Abstract
We have previously shown that oxidative stress within the tumor microenvironment causes phosphatidylserine (PS) to redistribute from the inner to the outer membrane leaflet of the endothelial cells (EC) creating a highly specific marker for the tumor vasculature. Because the distribution of phosphatidylethanolamine (PE) and PS within the membrane is coregulated, we reasoned that PE would also be localized in the outer membrane leaflet of tumor EC. To demonstrate this, the PE-binding peptide duramycin was biotinylated and used to determine the distribution of PE on EC in vitro and in vivo. Exposure of cultured EC to hypoxia, acidity, reactive oxygen species, or irradiation resulted in the formation of membrane blebs that were intensely PE-positive. When biotinylated duramycin was intravenously injected into tumor-bearing mice, it preferentially localized to the luminal surface of the vascular endothelium. Depending on tumor type, 13% to 56% of the tumor vessels stained positive for PE. PE-positive vessels were observed in and around hypoxic regions of the tumor. With the exception of intertubular vessels of the kidney, normal vessels remained unstained. To test the potential of PE as a biomarker for imaging, duramycin was conjugated to the near-infrared fluorophore 800CW and used for optical imaging of RM-9 prostate carcinomas. The near-infrared probe was easily detected within tumors in live animals. These results show that PE, like PS, becomes exposed on tumor vascular endothelium of multiple types of tumors and holds promise as a biomarker for noninvasive imaging and drug targeting.
Introduction
In normal cells, the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are asymmetrically distributed across the plasma membrane with essentially all the PS and the majority of the PE localized in the cell's inner membrane leaflet [1,2]. This membrane lipid asymmetry is maintained by a group of P-type ATPases known as aminophospholipid translocases (APTLs) that catalyze the active transport of PS and PE from the external to the internal leaflet of the plasma membrane [3]. Unlike normal cells, apoptotic cells and tumor cells lose their capacity to maintain PS asymmetry resulting in the appearance of the lipid in the cells' outer membrane leaflet. The expression of PS at the cell surface inhibits immune responsiveness and, in the case of apoptotic cells, also serves as a recognition ligand and binding site for phagocytes [4,5]. PE has also been shown to be exposed on apoptotic cells, but it has not been associated with a specific function [6].
Increases in intracellular [Ca2+] as a result of transcriptional activation or exposure to environmental stress can also cause loss of membrane asymmetry. Influx of exogenous Ca2+ or Ca2+ released from intracellular stores inhibits APTLs and, at the same time, activates ATP-binding cassette transporters and phospholipid scramblases that randomize all membrane phospholipids between leaflets resulting in a complete loss in plasma membrane lipid asymmetry [7,8]. Environmental stress also activates sphingomyelinases that cleave sphingomyelin (SM) to ceramide [9,10]. Ceramide destabilizes the bilayer, activates proapoptotic signaling pathways, and promotes membrane blebbing [11,12]. Thus, cellular stress and activation can promote the exposure of aminophospholipids on the cell surface through multiple pathways. Indeed, unlike quiescent normal endothelium, there is significant environmental stress imposed on the tumor endothelium by acidity, reactive oxygen species (ROS), and transient hypoxia, which results in the redistribution of PS to the cell surface [13]. Consequently, PS becomes exposed on vascular endothelium of all tumor types so far examined [14,15]. Because both PS and PE are coregulated by the same transporters, [2] the expression of cell surface PS on tumor EC raises the possibility that PE is also expressed on the surface of tumor blood vessels.
Duramycin (MWt = 2013 Da) is a highly specific PE-binding peptide produced by the bacteria Streptoverticillium cinnamoneus. Duramycin binds PE at a 1:1 molar ratio with a Kd of 4 to 6 nM, an unusually high affinity for a small, ligand-binding peptide [16]. Duramycin is the smallest polypeptide known to have a defined three-dimensional binding pocket and recognizes ethanolamine phospholipids with exclusive specificity [16] by fitting over the ethanolamine head group like a glove [17]. The hydrophobic binding pocket is stabilized by three internal thioether linkages that make the peptide resistant to heat and proteolytic degradation [18]. Pharmacokinetic studies in rats have shown that duramycin is rapidly cleared from the blood stream with a serum half-life of less than 4 minutes [19]. 99mTc-labeled duramycin binds to PE exposed on apoptotic and necrotic cells and has been used successfully for the in vivo imaging of acute myocardial infarction [19].
In this report, we used duramycin to show that PE becomes specifically exposed on the surface of tumor EC and that treatment of cultivated EC with known tumor-associated stresses causes the formation of PE-positive blebs on the cell membrane. In vivo studies revealed that labeled duramycin specifically localized to the vascular endothelium in multiple tumor types. Duramycin was also effective at imaging subcutaneous tumors. Taken together, these findings indicate that externalized PE may be a general marker of tumor vasculature and suggest that duramycin possesses the specificity and pharmacokinetic properties to make it an effective agent for specifically targeting the tumor vasculature with imaging agents and/or therapeutic drugs.
Materials and Methods
Materials
Duramycin from S. cinnamoneus, bovine serum albumin (BSA), o-phenylenediamine dihydrochloride (ODP), and vascular endothelial growth factor (VEGF) were purchased from Sigma (St Louis, MO). Sulfo-NHC-LC biotin was obtained from Pierce (Rockford, IL). IRDye 800CW NHS Ester was obtained from LI-COR Biosciences (Lincoln, NE). Dulbecco modified Eagle tissue culture medium, Dulbecco phosphate-buffered saline (PBS) containing Ca2+ and Mg2+, glutamine, and fetal bovine serum (FBS) were obtained from HyClone (Thermo Scientific, Logan, UT). Alexa Fluor 488-conjugated streptavidin, Alexa Fluor 350-conjugated streptavidin, and Prolong Gold mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Molecular Probes (Invitrogen, Carlsbad, CA). Horseradish peroxidase-conjugated streptavidin and Cy3-conjugated streptavidin were purchased from Jackson Immuno Research Laboratories (West Grove, PA). Immulon 1B 96-well microtiter plates were obtained from Thermo LabSystems (Franklin, MA). LUMITRAC 96-well microtiter plates were obtained from Greiner Bio-One (Monroe, NC). PE, phosphatidylcholine (PC), PS, SM, phosphoinositol (PI), phosphatidic acid (PA) and phosphatidylglycerol (PG) were obtained from Avanti Polar Lipids (Alabaster, AL).
Antibodies
The PS-targeting antibody bavituximab is produced under serum-free conditions by Peregrine Pharmaceuticals, Inc (Tustin, CA). Bavituximab requires 1:1 addition of human β2-glycoprotein to bind PS. Rituxan is a human monoclonal antibody that binds human CD20 and was obtained from the UT Southwestern Pharmacy. Rat antimouse CD31 antibody was purchased from BD Biosciences (San Jose, CA). Goat antihuman immunoglobulin G (IgG) conjugated to Cy2, goat antirat IgG conjugated to Cy3, and biotinylated goat antirat IgG secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA).
Synthesis of Duramycin Conjugates and Control Peptides
Duramycin was reacted through its free amino groups with an N-hydroxysuccinimide (NHS) ester of a long-chain (adipic) derivative of l-biotin at a molar ratio of 1:1.1. Briefly, NHS-biotin in dimethyl sulfoxide was rapidly mixed with duramycin in PBS. The reaction was allowed to proceed for 2 hours. Unreacted biotin was removed by dialysis. The product corresponding to one molecule of duramycin linked to one molecule of biotin was purified by high-performance liquid chromatography. Mass spectrometry confirmed a molecular mass of 2352 Da (Figure 1A). The duramycin-l-biotin conjugate (DLB) was supplemented with an equal weight of unlabeled duramycin for all assays, as its addition strengthened PE binding. The control peptide linear duramycin (linDUR) was synthesized by Biosynthesis, Inc (Lewisville, TX) and has the same sequence as duramycin except that thioether-linked amino acids were substituted with alanines. linDUR was biotinylated to produce the control peptide linear-duramycin-l-biotin (linDLB). linDLB was also supplemented with an equal weight of unlabeled duramycin for all assays.
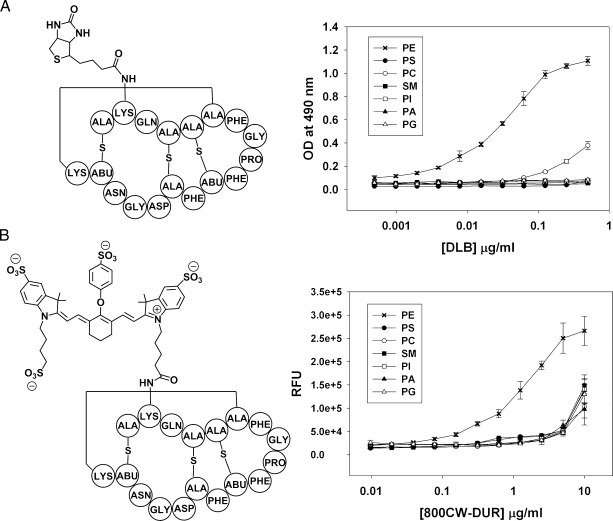
Figure 1.
Lipid specificity of the PE binding probes DLB and 800CW-DUR. (A) Structure of duramycin-linked biotin (DLB) and ELISA showing DLB binds specifically to PE. (B) Structure of duramycin conjugated to the near-infrared fluorophore IRDye 800CW (800CW-DUR) and ELISA showing that 800CW-DUR also retains binding specificity for PE. The structures show conjugation through the preferred lysine, although conjugation can also occur at the N-terminus.
Duramycin was reacted with the NHS ester of IRDye 800CW at a 1:1 molar ratio as described above. The product consisting of one molecule of duramycin linked to one molecule of 800CW was purified by high-performance liquid chromatography. Mass spectrometry confirmed a molecular mass of 2998 Da (Figure 1B). linDUR reacted with IRDye 800CW produced the control peptide referred to as 800CW-linDUR. Both 800CW-DUR and 800CW-linDUR were supplemented with an equal weight of unlabeled duramycin for all assays.
Cells
Adult bovine aortic endothelial (ABAE) cells were obtained form Clonetics (Walkersville, MD). RM-1 mouse prostate carcinoma, RM-9 mouse prostate carcinoma, 4T1 mouse breast carcinoma, B16 mouse melanoma, and A549 human lung carcinoma cells were obtained from American Type Cell Collection (Rockville, MD). MDA-MB-231 human breast carcinoma cells were provided by Dr Robert Kerbel (Sunnybrook Health Sciences Center, Toronto, Ontario, Canada). All cells were maintained in Dulbecco modified Eagle medium supplemented with 10% FBS and 2 mM l-glutamine. Cells were trypsinized with 0.25% trypsin and 2.1 mM EDTA (Mediatech Inc, Manassas, VA).
Growth of Subcutaneously Implanted Tumors
For localization studies, 106 RM-1, RM-9, or B16 cells were injected into the right-hind flank of male C57BL/6 mice (UTSW breeding core). A549 cells (5 x 106 cells) were injected into the right-hind flank of female athymic nu/nu mice (Charles River, Fredrick, MD). Tumors were allowed to grow to a volume of 0.7 to 1.0 cm3.
Orthotopic MDA-MB-231 and 4T1 Breast Carcinoma Models
Female nu/nu or severe combined immunodeficient mice were purchased from Charles River. MDA-MB-231 or 4T1 cells (5 x 106) suspended in 0.1 ml were implanted into the mammary fat pad as described previously [20]. Briefly, mice were anesthetized, and a 5-mm incision was made in the skin over the lateral thorax. The mammary fat pad was exposed to ensure the correct site of implantation, and tumor cells were injected with a 25-gauge needle. The incision was closed with a wound clip that was removed 5 to 7 days later.
MMTV-PyMT Transgenic Mice
Mouse mammary tumor virus (MMTV) promoter-driven expression of polyoma middle T antigen (PyMT) results in mammary gland-specific adenocarcinoma formation [21]. Female MMTV-PyMT mice were obtained from the laboratory of Dr Rolf Brekken at UT Southwestern Medical Center (Dallas, TX).
Antiphospholipid ELISAs
To test the binding of DLB and 800CW-DUR, phospholipids were dissolved in n-hexane (10 µg/ml), and 50 µl of this solution was added to wells of Immulon 1B or LUMITRAC 96-well plates. The solvent was evaporated at room temperature, and the plates were blocked for 1 hour with 5% FBS dissolved in PBS. DLB and 800CW-DUR were diluted in blocking buffer at an initial concentrations of 1 and 10 µg/ml, respectively, and twofold dilutions were performed in a separate 96-well plates (100 µl/well). Phospholipid-coated plates were washed with PBS, and DLB and 800CW-DUR were transferred from dilution plates to Immulon 1B and LUMITRAC plates, respectively. The plates were incubated for 1 hour at room temperature and then washed. Bound 800CW-DUR was detected using an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE). Bound DLB was detected using HRP-conjugated streptavidin (1:2000 in blocking buffer) and ODP substrate. The optical density of ODP was read at 490 nm using a microplate reader (BioTek Instruments, Winooski, VT).
Hemolysis Assay
Fresh mouse blood was treated with heparin (15 IU/ml) and centrifuged (300g for 5 minutes) to sediment the red cells. After three washes, the red cells were resuspended in 10x the original volume of blood. Duramycin or DLB was serially twofold diluted in PBS in 100-µl volumes in 96-well round-bottomed microplates. The red cell suspension (100 µl/well) was added. The plates were kept at room temperature for 1 hour. Supernatants were removed, and their absorbance was measured at 575 nm in an ELISA reader.
Immunohistochemical Detection of Exposed PE on the Surface of Cultured Endothelial Cells
ABAE cells were cultured in eight-well chamber slides (BD Falcon, Bedford, MA) until approximately 80% confluent. To induce PE exposure, cells were irradiated with 5 Gy 24 hours before staining. Untreated cells were used as a control. Cells were incubated with DLB (0.5 µg/ml) dissolved in culture media. linDLB (0.5 µg/ml) was used as a negative control. Bavituximab (1 µg/ml) was used as a positive control for detection of PS. The cells were then washed with PBS and fixed with 4% paraformaldehyde for 10 minutes. Excess aldehyde groups were quenched with 50 mM ammonium chloride for 5 minutes. Biotinylated peptides were detected by incubating Alexa Fluor 488- or Cy3-conjugated streptavidin (1:1000 in 1% BSA) for 30 minutes. Cell membranes were permeablized with 0.5% Triton X-100 (5 minutes), and cytoskeletons were stained with Texas Red-labeled phalloidin (1:200 in 1% BSA; 30 minutes). Cell nuclei were counterstained with DAPI.
Effect of ROS, Hypoxia, pH, and VEGF on PE Exposure in Cultured EC
For all treatments, ABAE cells were cultured in 75-cm2 flasks (Corning, Inc, Lowell, MA) until approximately 80% confluent. To mimic ROS in tumors, cells were treated with 10 µM H2O2 in serum-free medium for 1 hour. To study the effect of hypoxia, flasks of cells were placed in a humidified normoxic atmosphere (21% O2, 5% CO2) for 48 hours before being transferred to a humidified hypoxic atmosphere (1% O2, 5% CO2, 94% N2) in a sealed chamber (BioSpherix, Lacona, NY). The cells were incubated in the hypoxic chamber at 37°C for 24 hours and were then returned to a normoxic environment for 4 hours at 37°C. To study the effect of acidity, cells were incubated for 24 hours in bicarbonate-free medium adjusted to pH 5.8 with HCl at 37°C and in the absence of CO2. Untreated cells from an identical passage were used as controls. After all treatments, cells were trypsinized for 1 minute, washed, and resuspended in ice-cold FACS buffer (PBS with 10% FBS and 0.02% NaN3). To detect externalized PE, cells were incubated with DLB (0.5 µg/ml) for 1 hour. linDLB (0.5 µg/ml) was used as a negative control. Bavituximab (20 µg/ml) was used to detect exposed PS, and rituxan (20 µg/ml) was used as a nonbinding control for bavituximab. Cells were washed, and bound peptides were detected with Alexa Fluor 488-conjugated streptavidin (1:3000 in FACS buffer), and bound antibodies were detected with goat antihuman IgG-Cy2 (1:3000). Apoptotic cells were identified with propidium iodide. The cells were analyzed with a FACScan flow cytometer (BD Biosciences).
Localization of Duramycin Peptides in Tumor-Bearing Mice In Vivo
To detect PE on the surface of EC, DLB (100 µg) was injected intravenously into tumor-bearing mice. Bavituximab (150 µg) and linDLB (100 µg) were used as positive and negative controls, respectively. The mice were killed after 60 minutes and perfused with heparinized saline as described previously [15,22]. The tumor and major organs were removed and snap-frozen, and 10-µm cryosections were cut. Sections were incubated overnight with rat antimouse CD31 antibody (1:200 in 1% BSA). Goat antirat IgG-Cy3 was used to detect the CD31 antibody. DLB was detected with Alexa Fluor 488-conjugated streptavidin. Bavituximab was detected with goat antihuman IgG-Cy2. Cell nuclei were counterstained with DAPI. PE-positive vessels were classified as DLB+, CD31+-costained structures having vascular endothelial cell morphology.
Detection of Hypoxia in PE-Positive Tumors
One hundred micrograms of DLB was injected intravenously into male C57BL/6 mice bearing RM-9 tumors. Immediately afterward, 2 mg of pimonidazole HCl (Hypoxyprobe-1 kit, Burlington, MA) was injected intraperitoneally. After 1 hour, tumors were harvested and sections were prepared as described previously [22]. Sections were incubated overnight with rat antimouse CD31 IgG antibody (1:200) and fluorescein isothiocyanate-labeled antibody against the pimonidazole adduct (Hypoxyprobe-1 kit) (1:50) in 1% BSA. The following day, sections were stained with saturating amounts of Cy3-conjugated streptavidin (1:500) to detect DLB. After washing, the sections were incubated with biotinylated goat antirat IgG (1:500) followed with Alexa Fluor 350-conjugated streptavidin (1:500) to detect the CD31 antibody. Immunohistochemical staining for pimonidazole adduct formation was also used to compare levels of hypoxia in similar-sized (1-cm-diameter) RM-9 tumors and 4T1 tumors. For each tumor type, multiple fluorescent images were captured at low magnification (100x), and hypoxia staining was quantified using ImageJ software.
Imaging of Tumors with 800CW-DUR
In vivo near-infrared fluorescence imaging was performed using a Xenogen IVIS Lumina imaging system (Xenogen, Alameda, CA). 800CW-DUR (50 µg) was injected into a tail vein in male mice bearing RM-9 tumors. 800CW-linDUR (50 µg) was used as a control. Twenty-four hours after injection, mice were anesthetized with 2% isoflurane (Halocarbon, North Augusta, SC), and images were obtained. Fluorescence (photons/sec per squared centimeter per steradian) was detected using the NIR filter set (excitation between 605 and 780 nm and emission between 810 and 885 nm).
Results
Modification of Duramycin to Create the PE-Binding Probes DLB and 800CW-DUR
DLB was produced by reacting duramycin with the NHS ester of l-biotin (Figure 1A). Lipid binding was assessed by ELISA. Figure 1A shows that DLB binding was specific for PE with maximum binding occurring at ∼0.5 µg/ml (0.2 µM). Although some binding to PC was observed at the higher concentrations, binding to PS, PI, PA, and SM was undetectable. 800CW-DUR for noninvasive in vivo imaging was produced by reacting duramycin with the NHS ester of IRDye 800CW (Figure 1B). Similar to DLB, the IR probe (800CW-DUR) demonstrated specificity for PE up to ∼5 µg/ml (Figure 4B). Maximal binding occurred at ∼5 µg/ml (1.7 µM). Thus, modification of duramycin by conjugation to l-biotin did not change its affinity or specificity of binding to PE. DLB lysed 50% of murine red cells at 80 µM compared with 20 µM for duramycin itself, showing that biotinylation reduced the hemolytic activity of duramycin by fourfold.
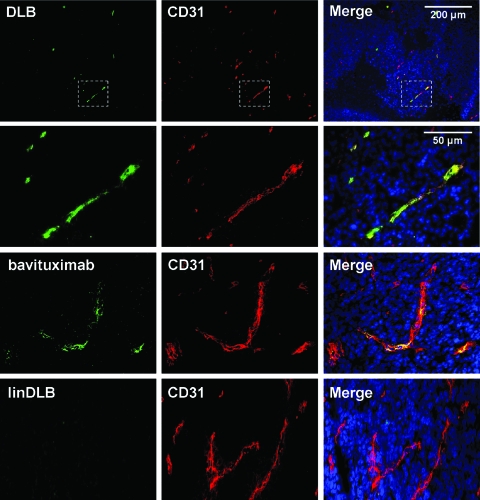
Figure 4.
Exposure of PE on vascular endothelial cells in tumors. One hundred micrograms of DLB was injected intravenously into mice bearing subcutaneous RM-9 prostate tumors. After 1 hour, mice were perfused, and tumors and major organs were harvested. Immunohistochemical staining of frozen sections revealed that DLB (green) colocalized with the pan-endothelial marker CD31 (red) in tumors. PE exposure was observed in multiple tumor types (Table 1). Bavituximab produced a similar staining pattern to DLB.
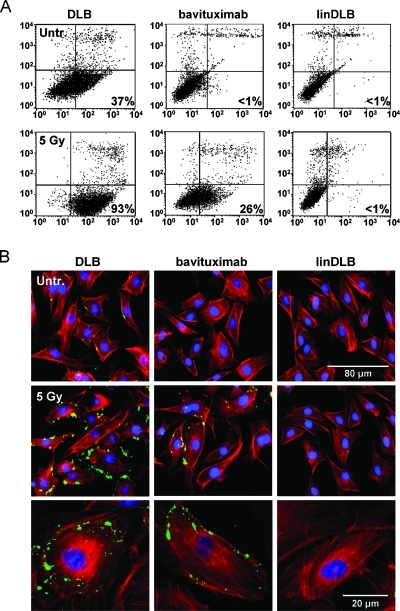
DLB Binds to PE on the Surface of Irradiated EC
We have previously shown that binding of fluorescent liposomes coated with duramycin or bavituximab to ABAE cells is enhanced by x-irradiation [2]. Here, we used biotinylated duramycin (DLB) with FACS analysis and immunofluorescence staining to detect PE on the surface of nonirradiated and irradiated ABAE cells. Figure 2A shows that DLB bound to 37% of control ABAE cells. Irradiation increased DLB binding to 93% of the cells and increased their median fluorescence intensity by fourfold to fivefold. Consistent with previous data showing that irradiation induces PS externalization, immunofluorescence analysis of the cells 24 hours after 5-Gy irradiation showed that both DLB (PE) and bavituximab (PS) bound to membrane blebs (Figure 2B).
Figure 2.
DLB binds PE exposed on cultured EC. DLB was used to detect PE on the surface of irradiated ABAE cells. (A) FACS analysis of DLB-labeled ABAE cells that were untreated (upper panels) or had been irradiated with 5 Gy 24 hours earlier (lower panels). (B) Cells were incubated with DLB or bavituximab, and binding was detected with streptavidin-Alexa Fluor 488 and Cy2-goat antihuman Ig, respectively. LinDLB was used as a negative control for DLB. The cells were counterstained with Texas Red-labeled phalloidin.
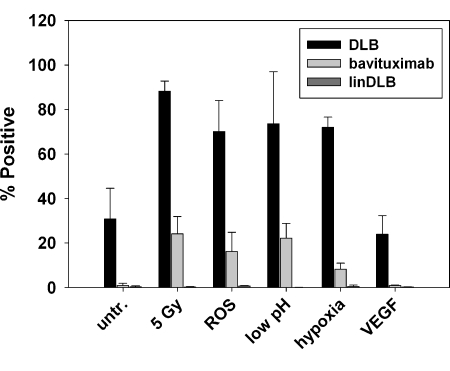
Induction of PE Exposure on Cultured EC by ROS, Hypoxia, and Low pH
Flow cytometry was used to assess PE levels on EC after treatment with different inducers of oxidative stress present in the tumor microenvironment. About 30% of cultured EC had exposed PE before treatment. Irradiation, H2O2, low pH, and hypoxia increased the percentage of cells expressing PE from ∼35% to between 70% and 90% (Figure 3). Treatment with VEGF did not influence the externalization of PE. Similar trends were seen when bavituximab was used to stain externalized PS. Bavituximab did not stain resting EC, but stained 10% to 25% of the cells after they had been treated with irradiation, H2O2, low pH, or hypoxia.
Figure 3.
Multiple stresses associated with the tumor microenvironment induce increased PE exposure on cultured EC. Flow cytometry was used to assay PE levels on ABAE cells after 1) treatment with 10 µM H2O2 to simulate ROS, 2) incubation in medium at pH 5.8 for 24 hours, 3) incubation under hypoxic conditions (1% O2, 94% N2, 5% CO2) followed by reoxygenation for 4 hours (5% CO2 in air), and 4) treatment with 20 ng/ml VEGF. Irradiation at 5 Gy was used as a positive control. Bavituximab was used to detect PS externalization. Rituxan gave no staining (not shown). LinDLB was used as a negative control for DLB.
DLB Localizes to Tumor Blood Vessels
Intravenously injected DLB localized to tumor vessels in all tumors examined (Table 1 and Figure 4). PE-positive vessels were identified from colocalization of DLB with CD31-stained structures having a vascular morphology. Among the implanted solid tumor models, the endothelium in syngeneic RM-1 and RM-9 prostate carcinomas displayed the highest levels of exposed PE with ∼50% of the vessels staining positive. Orthotopically implanted human (MD-AMB-231) and mouse (4T1) breast carcinomas displayed ∼29% and ∼13% PE-positive blood vessels, respectively. Spontaneously developing breast tumors in MMTV-PyMT transgenic mice contained 44% PE-positive vessels (Figure W1).
Table 1.
Percentage of PE-Positive Blood Vessels in Various Tissues.
| Tissues | DLB | Bavituximab | linDLB |
| Tumors | |||
| RM-1 | 47 ± 9 | 58 ± 12 | 0 |
| RM-9 | 56 ± 3 | 60 ± 8 | 0 |
| MDA-MB-231 | 29 ± 5 | 31 ± 8 | 0 |
| 4T1 | 13 ± 3 | 28 ± 5 | 0 |
| B16 | 17 ± 5 | 18 ± 7 | 0 |
| A549 | 21 ± 7 | 23 ± 6 | 0 |
| MMTV-PyMT | 41 ± 3 | 0 | |
| Normal | |||
| Heart | 0 | 0 | 0 |
| Lung | 0 | 0 | 0 |
| Liver | 0 | 0 | 0 |
| Kidney | 45 ± 10 | 0 | 0 |
| Spleen | 0 | 0 | 0 |
| Stomach | 0 | ||
| Intestine | 0 | ||
| Bladder | 0 | ||
| Muscle | 0 | ||
| Fat | 0 | ||
| Brain | 0 | ||
| Testes | 0 |
The fraction of PE-positive blood vessels in each tumor model correlated with the fraction of PS-positive blood vessels detected with bavituximab (Table 1 and Figure 4). Coinjection of DLB and bavituximab into mice bearing subcutaneous RM-9 tumors revealed that the same tumor blood vessels were positive for both PE and PS (Figure W2). The pattern of DLB staining generally resembled that of bavituximab, with both demonstrating relatively homogeneous costaining with CD31. However, some DLB-positive vessels also exhibited punctate staining (as in Figure W2) that may indicate regions of endothelium having membrane blebs similar to those seen on cells in tissue culture. DLB bound specifically to PE exposed on the tumor endothelium and did not seem to extravasate from the blood vessels and bind tumor cells or become trapped within the interstitium. Intravenously injected linDLB did not localize to tumor blood vessels.
The binding of DLB to vasculature was largely restricted to tumor blood vessels. DLB did not localize to the endothelium in the heart, lung, liver, spleen, stomach, intestine, muscle, fat, brain, or testis (Table 1 and Figure W3). In the kidney, DLB did not stain endothelium in the glomeruli or larger vessels but did stain endothelium in intertubular vessels between the distal tubules. DLB also showed diffuse staining of the distal kidney tubules themselves and, to a lesser extent, of proximal tubules. DLB uptake by the kidneys was not unexpected because the urinary system is the main route by which duramycin is eliminated from the body [23,24]. DLB also stained a population of CD31-negative cells in the liver, which appeared from their morphology to be Kupffer cells.
DLB Binding Is High in Hypoxic Areas of Tumors
Abnormal angiogenesis in tumors often results in malformed blood vessels that fail to provide adequate blood flow throughout the tumor. Therefore, specific areas within a tumor are subject to acute ischemic hypoxia that is often transient [25,26]. Because hypoxia induced PE exposure in in vitro-cultivated EC (Figure 3), we determined whether tumors growing in mice also expressed PE in hypoxic regions. Mice bearing similar-sized (1-cm-diameter) RM-9 or 4T1 tumors were injected with DLB to detect PE and pimonidazole to assess hypoxia. The mice were killed 1 hour after injection and perfused with saline, and frozen sections of tumors were examined by immunohistochemistry. The results presented in Figure 5 show a correlation between the distribution of PE-positive (red) EC and hypoxic regions (green) of the tumor. RM-9 tumors, in which PE-positive vessels were abundant, had large areas of hypoxia throughout the tumor, whereas 4T1 tumors, in which PE-positive vessels were sparse, had smaller, more localized areas of hypoxia and much less hypoxia overall (Figure W4). Hypoxic regions were most pronounced around necrotic regions and in central areas of the tumor. Consistent with previous localization experiments, DLB stained greater than 50% of the blood vessels in the RM-9 tumors. Most of the DLB-positive vessels were located in and around the hypoxic regions (Figure 5, top right panel). The control peptide linDLB did not bind tumor EC (not shown).
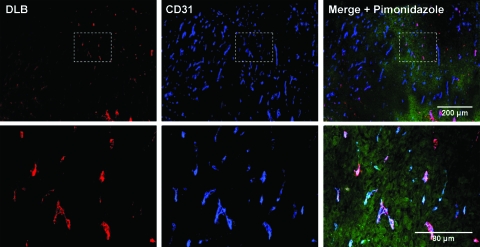
Figure 5.
PE exposure on tumor vessels is predominately in hypoxic areas. Mice bearing subcutaneous RM-9 prostate tumors were injected with DLB to detect PE and pimonidazole HCl to detect hypoxia. Immunohistochemical staining of frozen sections demonstrated that PE exposure on tumor vessels occurred predominantly in hypoxic areas of the tumor. Blue indicates CD31; green, hypoxia; red, DLB.
In Vivo Tumor Imaging with 800CW-DUR
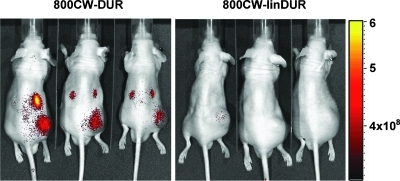
To determine the ability of duramycin to target tumor vasculature in vivo, mice bearing subcutaneously implanted RM-9 tumors were injected with 800CW-DUR. The distribution of the probe was detected with the Xenogen IVIS imaging system (Xenogen, Alameda, CA) 24 hours later. Images are shown in Figure 6. Remarkable delineation of the implanted tumors was achieved in three of three mice. No other normal tissues showed detectable accumulation of label, with the exception of the kidneys that were strongly labeled.
Figure 6.
In vivo imaging of RM-9 tumors with 800CW-DUR. Male athymic nu/nu mice bearing subcutaneous RM-9 tumors in their right-hind flank were injected with 50 µg of 800CW-DUR. 800CW conjugated to linear duramycin was used as a negative control (800CW-linDUR). The mice were imaged after 24 hours with a Xenogen IVIS Lumina imaging system. Fluorescence intensity is displayed as photons per second per squared centimeter per steradian.
Discussion
The major finding of this study is that PE becomes exposed on the luminal surface of the vascular endothelium of tumors. PE exposure is the most prominent in and around regions of hypoxia and is probably induced by oxidative stresses in the tumor microenvironment.
The PE-binding peptide duramycin tagged with a single molecule of L-biotin was used to assess the presence of PE on the cell surface. Biotinylation seems to have reduced the ability of duramycin to disrupt cell membranes because the hemolytic activity of DLB was four-fold lower than that of duramycin itself. This is likely attributable to the increased hydrophilic character imparted by the water-soluble biotin moiety. This change may explain why DLB stained cell surface structures but not intracellular membranes at the concentrations tested.
DLB staining indicated a fourfold to fivefold increase in exposure of PE on irradiated EC. DLB strongly stained membrane blebs that formed on the surface after irradiation. The pattern of DLB staining in irradiated ABAE closely resembled that of bavituximab, suggesting that both PE and PS preferentially localize to membrane patches and blebs [2]. Unlike PS, basal levels of PE are exposed on normal (untreated) ABAE cells, either naturally or because ABAE cells do not readily enter a fully resting state in culture. These observations are consistent with data indicating that essentially all the membrane PS and ∼80% of the membrane PE are restricted to the cells plasma membrane inner membrane leaflet [27]. Increased PE exposure has also been shown at the cell surface along the cleavage furrow in Chinese hamster ovary cells during cytokinesis and in a subset of EC in the rat aorta [28,29]. Although PS and PE are both externalized during apoptosis and in response to stress, PS is more highly restricted to the inner membrane leaflet in healthy cells.
FACS analysis revealed that stress conditions associated with the tumor microenvironment induced significant PE exposure when applied to in vitro-cultivated EC. Tumors are known to contain high levels of ROS owing to a number of dysregulated metabolic processes. These include growth factor-mediated activation of mitochondria, inflammatory responses from tumor-infiltrating leukocytes, and activation/overexpression of enzymes such as NADPH-oxidase [30–32]. Transient hypoxia in tumors can lead to ROS generation by this last mechanism (i.e., activation of NADPH-oxidase) [13]. In addition to high ROS levels, the tumor microenvironment is also often characterized by low pH relative to normal tissues. Tumor cells can undergo high rates of glycolysis irrespective of oxygen tension (the Warburg effect) and generate lactic acid as a byproduct [33]. We found that the fraction of ABAE that expressed PE at the cell surface after treatment with ROS (H2O2), hypoxia, or acidic medium significantly increased (Figure 3). These stresses also induced PS exposure. These observations suggest that ROS, hypoxia, and acidic pH, possibly in concert with other factors (e.g., inflammatory cytokines and thrombin), promote the exposure of PE and PS in tumors.
Without exception, increased PE exposure was found on the tumor endothelium in all the tumors examined. These models included syngeneic, human xenografts, subcutaneous, orthotopic, and transgenic tumors. This suggests that, much like PS, PE functions as a broad tumor marker common to many malignancies. The percentage of PE-positive vessels varied between models but corresponded to the fraction of PS-positive vessels (determined by bavituximab binding) for each type of tumor. Moreover, the exposure of PE and PS occurred on the same tumor vessels, suggesting that the same mechanism is responsible for the redistribution of both PE and PS. Tumor-bearing mice were coinjected with DLB and pimonidazole HCl to assess hypoxia in areas immediately surrounding PE-positive blood vessels. A general correlation between DLB binding and pimonidazole staining showed that PE-positive vessels were concentrated in hypoxic portions of the tumor. RM-9 tumors, which have abundant PE-positive vessels, were markedly hypoxic, whereas 4T1 tumors, which have relatively few PE-positive vessels, were largely not hypoxic. These results further indicate that hypoxia and other stresses in hypoxic tumor regions drive PE exposure on tumor vasculature.
Despite evidence from in vitro experiments that normal EC contain basal levels of PE on their surface, DLB did not localize to endothelium in heart, lung, liver, spleen, stomach, intestine, muscle, fat, brain, or testis. This difference might be because EC grown in vitro are activated because of sustained low levels of chronic stress that promote higher levels of exposed PE, whereas EC in normal tissues in vivo attain true quiescence. The transport enzymes that maintain membrane lipid asymmetry have a higher affinity for PS, and its rate of PS transport is approximately 10-fold higher than its rate of PE transport [27,34]. This could explain why stress has a more profound effect on the redistribution of PE.
The selective exposure of PE on tumor vascular endothelium suggested that it might serve as a marker for imaging tumor vasculature. Being luminally exposed and in direct contact with the blood, we reasoned that fluorescently labeled derivatives of duramycin would localize rapidly and specifically to tumor endothelium. To test this hypothesis, we labeled duramycin with a near-infrared dye, 800CW, and injected it into mice bearing RM-9 prostate tumors. The biodistribution of injected 800CW-DUR confirmed that the levels of exposed PE are indeed high in tumors. 800CW-DUR binding to RM-9 tumors allowed them to be clearly distinguished above background fluorescence, suggesting that PE-specific probes may be a powerful tool for tumor imaging. However, both 800CW-DUR and DLB showed high uptake in the kidneys, as observed by Zhao and Bugenhagen [19] with 99mTc-duramycin. Immunohistochemical analysis revealed that DLB localized strongly to the distal kidney tubules and less strongly to the proximal kidney tubules. DLB also stained intertubular blood vessels between distal tubules, although it did not bind endothelium in larger renal vessels or the glomeruli. Other studies have shown that duramycin forms ion channels in artificial membranes and can disrupt mitochondrial membranes at concentrations greater than 5 µM [35,36]. Cinnamycin has also been shown to be toxic and promote its own binding by inducing flipping of membrane lipids [37]. It is possible that, as the kidneys filter DLB or 800CW-DUR from the blood, local concentrations of the drug in the collecting ducts and the associated vasculature increase to the extent that it disrupts membranes and exposes PE.
In conclusion, the data presented in this report demonstrate that PE becomes exposed on the luminal surface of tumor vascular endothelium. PE seems to be a broad marker found in a number of mouse models of solid malignancies including spontaneously developing tumors. We demonstrate that PE on tumor vasculature can be imaged with PE-targeting probes. These probes are small compared with PS-targeting antibodies and annexins, and clear rapidly from the bloodstream making them particularly suitable for imaging purposes. In addition, PE is more abundant than PS, giving the potential for stronger signals. Indeed, duramycin labeled with 99mTc is being developed for imaging exposed PE in cardiac ischemia [19]. The results reported herein suggest that PE also has potential as a marker for imaging human malignancies.
Supplementary Material
Acknowledgments
The authors thank Shuzhen Li for her expert technical assistance and Alan J. Schroit for his comments on the article.
Footnotes
This research was supported in part by a sponsored research agreement with Peregrine Pharmaceuticals, Inc. (Tustin, CA) and funding from the Gillson Longenbaugh Foundation (TX). Imaging was facilitated by the Southwestern Small Animal Imaging Resource (SW-SAIR), which is supported in part by the NCI U24 CA126608, the Harold C. Simmons Cancer Center, which is supported in part by an NCI Cancer Center Support Grant, CA142543-01, and the Department of Radiology.
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Williamson P, Schlegel RA. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol. 1994;11:199–216. doi: 10.3109/09687689409160430. [DOI] [PubMed] [Google Scholar]
- 2.Marconescu A, Thorpe PE. Coincident exposure of phosphatidylethanolamine and anionic phospholipids on the surface of irradiated cells. Biochim Biophys Acta. 2008;1778:2217–2224. doi: 10.1016/j.bbamem.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Devaux PF. Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 5.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232:430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 7.Bitbol M, Fellmann P, Zachowski A, Devaux PF. Ion regulation of phosphatidylserine and phosphatidylethanolamine outside-inside translocation in human erythrocytes. Biochim Biophys Acta. 1987;904:268–282. doi: 10.1016/0005-2736(87)90376-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 9.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335(pt 3):465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi Y, Ohba T, Miyata H, Ohki K. Rapid phase change of lipid microdomains in giant vesicles induced by conversion of sphingomyelin to ceramide. Biochim Biophys Acta. 2006;1758:145–153. doi: 10.1016/j.bbamem.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Kornberg RD, McConnell HM. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971;10:1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- 13.Zulueta JJ, Yu FS, Hertig IA, Thannickal VJ, Hassoun PM. Release of hydrogen peroxide in response to hypoxia-reoxygenation: role of an NAD(P)H oxidase-like enzyme in endothelial cell plasma membrane. Am J Respir Cell Mol Biol. 1995;12:41–49. doi: 10.1165/ajrcmb.12.1.7529030. [DOI] [PubMed] [Google Scholar]
- 14.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 15.Ran S, He J, Huang X, Soares M, Scothorn D, Thorpe PE. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin Cancer Res. 2005;11:1551–1562. doi: 10.1158/1078-0432.CCR-04-1645. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto K, Hayakawa T, Murate M, Makino A, Ito K, Fujisawa T, Kobayashi T. Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophysical Journal. 2007;93:1608–1619. doi: 10.1529/biophysj.106.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi F, Nagashima K, Terui Y, Kawamura Y, Matsumoto K, Itazaki H. The structure of PA48009: the revised structure of duramycin. J Antibiot (Tokyo) 1990;43:1421–1430. doi: 10.7164/antibiotics.43.1421. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann N, Freund S, Fredenhagen A, Jung G. Solution structures of the lantibiotics duramycin B and C. Eur J Biochem. 1993;216:419–428. doi: 10.1111/j.1432-1033.1993.tb18159.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Li Z, Bugenhagen S. 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J Nucl Med. 2008;49:1345–1352. doi: 10.2967/jnumed.107.048603. [DOI] [PubMed] [Google Scholar]
- 20.Price JE. Metastasis from human breast cancer cell lines. Breast Cancer Res Treat. 1996;39:93–102. doi: 10.1007/BF01806081. [DOI] [PubMed] [Google Scholar]
- 21.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows FJ, Watanabe Y, Thorpe PE. A murine model for antibody-directed targeting of vascular endothelial cells in solid tumors. Cancer Res. 1992;52:5954–5962. [PubMed] [Google Scholar]
- 23.Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther. 2010;87:39–47. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Montfoort JE, Hagenbuch B, Groothuis GM, Koepsell H, Meier PJ, Meijer DK. Drug uptake systems in liver and kidney. Curr Drug Metab. 2003;4:185–211. doi: 10.2174/1389200033489460. [DOI] [PubMed] [Google Scholar]
- 25.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 26.Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 27.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci USA. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Wells CW, Esmon CT, Zhao M. Phosphatidylethanolamine at the endothelial surface of aortic flow dividers. J Thromb Haemost. 2009;7:227–229. doi: 10.1111/j.1538-7836.2008.03193.x. [DOI] [PubMed] [Google Scholar]
- 30.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 31.Fantone JC, Ward PA. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- 32.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Lazaro M. TheWarburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem. 2008;8:305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- 34.Zachowski A, Favre E, Cribier S, Herve P, Devaux PF. Outside-inside translocation of aminophospholipids in the human erythrocyte membrane is mediated by a specific enzyme. Biochemistry. 1986;25:2585–2590. doi: 10.1021/bi00357a046. [DOI] [PubMed] [Google Scholar]
- 35.Sheth TR, Henderson RM, Hladky SB, Cuthbert AW. Ion channel formation by duramycin. Biochim Biophys Acta. 1992;1107:179–185. doi: 10.1016/0005-2736(92)90345-m. [DOI] [PubMed] [Google Scholar]
- 36.Sokolove PM, Westphal PA, Kester MB, Wierwille R, Sikora-VanMeter K. Duramycin effects on the structure and function of heart mitochondria. I. Structural alterations and changes in membrane permeability. Biochim Biophys Acta. 1989;983:15–22. doi: 10.1016/0005-2736(89)90374-x. [DOI] [PubMed] [Google Scholar]
- 37.Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, et al. Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J Biol Chem. 2003;278:3204–3209. doi: 10.1074/jbc.M210347200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.