a high mortality rate. To date, few studies have investigated the therapeutic effects of A-thanatin in vivo. An obvious increase in the survival rate of ESBL-EC–infected mice has, to our knowledge, first been observed in this study, which indicated a high efficacy of A-thanatin against ESBL-EC infection in a dose-dependent manner. At present, more and more multidrug-resistant strains are posing a serious threat to human health. Compared with the increasingly restrictive clinical application of traditional antibiotics (such as ampicillin and ciprofloxacin), A-thanatin may be a potential agent for infection treatment, especially for MDR strains.
Most antimicrobial peptides target the cell membrane, so toxicity issues have not been unexpected, especially hemolytic activity (cytotoxicity to RBCs) [21]. Low selectivity is a disadvantage of antimicrobial peptides. Their toxicity seriously hampers systemic applications; therefore, almost all peptides currently in clinical use are for topical treatment (eg, daptomycin) [22]. Several antimicrobial peptides with excellent bactericidal activity have been abandoned because of toxic effects [21]. A-melittin is a high-performance antibiotic agent that has recently been researched in depth; however, its cytotoxicity remains a barrier to its clinical application [23, 24]. Our current study showed little hemolysis in the A-thanatin–treated group. Meanwhile, efficient antibacterial actions were also observed in vitro and in vivo, which implied a high selectivity between prokaryocyte and eukaryocyte. As a result, it could be inferred that A-thanatin might be used safely in vivo. Indeed, more work is required to establish the safety of A-thanatin before reliable clinical use will be possible.
Clinical application requires good stability of therapeutic peptides. Considerable efforts have been made to achieve this goal, such as modifying the N- and C-terminals of peptides, replacing amino acids with nonnatural residues, cyclizing peptides, and reducing the peptide size [25]. In this study, the decrease in the activity of K4-S4(1–16)a in plasma may be caused by plasma protein binding or protease-associated degradation. Conversely, A-thanatin retained identical activity against ESBL-EC, suggesting a good stability in plasma.
Whether the biological activity of A-thanatin depends on membrane permeabilization remains controversial. It has been confirmed that A-thanatin caused membrane permeabilization by observing the morphologic changes of ESBL-EC. The findings of SEM and TEM corroborate a time-dependent permeabilization of A-thanatin. Moreover, staining samples with the permeant fluorescent probe SYTO 9 and impermeant fluorescent probe propidium iodide further revealed a gradual enhancement of membrane permeability with increase of A-thanatin dose. We therefore speculate that low-dose A-thanatin or a short incubation time leads to minor permeabilization, which causes bacterial swelling and holes in the membrane, whereas higher concentrations and prolonged incubation times result in cell content pouring out because of severe membrane damage that eventually leads to bacterial death. Thus, we conclude that the cytoplasmic membrane is an important target of the bactericidal action of A-thanatin. A-thanatin killed ESBL-EC in a time-dependent and dose-dependent manner.
In addition to direct bacterial killing, A-thanatin also induced significant aggregation of ESBL-EC. Although it has been confirmed that A-thanatin can promote bacterial aggregation in vitro, the underlying mechanism remains unclear. A very recent study found that rBPI21, which is a bactericidal permeability-increasing protein, can interact with LPS electrostatically, thereby promoting its aggregation and neutralizing its toxicity [26]. One possible explanation of this phenomenon is that binding of the cationic peptide caused a reduction in the surface charge density of the lipopolysaccharide and thus reduced the electrostatic repulsion between bacteria [7]. This promotion of bacterial aggregation by A-thanatin might enhance phagocytosis, inhibit microbial colonization and invasion, and prevent marked sepsis by neutralizing LPS toxicity.
In summary, this study has demonstrated that A-thanatin is highly effective against ESBL-EC, with satisfactory stability in plasma and little hemolysis. Favorable in vivo therapeutic effects were also observed in a septicemic animal model. Membrane permeabilization may be a biological action of A-thanatin. Because increasing multidrug resistance limits the available therapeutic options, A-thanatin could provide a novel strategy for treating ESBL-EC and other MDR infections. Future studies should attempt to fully characterize the usefulness of A-thanatin as a clinical therapy for infectious diseases.
Acknowledgments
We thank Xiuli Xu (Xijing Hospital, Shaanxi, China), Shanmei Wang (Henan Provincial People's Hospital, Henan, China), Lixia Zhang (Shaanxi Provincial People's Hospital, Shaanxi, China), and Yi Zhang (Second Affiliated Hospital of Medical College of Xi'an Jiaotong University, Shaanxi, China), for clinical strains.
References
- 1.Jackson LA, Benson P, Neuzil KM, Grandjean M, Marino JL. Burden of community-onset Escherichia coli bacteremia in seniors. J Infect Dis. 2005;191:1523–1529. doi: 10.1086/429344. [DOI] [PubMed] [Google Scholar]
- 2.Leverstein-van Hall MA, Joseph M, M Blok HE, T Donders AR, Paauw A, Fluit AC, Verhoef J. Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J Infect Dis. 2003;187:251–259. doi: 10.1086/345880. [DOI] [PubMed] [Google Scholar]
- 3.Khanfar HS, Bindayna KM, Senok AC, Botta GA. Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: trends in the hospital and community settings. J Infect Dev Ctries. 2009;3:295–299. doi: 10.3855/jidc.127. [DOI] [PubMed] [Google Scholar]
- 4.Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect. 2007;55:254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Cirioni O, Giacometti A, Ghiselli R, et al. Potential therapeutic role of histatin derivative P-113d in experimental rat models of Pseudomonas aeruginosa sepsis. J Infect Dis. 2004;190:356–364. doi: 10.1086/421913. [DOI] [PubMed] [Google Scholar]
- 6.Cirioni O, Giacometti A, Ghiselli R, et al. Temporin A alone and in combination with imipenem reduces lethality in a mouse model of staphylococcal sepsis. J Infect Dis. 2005;192:1613–1620. doi: 10.1086/496888. [DOI] [PubMed] [Google Scholar]
- 7.Fehlbaum P, Bulet P, Chernysh S, et al. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc Natl Acad Sci U S A. 1996;93:1221–1225. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu GQ, Ding JX, Li LX, Wang HL, Zhao R, Shen ZL. Activity of the antimicrobial peptide and thanatin analog S-thanatin on clinical isolates of Klebsiella pneumoniae resistant to conventional antibiotics with different structures. Curr Microbiol. 2009;59:147–153. doi: 10.1007/s00284-009-9410-2. [DOI] [PubMed] [Google Scholar]
- 9.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 11.Khandelia H, Ipsen JH, Mouritsen OG. The impact of peptides on lipid membranes. Biochim Biophys Acta. 2008;1778:1528–1536. doi: 10.1016/j.bbamem.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Giuliani A, Pirri G, Bozzi A, Di Giulio A, Aschi M, Rinaldi AC. Antimicrobial peptides: natural templates for synthetic membrane-active compounds. Cell Mol Life Sci. 2008;65:2450–2460. doi: 10.1007/s00018-008-8188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feder R, Dagan A, Mor A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J Biol Chem. 2000;275:4230–4238. doi: 10.1074/jbc.275.6.4230. [DOI] [PubMed] [Google Scholar]
- 14.Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother. 2002;46:689–694. doi: 10.1128/AAC.46.3.689-694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS (National Committee for Clinical Laboratory Standards) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard, 6th edition. M7-A6. NCCLS, Wayne, PA. 2003. [Google Scholar]
- 16.Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, Mor A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J Biol Chem. 2002;277:16941–16951. doi: 10.1074/jbc.M111071200. [DOI] [PubMed] [Google Scholar]
- 17.Tossi A, Scocchi M, Zanetti M, Gennaro R, Storici P, Romeo D. An approach combining rapid cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. Methods Mol Biol. 1997;78:133–150. doi: 10.1385/0-89603-408-9:133. [DOI] [PubMed] [Google Scholar]
- 18.Radzishevsky IS, Rotem S, Zaknoon F, Gaidukov L, Dagan A, Mor A. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob Agents Chemother. 2005;49:2412–2420. doi: 10.1128/AAC.49.6.2412-2420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 20.Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;13:pii=19044. [PubMed] [Google Scholar]
- 21.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 23.Maher S, McClean S. Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem Pharmacol. 2008;75:1104–1114. doi: 10.1016/j.bcp.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Liu SI, Cheng HH, Huang CJ, et al. Melittin-induced [Ca2+]i increases and subsequent death in canine renal tubular cells. Hum Exp Toxicol. 2008;27:417–424. doi: 10.1177/0960327108094606. [DOI] [PubMed] [Google Scholar]
- 25.Oyston PC, Fox MA, Richards SJ, Clark GC. Novel peptide therapeutics for treatment of infections. J Med Microbiol. 2009;58:977–987. doi: 10.1099/jmm.0.011122-0. [DOI] [PubMed] [Google Scholar]
- 26.Domingues MM, Castanho MA, Santos NC. rBPI(21) promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes. PLoS One. 2009;4:e8385. doi: 10.1371/journal.pone.0008385. [DOI] [PMC free article] [PubMed] [Google Scholar]