Abstract
Background. Chikungunya virus (CHIKV) infection induces arthralgia. The involvement of inflammatory cytokines and chemokines has been suggested, but very little is known about their secretion profile in CHIKV-infected patients.
Methods. A case-control longitudinal study was performed that involved 30 adult patients with laboratory-confirmed Chikungunya fever. Their profiles of clinical disease, viral load, and immune mediators were investigated.
Results. When patients were segregated into high viral load and low viral load groups during the acute phase, those with high viremia had lymphopenia, lower levels of monocytes, neutrophilia, and signs of inflammation. The high viral load group was also characterized by a higher production of pro-inflammatory cytokines, such as interferon-α and interleukin (IL)–6, during the acute phase. As the disease progressed to the chronic phase, IL-17 became detectable. However, persistent arthralgia was associated with higher levels of IL-6 and granulocyte macrophage colony-stimulating factor, whereas patients who recovered fully had high levels of Eotaxin and hepatocyte growth factor.
Conclusions. The level of CHIKV viremia during the acute phase determined specific patterns of pro-inflammatory cytokines, which were associated with disease severity. At the chronic phase, levels of IL-6, and granulocyte macrophage colony-stimulating factor found to be associated with persistent arthralgia provide a possible explanation for the etiology of arthralgia that plagues numerous CHIKV-infected patients.
Chikungunya fever (CHIKF) has emerged as an important infection in South-East Asia, the Indian Ocean region, and Europe [1–6]. CHIKF is an acute illness characterized by fever, rash, and arthralgia, with occasional involvement of the nervous system and liver. Incapacitating arthralgia has been reported to persist for years in some patients [7–9]. The causative agent of CHIKF, Chikungunya virus (CHIKV), is a linear single-stranded, positive-sense RNA virus with a length of ∼11.8 kb. It belongs to the Alphavirus genus in the Togaviridae family and is known to induce prominent joint pathologies [8, 9]. Of concern, re-emerging CHIKV has been associated with considerable morbidity and some fatalities, whereas CHIKF was considered to be relatively benign previously. Although clinical features of acute CHIKV infection have been described [10–12], little is known about its long-term sequelae and the pathogenesis of arthropathy.
It has been proposed that CHIKV-induced arthralgia has an immunopathologic origin [13–15]. It has been suggested that interleukin (IL)–1β, IL-6, and RANTES are associated with disease severity in acutely infected patients and that these markers can be used to identify patients with poor prognosis for special monitoring [16].
Treatment for CHIKF is largely palliative and based primarily on anti-inflammatory drugs [7]. Because of the prominent involvement of the immune system and cytokines throughout the acute and chronic disease phases, there is potential for the development of therapeutic modulators against the immunopathologic effects of CHIKV infection. We therefore conducted this longitudinal study as a follow-up to an earlier report [16] to further define circulating levels of immune mediators, from the acute to convalescent and chronic phases of the infection, in 30 laboratory-confirmed cases of CHIKF in Singapore.
MATERIALS AND METHODS
Ethical Approval
Written informed consent was obtained from all participants. The study was approved by the National Healthcare Group's domain-specific ethics review board (DSRB Reference No. B/08/026)
Patients and Plasma Collection
The study included 30 patients with CHIKF who were admitted to the Communicable Disease Centre at Tan Tock Seng Hospital from 1 August through 23 September 23 2008. The outbreak occurred in an industrial area where the source population comprised predominantly male workers, with only 4 female individuals among the 30 patients in this study. Plasma specimens were collected at 4 times after illness onset: (1) on the day of admission to the hospital (acute phase; median, 4 days after illness onset), (2) on the day of discharge from the hospital (early convalescent phase; median, 10 days after illness onset), (3) 4–6 weeks after illness onset (late convalescent phase), and (4) 2–3months after illness onset (chronic phase). All patients were confirmed to have CHIKF by reverse-transcription polymerase chain reaction (RT-PCR). Crossing-point values were used to derive viral loads from a standard curve generated by 10-fold serial dilutions of a 1×108 plaque forming unit (pfu)/mL stock. Data on demographic characteristics and premorbid conditions were collected at hospital admission. Routine hematological, biochemical laboratory test findings and CHIKV load were monitored for the first and second collections. Patients’ clinical features were evaluated at all 4 collection times. Illness was defined as severe if a patient had either a maximum temperature >38.5°C, a maximum pulse rate >100 beats/min, or a nadir platelet count <100 × 109cells/L [16]. Patients who do not fulfill these criteria were classified as having mild illness. In addition, plasma specimens were collected from 8 healthy volunteers as controls. All plasma specimens collected were aliquoted and stored at −80°C until use.
Multiplex Microbead Immunoassay for Cytokine Quantification
Plasmatic cytokine levels were measured using the Biosource Human Cytokine Assay Kit (Invitrogen) according to the manufacturer's instruction, as described elsewhere [16]. Results were acquired using the Luminex 200 instrument (Millipore), with IS 2.3 software, based on standard curves plotted through a 5-parameter logistic curve setting.
Data Analysis
Continuous variables were compared among different disease phases and patient groups with use of a nonparametric Mann–Whitney U test (1-tailed) analysis. Patient group comparisons included (1) patients with CHIKF and high viral load (HVL) versus patients with CHIKF and low viral load (LVL), (2) patients with CHIKF versus normal control subjects, and (3) patients with CHIKF and persistent arthralgia versus patients with CHIKF and full recovery. P values < .05 were considered to be statistically significant. In addition, the relationships between CHIKV load and cytokines and between C-reactive protein (CRP) and cytokines were determined using Pearson's correlation analysis. Pearson's correlation coefficient (r) >.6 was considered to be strong. Two-way hierarchical clustering was done as described elsewhere [16].
RESULTS
Characteristics of Patients with CHIKV Infection
Of the 30 patients with CHIKF in the study, 26 were male and 4 were female; their enrollment from an industrial area, where the outbreak occurred, explains the high male-to-female ratio. Their ages ranged from 23 to 67 years (mean, 39 years; median, 36.5 years) (Table 1). None had underlying medical conditions. Slightly more than half (53.3%) had severe illness from CHIKV infection, but the majority (25 of 30) recovered without any remarkable symptoms. Arthralgia (60.0%), fever (46.7%), myalgia (46.7%), and rash (43.3%) were the most common presenting symptoms at hospital admission. Joint swelling occurred in 4 patients; 3 had swelling of the fingers, and 1 had both knee and shoulder swelling. At hospital discharge, all joint swelling had resolved. None of the 4 patients had persistent arthralgia. A small proportion of patients continued to have rash (10%) during the late convalescent phase (4–6 weeks after illness onset), which was resolved by the chronic phase (2–3 months after illness onset). Arthralgia or joint pains persisted at the chronic phase (month 2–3 after illness onset) in 4 other patients (13.3%). Most laboratory parameters were unremarkable at the time of hospital admission, except for CRP level; the mean peak CRP level for the 30 patients with CHIKF was 24.29 ± 24.07 mg/dL (normal range, 0.0–5.0 mg/dL).
Table 1.
Demographic and Epidemiologic Data for 30 Patients with Confirmed Chikungunya Virus (CHIKV) Infection
| Patient (Sex, Age in years) | Duration of fever, Days | Acute illness severitya | CHIKV load, pfu/mL | CRP level, mg/dL | Lymphocytes, % | Neutrophils, % | Monocytes, % | Clinical outcomeb |
| CHIKV 1 (M, 40) | 3 | Severe | 5.62E+08 | 77.9 | 5.9 | 84.2 | 8.7 | Complete recovery |
| CHIKV 2 (M, 23) | 8 | Severe | 3.45E+08 | 93.7 | 5.8 | 84.0 | 9.6 | Complete recovery |
| CHIKV 3 (M, 62) | 7 | Severe | 3.45E+08 | 46.7 | 13.6 | 73.8 | 10.2 | Lethargy, weakness |
| CHIKV 4 (M, 43) | 5 | Severe | 3.45E+08 | 46.1 | 5.1 | 85.3 | 9.1 | Complete recovery |
| CHIKV 5 (M, 29) | 6 | Severe | 2.12E+08 | 58.9 | 6.3 | 79.4 | 14.2 | Complete recovery |
| CHIKV 6 (M, 35) | 7 | Severe | 1.14E+07 | 0.4 | 32.3 | 57.9 | 8.5 | Complete recovery |
| CHIKV 7 (M, 30) | 4 | Severe | 1.14E+07 | 29.6 | 11.5 | 77.6 | 10.6 | Complete recovery |
| CHIKV 8 (M, 35) | 4 | Severe | 2.64E+06 | 9.0 | 11.6 | 74.1 | 13.5 | Complete recovery |
| CHIKV 9 (M, 26) | 3 | Severe | 9.97E+05 | 20.8 | 4.3 | 87.7 | 7.9 | Complete recovery |
| CHIKV 10 (M, 28) | 4 | Severe | 3.76E+05 | 22.8 | 15.9 | 75.7 | 7.5 | Complete recovery |
| CHIKV 11 (M, 49) | 2 | Severe | 3.76E+05 | 25.7 | 13.3 | 74.8 | 11.6 | Complete recovery |
| CHIKV 12 (M, 50) | 6 | Severe | 2.31E+05 | 36.9 | 5.9 | 80.0 | 8.6 | Complete recovery |
| CHIKV 13 (M, 38) | 3 | Severe | 2.31E+05 | 34.2 | 30.3 | 56.9 | 12.3 | Complete recovery |
| CHIKV 14 (M, 60) | 3 | Mild | 1.42E+05 | 56.4 | 9.1 | 84.4 | 5.9 | Complete recovery |
| CHIKV 15 (F, 62) | 7 | Severe | 5.36E+04 | 8.5 | 24.3 | 91.0 | 3.0 | Complete recovery |
| CHIKV 16 (M, 45) | 0 | Mild | 5.36E+04 | 2.1 | 20 | 57.0 | 12.0 | Complete recovery |
| CHIKV 17 (M, 34) | 3 | Mild | 5.36E+04 | 9.2 | 45.4 | 51.0 | 15.9 | Complete recovery |
| CHIKV 18 (M, 29) | 2 | Severe | 2.02E+04 | 46.9 | 18.6 | 70.5 | 13.3 | Persistent arthralgia |
| CHIKV 19 (F, 67) | 7 | Mild | 2.02E+04 | 10.7 | 6 | 68.0 | 19.0 | Complete recovery |
| CHIKV 20 (M,24) | 3 | Mild | 2.02E+04 | 9.3 | 45.6 | 41.0 | 12.6 | Complete recovery |
| CHIKV 21 (M, 34) | 0 | Mild | 2.02E+04 | 1.0 | 33.8 | 41.9 | 17.4 | Complete recovery |
| CHIKV 22 (M, 28) | 7 | Mild | 1.24E+04 | 2.9 | 32.6 | 56.8 | 8.2 | Complete recovery |
| CHIKV 23 (M, 42) | 2 | Mild | 1.24E+04 | 12.4 | 24.3 | 67.0 | 7.9 | Complete recovery |
| CHIKV 24 (F, 40) | 6 | Mild | 9.37E+03 | 17.3 | 69.9 | 69.9 | 15.4 | Persistent arthralgia |
| CHIKV 25 (F, 31) | 6 | Mild | 7.64E+03 | 32.5 | 11.7 | 81.8 | 6.0 | Persistent arthralgia |
| CHIKV 26 (M, 46) | 9 | Mild | 7.64E+03 | 4.9 | 28.2 | 57.9 | 12.7 | Complete recovery |
| CHIKV 27 (M, 26) | 4 | Mild | 7.64E+03 | 7.8 | 36.1 | 37.2 | 17.3 | Persistent arthralgia |
| CHIKV 28 (M,28) | 5 | Severe | 7.64E+03 | 0.3 | 18 | 60.0 | 14.0 | Complete recovery |
| CHIKV 29 (M, 47) | 8 | Mild | 4.69E+03 | 2.0 | 28 | 49.0 | 21.0 | Complete recovery |
| CHIKV 30 (M, 39) | 1 | Mild | BDL | 1.8 | 30.2 | 49.3 | 8.5 | Complete recovery |
NOTE. BDL, below detection limit (1E + 02 pfu/mL); CRP, C-reactive protein.
All patients do not have any pre-morbid conditions.
The 4 clinical parameters, CRP level (normal range, 0.0–5.0 mg/dL), lymphocyte percentage (normal range, 20%–45%), neutrophil percentage (normal range, 45%–75%), and monocyte percentage (normal range, 3%–10%), were determined at admission to the hospital at acute phase (median, 4 days after infection onset).
Severity was defined as having a temperature >38.5°C, pulse rate >100 beats/min, or platelet count <100 x 109 cells/L.
Clinical outcome at chronic phase (2-3 months after infection onset).
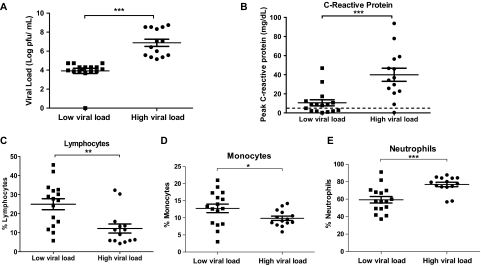
At hospital admission, viremia was observed in all except one patient. The mean CHIKV load in viremic patients was 6.33 × 107 pfu/mL (range, 4.69 × 103–5.62 × 108 pfu/mL), well within the reported range [17, 18]. At hospital discharge, only half of the patients continued to have detectable viral loads (range, 2.88 × 103–3.29 × 104 pfu/mL). On the basis of their viral loads at admission to hospital, the 30 patients could be divided into 2 distinct groups: HVL group and LVL group (Figure 1A). Thirteen (93%) of 14 patients with HVL (mean viral load, 1.31 × 108 pfu/mL; range, 1.42 × 105–5.63 × 108 pfu/mL) presented with severe clinical illness, compared with only 3 (19%) 16 patients with LVL (mean viral load, 1.95 × 104 pfu/mL; range, 1 × 102–5.36 × 104 pfu/mL) (Table 1, Figure 1A).
Figure 1.
Association of laboratory parameters with viral load. A, Patients were separated into 2 groups according to their viral load: high viral load (HVL; n = 14) and low viral load (LVL; n = 16). Comparisons of C-reactive protein (B), lymphocyte (C), monocyte (D), and neutrophil (E) levels between HVL and LVL groups. Data are presented as mean ± standard error of the mean (SEM). *P < .05; **P < .01; ***P < .001, Mann–Whitney U test, 1-tailed.
The magnitude of CRP level elevation in the HVL group (median, 29.6; interquartile range [IQR], 21.8–41.5 mg/dL) was significantly greater, compared with that in the LVL group (median, 7.8; IQR, 2–12.4 mg/dL; P < .001) (Figure 1B). More pronounced lymphopenia was observed in the HVL group during the acute phase, with a significantly lower proportion of lymphocytes (median, 10.3%; IQR 5.9%–13.5%), compared with the LVL group (median, 27.4%; IQR, 15.2%–32.2%; P < .01) (Figure 1C). After the acute phase of infection, lymphocyte counts in all patients were restored to normal levels by the early convalescent phase (median, 26.6%; IQR, 21.6%–31.1%). Lower levels of monocytes were also more pronounced in the HVL group (median, 9.4%; IQR, 8.5%–11.4%), compared with the LVL group (median, 13.0%; IQR, 8.4%–16.3%; P < .05) (Figure 1D). Neutrophilia was observed in the HVL group (median, 78.5%; IQR, 74.3%–84.2%), compared with the LVL group (median, 57.5%; IQR, 49.2%–68.5%; P < .001) (Figure 1E).
Immune Mediator Profile Over Time During CHIKV Infection
The levels of 7 cytokines (interferon [IFN]–γ, IL-2, IL-5, IL-10, IL-13, tumor necrosis factor–α, and vascular endotherial growth factor) were below the detection limit in most patients across all 4 collections. For mediators that were detected in our patients, we observed 4 patterns representing different kinetics of production. The first profile revealed cytokines, cytokine-related factors, and chemokines that peak at the acute phase (median, 4 days after illness onset) (Figure 2A) and represented the innate immune response at infection. Levels of cytokines, such as IFN-α, IL-1Ra, IL-6, IL-7, IL-8, IL-12, and IL-15, were significantly increased in patients with CHIKF, compared with control subjects. The second profile revealed mediators that peaked during the early convalescent phase (median, 10 days after illness onset) (Figure 2B). The cytokine profile of this group was characterized by an increased secretion of T cell cytokines/chemokines (IL-2R), with a bias toward Th2 type factors (IL-4, Eotaxin). Other factors, such as hepatocyte growth factor (HGF), FGF-basic, granulocyte colony-stimulating factor, and chemokines MIG and MIP-1a in patients with CHIKF were also above the median values in control subjects. The third profile represents mediators (RANTES and EGF) that peaked at the late convalescent phase (4–6 weeks after illness onset) (Figure 2C). Finally, the fourth profile revealed cytokines (IL-17) that were detected only during the chronic phase (2–3 months after illness onset) (Figure 2D).
Figure 2.
Kinetic profiles of immune mediator expression over time during Chikungunya virus (CHIKV) infection. A, Acute phase, median 4 days after illness onset; B; Early convalescent phase, median 10 days after illness onset; C, Late convalescent phase, 4–6 weeks after illness onset; and D, chronic phase, 2–3 months after illness onset. Comparisons between patients with CHIKF and normal healthy control subjects were analyzed using a 1-tailed Mann–Whitney U test. Horizontal dotted lines represent median values for healthy control subjects. Samples from all 30 patients were analyzed for each time. Data are presented as mean ± standard error of the mean (SEM). (*P < .05; **P < .01; ***P < .001).
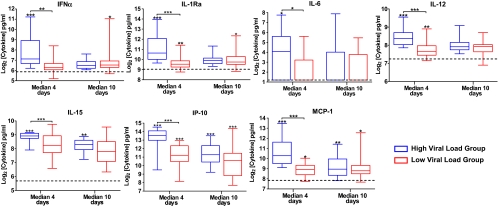
Acute Viral Load Influences Production of Immune Mediators
Qualitative analysis of the profile of mediators in Figure 3 revealed a clear difference between the HVL and the LVL groups, mainly during the acute phase. We found that levels of inflammatory mediators IFN-α, IL-1Ra, IL-6, IL-12, IL-15, IP-10, and MCP-1 were significantly higher in the HVL group than in the LVL group (Figure 4). In addition, the duration of production of these mediators was shorter in the LVL group than in the HVL group. A significantly strong positive correlation was found between CHIKV load and IP-10 (Pearson's r = .69; P < .0001), MCP-1 (Pearson's r = .78; P < .0010), IFN-α (Pearson's r = .72; P < .0001), IL-12 (Pearson's r = .72; P < .001), IL-1Ra (Pearson's r = .77; P < .0001), and IL-6 (Pearson's r = .76; P < .001).
Figure 3.
Patterns of acute phase immune mediators, shown by 2-way hierarchical clustering. Each colored cell in the 3 hit maps represents the relative levels of expression of a particular cytokine in a patient. Green indicates low production, and red indicated high production. The first and second hit maps from the left represent the qualitative cytokine expression profiles of patients in the high viral load (HVL) and low viral load (LVL) groups, respectively. Different disease phases are illustrated: acute phase (median, 4 days after illness onset), early convalescent phase (median, 10 days after illness onset), late convalescent phase (4–6 weeks after illness onset), and chronic phase (2–3 months after illness onset).
Figure 4.
Expression of inflammatory mediators during acute Chikungunya virus (CHIKV) infection. Levels of IFN-α, IL-1Ra, IL-6, IL-12, IL-15, IP-10, and MCP-1 in infected patients were compared with those in healthy control subjects with use of the nonparametric, 1-tailed Mann–Whitney U test. Horizontal dotted lines represent median values for healthy control subjects. Data are presented as mean ± standard error of the mean (SEM). (*P < .05; **P < .01; ***P < .001). Different disease phases are illustrated: acute phase (median, 4 days after illness onset), early convalescent phase (median, 10 days after illness onset), late convalescent phase (4–6 weeks after illness onset), and chronic phase (2–3 months after illness onset).
Markers Associated with Chronic Joint Pain
Four patients reported persistent arthralgia during the chronic phase (2–3 months after illness onset). We compared the mediator profiles in these 4 patients with those in patients who had fully recovered. Plasma levels of 2 cytokines (IL-6 and GM-CSF) were significantly higher in patients with persistent arthralgia (P < .05) (Figure 5A) during this chronic phase, whereas chemokines Eotaxin and HGF were significantly lower, compared with individuals who had fully recovered (P < .05) (Figure 5B).
Figure 5.
Cytokines implicated in chronic arthropathy. A, Levels of IL-6 and GM-CSF in patients who experienced persistent joint pain, compared with those in persons who made a full recovery at 2–3 months after Illness onset. B, Eotaxin and HGF levels were significantly lower in patients who still had persistent joint pain, compared with those who recovered completely. Horizontal dotted lines represent median values for healthy control subjects. *P < .05, Mann–Whitney U test, 1-tailed.
DISCUSSION
Despite CHIKV being first isolated in 1952, the mechanisms leading to the prominent pathologies of arthralgia and arthritis in CHIKV infection have not been well studied [13, 15]. It was only recently that a role for cytokines has been proposed for the severity observed during acute CHIKV infection, both in humans [16] and in a nonhuman primate model [19]. However, it is still unknown whether cytokines and other mediators, such as chemokines, are involved in chronic joint pathologies induced by CHIKV infection.
In this study, longitudinal immune mediator profiles were determined in patients with CHIKF with different clinical features and viral loads. We first observed that viral load decreased after an intense but relatively short viremic phase, during which patients experienced fever and typical clinical features of CHIKF. During this CHIKF outbreak in August 2008, patients were observed to have viremia lasting a median of 6 days [20]. This was comparable to the earlier local Chikungunya cohort, in which 40% continued to have detectable viral loads after day 5 of illness [6], and cohorts from the Indian Ocean region, in which viral RNA–positive results were observed in 60%, 50%, and 40% of individuals at day 5, 6, and 7 after illness onset, respectively [17].
In our study, acute disease severity in patients was defined at admission by either maximum temperature >38.5°C, a maximum pulse rate >100 beats/min, or a nadir platelet count <100 ×109 cells/L [14, 19]. More patients from the HVL group (95%) had severe CHIKF illness than those in the LVL group (19%). We next observed that patients with high viral loads had significantly more elevated CRP levels, lymphopenia, and lower monocyte levels during the acute phase. Of interest, neutrophilia was also observed. This suggests that viremia drives disease pathogenesis and the associated signs and symptoms of CHIKF (Figure 1).
Differences in viral load between the different patients could be attributed to host genetic factors controlling virus invasion and replication. Such a phenomenon has been observed for other viruses [21–23]. Further studies are needed to understand this in CHIKV-host interactions. Another explanation is differences in the innate immune response, because recent reports have shown that a swift response of IFN-α, induced by CHIKV infection in vitro and in vivo [14, 19, 24], could control CHIKV replication efficiently [24].
In a previous study, a limited number of samples (n = 10) taken during the acute phase of CHIKF illness in an earlier outbreak, showed a significant increased production of several inflammatory cytokines. However, because of the small cohort size, only striking differences could be observed [16]. In the present study, a larger cohort with longitudinal sampling allowed us to further define markers that could be involved in severe illness and at various phases of CHIKF disease course.
Four unique immune mediator profiles were observed on the basis of kinetics of production that represented the different phases of anti-CHIKV immune response. The first profile, defined by a rapid but short-lasting production of mediators, was characteristic of an early antiviral innate immune response (Figure 2A). A high viral load was associated with high levels of cytokines, likely inflammatory responses involved in antiviral defense. IFN-α levels were significantly higher in the HVL group than in the LVL group only during the acute viremic phase (median, 4 days after illness onset). IFN-α was induced by the infection [25], but its production levels depended on the viral load, because it occurred earlier in the HVL group than in the LVL group (Figure 4). In the LVL group, some patients had higher levels of IFN-α only during the early convalescent phase (median, 10 days after illness onset) (Figure 4), confirming recent findings obtained with a cohort from Reunion Island [14]. A certain threshold in viral load is required before IFN-α is induced. Alternatively, it could be attributable to a delayed innate immune response. This could perhaps explain the clinical outcome of persistent arthralgia experienced by patients in the LVL group (Table 1).
High levels of IL-15 and IL-12 were found to be significantly produced, compared with healthy control subjects, in the first 2 profiles (Figure 2A and B). Both cytokines (together with IFN-α and IL-7, for which levels were also increased) are cytokines known to activate natural killer (NK) cells [26]. NK cells have been recently shown, in humans and nonhuman primates with CHIKV infection, to increase in number early after infection and, thus, may participate in early control of CHIKV [19, 27]. IL-12 is also important for the development of Th-1 cells that have potent antiviral activities [28]. Other mediators in the first profile are chemoattractants, such as IL-8, MCP-1, and IP-10, which could mobilize immune cells, such as neutrophils and monocytes [29, 30], allowing them to migrate to the skin or to the lymph nodes—2 sites of early viral replication, as shown in the nonhuman primate model of CHIKV infection [19].
As in our previous study [16], IL-6 was observed to be the only pyretic detected during the acute phase of CHIKV infection. It was induced only in the HVL group, in which the majority of patients (80%) had high fever (temperature, >38.5°C) (Figure 2A and 4). It is also known to be the major inducer of acute phase proteins, such as CRP, in humans [31, 32]. Patients in the HVL group showed increased levels of CRP, a marker of systemic inflammation [33, 34]. CRP levels were also found to be strongly correlated with IL-6 levels (Pearson's r = .70; P < .001). Previously, CRP levels were shown to be elevated during the acute phase of CHIKV infection [35]. During the 2005–2006 CHIKF outbreaks in Reunion Island, CRP levels in hospitalized patients with CHIKF were found to be significantly higher than those in nonhospitalized patients [36, 37]. CRP measurement is a routine biochemical test used in the clinical management of patients who receive a diagnosis of infection, because of its low cost and ready availability. If no means exist to assess viral loads, an elevated CRP level could be used as a surrogate marker for high viral load in acutely ill patients with CHIKF. This could help in the early identification and close monitoring of patients with CHIKF who are at risk of severe illness. The high IL-6 levels, together with the increased levels of IL-8 and granulocyte colony-stimulating factor, could also explain the observed neutrophilia and part of the joint pathologies during the acute phase, because these immune mediators have been previously associated with rheumatoid arthritis [38]. The second profile represents mediators that peak at the early convalescent phase (median, 10 days after illness onset), representing mediators involved in both the innate immune response and in the establishment of an antiviral T cell response.
The third profile represents factors detected during the late convalescent phase (4–6 weeks after illness onset), with a distinct increased in RANTES and EGF levels, indicating recovery from thrombocytopenia, because these cytokines are produced mainly by platelets. In the chronic phase of the infection (fourth profile) at 2–3 months after illness onset, high levels of IL-17 were seen. This cytokine has a role in bone tissue inflammation and destruction and has been implicated in the etiology of rheumatoid arthritis. Of note, previous studies have proposed that, in patients with rheumatoid arthritis, neutrophilia drives production of IL-17, which destroys the extracellular matrix and causes bone resorption, further inducing IL-6 production [39]. This is consistent with our data indicating that patients with persistent arthralgia have higher levels of IL-6 and GM-CSF, whereas patients who recovered have normal levels. Recovered patients also had increased levels of HGF, a hormone that facilitates cartilage repairs [40], and increased levels of Eotaxin, a type-2 cytokine, suggesting a local anti-inflammatory response.
Two of the 4 patients who had persistent arthralgia during the chronic phase (2–3 months after infection onset) were female. These female patients represented two-thirds (66.7%) of the female study cohort who presented with arthralgia, compared with 2 (13.3%) of 15males. It was observed in another study that female patients (100%) were significantly more likely than male patients (26.5%) to have persistent arthralgia at week 6 of illness [20]. Our study has shed some light on the possible pathogenesis of arthropathy and arthralgia in patients with CHIKF. However, more work is needed to understand the immunopathology of joint swelling in CHIKV infection.
In conclusion, the immune mediator profiles observed through this longitudinal study have provided a better understanding of CHIKF immunopathology. A positive link between CHIKV infection and cytokine induction was clearly seen throughout different phases of the disease, with specific cytokines being involved at different phases of the infection, corroborating observations from a previous study [16]. There are statistical limitations with this study, mainly in terms of power, because of the small number of samples from each patient. However, we believe that the use of sequential samples from the same individual throughout the disease course adds strength to our study. The discovery of specific immune mediators involved in disease progression not only gives a better idea of possible therapeutic interventions, but also provides plausible mechanisms in the complex area of cytokine signaling that could explain the pathogenesis of CHIKF and other immune-mediated diseases.
Funding
The study was supported by the Biomedical Research Council, A*STAR, and the President's Graduate Fellowship from the Yong Loo Lin School of Medicine, National University of Singapore (to ZH).
Acknowledgments
We thank the study participants and healthy volunteers, for their participation in the study; research staff from the Communicable Disease Centre/Tan Tock Seng Hospital, namely Meng-Li Teo, for assistance in blood sample preparation; Clement Kan, Amy Chan, and Mar-Kyaw Win, for patient enrollment, study coordination, and data entry; clinical staff of Communicable Disease Centre/Tan Tock Seng, for patient enrollment and care; and Keh-Chuang Chin from SIgN, for critically reading this manuscript.
Author Contributions All authors discussed the results and commented on the manuscript. LFPN, AC, and YSL conceived and designed the experiments. EKSO, ZH and DJCK performed the experiments. AC, LFPN, EKSO, ZH, JMC, TB, HY, and LR analyzed the data. AC, FD, and YSL contributed materials. LFPN, AC, ZH, and EKSO wrote the article.
References
- 1.AbuBakar S, Sam IC, Wong PF, MatRahim N, Hooi PS, Roslan N. Reemergence of endemic Chikungunya, Malaysia. Emerg Infect Dis. 2007;13:147–9. doi: 10.3201/eid1301.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrame A, Angheben A, Bisoffi Z, et al. Imported Chikungunya infection, Italy. Emerg Infect Dis. 2007;13:1264–6. doi: 10.3201/eid1308.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonn D. How did Chikungunya reach the Indian Ocean? Lancet Infect Dis. 2006;6:543. doi: 10.1016/s1473-3099(06)70559-x. [DOI] [PubMed] [Google Scholar]
- 4.Enserink M. Tropical disease follows mosquitoes to Europe. Science. 2007;317:1485. doi: 10.1126/science.317.5844.1485a. [DOI] [PubMed] [Google Scholar]
- 5.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai. 2004;87:464–72. [PubMed] [Google Scholar]
- 6.Leo YS, Chow AL, Tan LK, Lye DC, Lin L, Ng LC. Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis. 2009;15:836–7. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrel RN, De Lamballerie X, Raoult D. Chikungunya outbreaks-the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 8.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 9.Powers AM, Logue CH. Changing patterns of Chikungunya virus: Re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 10.Toivanen A. Alphaviruses: An emerging cause of arthritis? Curr Opin Rheumatol. 2008;20:486–90. doi: 10.1097/BOR.0b013e328303220b. [DOI] [PubMed] [Google Scholar]
- 11.Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: Clinical presentation and course. Clin Infect Dis. 2007;45(1):e1–4. doi: 10.1086/518701. [DOI] [PubMed] [Google Scholar]
- 12.Economopoulou A, Dominguez M, Helynck B, et al. Atypical Chikungunya virus infections: Clinical manifestations, mortality risk factors for severe disease during the 2005-2006 outbreak on Reunion. Epidemiol Infect. 2009;137(4):534–41. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 13.Couderc T, Chrétien F, Schilte C, et al. A mouse model for Chikungunya: young age and inefficient Type I interferon signaling are risk factors for severe disease. PloS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilte C, Couderc T, Chretien F, et al. Type I IFN controls Chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207:429–42. doi: 10.1084/jem.20090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler SA, Lu L, da Rosa AP, Xiao SY, Tesh RB. An animal model for studying the pathogenesis of Chikungunya virus infection. Am J Trop Med Hyg. 2008;79:133–9. [PubMed] [Google Scholar]
- 16.Ng LFP, Chow A, Sun YJ, et al. IL-1β, IL-6, RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14:416–22. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parola P, De Lamballerie X, Jourdan J, et al. Novel Chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–9. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labadie K, Larcher T, Joubert C, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Win MK, Chow A, Dimatatac F, Go CJ, Leo YS. Chikungunya fever in Singapore: Acute clinical laboratory features, and factors associated with persistent arthralgia. J Clin Virol. 2010;49:111–4. doi: 10.1016/j.jcv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Coffey LL, Mertens E, Brehin AC, et al. Human genetic determinants of dengue virus susceptibility. Microbes Infect. 2009;11:143–56. doi: 10.1016/j.micinf.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Tang J, Shelton B, Makhatadze NJ, et al. Distribution of chemokine receptor CCR2 CCR5 genotypes and their relative contribution to human immunodeficiency virus Type 1 (HIV-1) seroconversion, early HIV-1 RNA concentration in plasma, and later disease progression. J Virol. 2002;76(2):662–72. doi: 10.1128/JVI.76.2.662-672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokireddy S, Sarojamma V, Ramakrishna V. Genetic predisposition to Chikungunya – a blood group study in Chikungunya affected families. Virol J. 2009;6:77. doi: 10.1186/1743-422X-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Her Z, Malleret B, Chan M, et al. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–13. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 25.Sourisseau M, Schilte C, Casartelli N, et al. Characterization of reemerging Chikungunya virus. PloS Pathog. 2007;4:e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aspinall R, Henson S, Pido-Lopez J, Ngom PT. Interleukin-7: An interleukin for rejuvenating the immune system. Ann N Y Acad Sci. 2004;1019:116–22. doi: 10.1196/annals.1297.021. [DOI] [PubMed] [Google Scholar]
- 27.Hoarau JJ, Bandjee MC, Trotot PK, et al. Persistent chronic inflammation infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–27. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 29.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey CE, Post JJ, Palladinetti P, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity lobular inflammation. J Leukoc Biol. 2003;74(3):360–9. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- 31.Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: New evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25(6):1231–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 32.Vaisman N, Leibovitz E, Dagan R, Barak V. The involvement of IL-6 IL-8 in acute invasive gastroenteritis of children. Cytokine. 2003;22(6):194–7. doi: 10.1016/s1043-4666(03)00177-7. [DOI] [PubMed] [Google Scholar]
- 33.Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19:104–8. doi: 10.1016/j.ejim.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Xu HL, Ye X, Steinberg H, Liu SF. Selective blockade of endothelial NF-kappaB pathway differentially affects systemic inflammation multiple organ dysfunction and injury in septic mice. J Pathol. 2010;220(4):490–8. doi: 10.1002/path.2666. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy AC, Fleming J, Solomon L. Chikungunya viral arthropathy: A clinical description. J Rheumatol. 1980;7:231–6. [PubMed] [Google Scholar]
- 36.Borgherini G, Poubeau P, Staikowsky F, et al. Outbreak of Chikungunya on Reunion Island: Early clinical laboratory features in 157 adult patients. Clin Infect Dis. 2007;44(11):1401–7. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 37.Staikowsky F, Talarmin F, Grivard P, et al. Prospective study of Chikungunya virus acute infection in the island of La Reunion during the 2005-2006 outbreak. LoS One. 2009;4(10):e7603. doi: 10.1371/journal.pone.0007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):554–9. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]
- 39.Miossec P, Korn T, Kuchroo VK. Interleukin-17 type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 40.Hegab A, Kubo H, Yamaya M, et al. Intranasal HGF administration ameliorates the physiologic morphologic changes in lung emphysema. Mol Ther. 2008;16(8):1417–26. doi: 10.1038/mt.2008.137. [DOI] [PubMed] [Google Scholar]