Abstract
A high expression of short and immature O-glycans is one of the prominent features of breast cancer cells, which would be attributed to the upregulated expression of glycosyltransferases. Therefore, a detailed elucidation of glycosyltransferases and their substrate(s) may improve our understandings for their roles in mammary carcinogenesis. Here we report that overexpression of polypeptide N-acetylgalactosaminyltransferase 6 (GALNT6), a glycosyltransferase involved in the initial step of O-glycosylation, has transformational potentials through disruptive acinar morphogenesis and cellular changes similar to epithelial-to-mesenchymal transition in normal mammary epithelial cell, MCF10A. As one of the critical O-glycan substrates, we identified fibronectin that was O-glycosylated in vivo and thereby stabilized by GALNT6. Because knockdown of fibronectin abrogated the disruptive proliferation caused by introduction of GALNT6 into epithelial cells, our findings suggest that GALNT6-fibronectin pathway should be a critical component for breast cancer development and progression.
Introduction
Alterations in O-type glycosylation of breast cancer cells induce diverse biologic and pathologic consequences influencing growth and survival of the cells and their ability for invasion and metastasis [1]. Accumulating evidences have shown that aberrant expression of glycosyltransferases confers the altered O-glycan structures in breast cancer cells [2,3]. In this regard, we previously characterized oncogenic roles of GALNT6 that was upregulated exclusively in breast cancer cells and regulated cell proliferation and cytoskeleton structure [4]. In accordance to biologic relevance, GALNT6 expression was related with poor prognosis of breast cancer patients, indicating its application as a molecular marker for risk of cancer metastasis [5]. However, the molecular mechanism of how GALNT6 contributes to breast malignancy by enhancing the O-glycosylation remains unclear.
The three-dimensional culture of MCF10A mammary epithelial cells was developed to assess oncogenic ability by monitoring the disruption of well-ordered architecture of mammary gland, which is regarded as an early aspect of mammary carcinogenesis [6]. Under the three-dimensional culture conditions, MCF10A cells form the acinar structure composed of a monolayer of polarized cells, but the well-organized architecture was disrupted by the introduction of certain cancer-related genes [7–9]. In addition to investigating cell morphogenesis, the three-dimensional culture-based monitoring system provides more accurate physiological conditions to assess oncogenic functions related to invasive behavior and epithelial-to-mesenchymal transition (EMT) [7].
In this study, we generated GALNT6-expressing MCF10A stable transfectants and clarified transformational potentials of GALNT6 by the three-dimensional culture method in the aspects of disruption of acinar structure formation as well as EMT-like cellular alterations.
Materials and Methods
Cell Lines
Human breast cancer cell lines (T47D and HCC1500) and an immortalized breast epithelial cell line (MCF10A) were purchased from American Type Culture Collection (Rockville, MD) and Cambrex Bioscience (Walkersville, MD), respectively. Detailed cell culture and test methods were previously described [4]. The cell viability was assessed by Cell Counting Kit 8 (Dojindo, Kumamoto, Japan).
Three-dimensional Cell Culture with MCF10A-GALNT6 Stable Transfectants
Each of the pCAGGS-HAc expression vectors of mock (no insert), GALNT6 wild-type (WT), and inactivated GALNT6 mutant (H271D) was transfected into MCF10A cells using FuGENE6 transfection reagent (Roche, Basel, Switzerland), as previously described [4]. Then, the positive clones were selected under incubation with culture medium containing 0.4 mg/ml of Geneticin (Invitrogen, Carlsbad, CA) and validated by Western blot analysis and immunocytochemical staining. The cells were seeded on the growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) and maintained in mammary epithelial cell medium media (Cambrex Bioscience) with 2% of the Matrigel [6]. Each size of cell colonies was quantified by ImageJ software by National Institutes of Health (Bethesda, MD) [10].
Western Blots and Immunocytochemical Staining
SDS-PAGE, immunoprecipitation, and immunocytochemical staining were performed as described previously [4], with anti-E- or N-cadherins (BD Biosciences), anti-fibronectin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin (Sigma-Aldrich, St Louis, MO), anti-integrin α6 (Chemicon, Billerica, MA), and anti-active caspase-3 (Abcam, Cambridge, UK) antibodies. The cytoskeleton structure was visualized by staining with Alexa Fluor-488/594 phalloidins (Molecular Probe, Eugene, OR). The GalNAc (N-acetyl-α-d-galactosamine)-conjugated glycoproteins were detected by Vicia villosa (VVA) lectin (Vector, Burlingame, CA) with or without incubation with recombinant GALNT6 proteins in vitro [4].
Knockdown Experiments
To deplete endogenous expression of fibronectin, we introduced a synthesized oligo-duplex small interfering RNA (siRNA) against the FN1 gene (5′-AAGTGGTCCTGTCGAAGTATT-3′) into MCF10A-GALNT6 stable transfectants once a week [11]. We used si-GALNT6 (5′-GAGAAAUCCUUCGGUGACA-3′) to deplete endogenous expression of GALNT6 and used si-EGFP (5′-GAGAAAUCCUUCGGUGACA-3′) as a control [4]. Semiquantitative reverse transcription-polymerase chain reaction was performed as described previously [12]. The polymerase chain reaction primer sequences were 5′-CTGCAGTATATCCGCTTAGCC-3′ and 5′-TAAGTCCATGCAAAGGAGACTAGC-3′ for ACTB and 5′GGAGTTGATTATACCATCACTG-3′ and 5′-TTTCTGTTTGATCTGGACCT-3′ for FN1 (GenBank no. NM_002026).
Statistical Analysis
Statistical significance was examined by Student's t test using R statistical package [13]. A difference of P < .05 was considered to be statistically significant.
Results
Breast Cancer Cells Express Multiple O-glycan Substrates of GALNT6
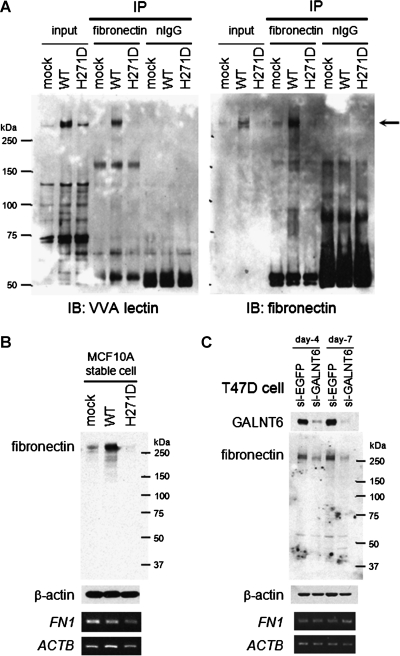
MUC1 was one of important substrates of GALNT6 in breast cancer cells (T47D, MCF7, and SKBR3). However, our previous findings indicated the presence of additional O-glycan substrates that would be O-glycosylated by GALNT6 [4]. Indeed, VVA-lectin Western blot analysis indicated the presence of multiple substrates whose O-glycosylations were diminished by knockdown of GALNT6 (Figure 1A, lane 1 vs 4). The glycosylations of these candidate bands were restored by in vitro incubation with recombinant WT GALNT6 protein (Figure 1A, lane 4 vs 5). This result was in concordance with our previous speculation that GALNT6 might have additional functions through O-glycosylation of unidentified substrates [4].
Figure 1.
Overexpression of GALNT6 in MCF10A cell. (A) Each of the T47D cell lysates from si-EGFP and si-GALNT6 was incubated with recombinant GALNT6 proteins (WT and H271D) before VVA-lectin Western blot analysis. (B) Overexpression of WT-GALNT6 in MCF10A cell augmented a few of the O-glycan bands (left) but showed no influence on the cell proliferation (right). Arrows indicate candidate O-glycan substrates of GALNT6 (A, B). Overexpression of WT-GALNT6 induced EMT-like cell morphologic changes (C) that were validated by Western blots with EMT markers, E- and N-cadherins (D). The representative images of epithelial and mesenchymal cells (C) were modified from Lee et al. [14]. Scale bars, 100 µm.
Overexpression of GALNT6 Induces EMT-like Morphologic Alterations
To investigate unidentified substrates of GALNT6, we generated transfectants stably expressing WT- or enzyme-dead- (H271D) GALNT6 in MCF10A cell that expressed a very low level of GALNT6 and MUC1 proteins [4]. In contrast to T47D cells (Figure 1A), VVA-lectin analysis recognized only a few bands that showed intensity enhancement by overexpression of GALNT6 (Figure 1B, left) and we observed no effect on the proliferation of MCF10A cells (Figure 1B, right). It might be due to the lower expression of some GALNT6 substrates in MCF10A cells. However, we observed significant morphologic changes from epithelial-like cells to mesenchymal-like cells by introduction of WT-GALNT6 (Figure 1C). Because such EMT-like changes have been strongly implicated in relation to cancer progression with abolished cell adhesion [14], we further performed Western blot analysis using the antibody to E-cadherin that is the representative cell adhesion molecule and an epithelial cell maker and found that E-cadherin expression was reduced by the overexpression of WT-GALNT6. However, E-cadherin expression level was elevated by H271D-GALNT6 induction than transfection of mock control, indicating a dominant negative effect of H271D-GALNT6 to the weakly expressed endogenous GALNT6 protein (Figure 1D, left). Interestingly, a mesenchymal cell marker, N-cadherin was inversely correlated with the expression levels of E-cadherin (Figure 1D, right) as known as “cadherin switching” that was supposed to be regulated in transcriptional levels [15]. Hence, it is likely that substrates of GALNT6 and their downstream targets might affect such a competitive transcription of two cadherins. In addition, the reduction of E-cadherin was also observed in HCC1500 breast cancer cells, which showed a low level of GALNT6, by the overexpression of GALNT6 (Figure W1).
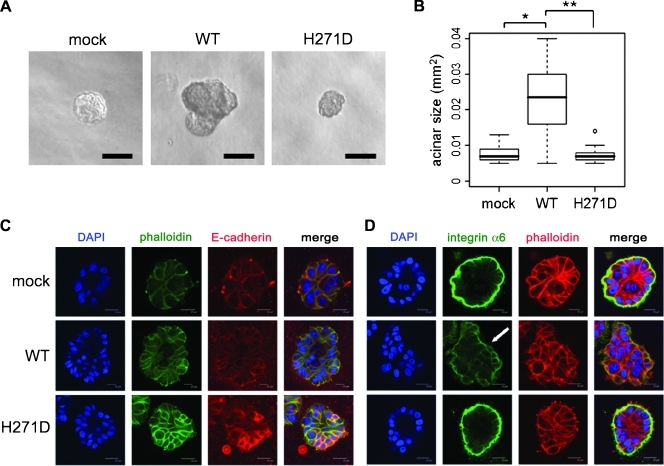
Overexpression of GALNT6 Disrupts Acinar Formation of MCF10A Cells in Three-dimensional Culture
The three-dimensional cell culture enabled us to monitor cell morphogenesis in a relatively physiological condition compared with the conventional two-dimensional cell culture. Hence, we performed three-dimensional cell culture of MCF10A-GALNT6 stable cells and observed irregular and invasive proliferation of these cells into Matrigel matrix after 10 days of incubation (Figure 2A). Subsequently, we quantified each acinar size and found that the disruptive proliferation of MCF10A cells was induced by the overexpression of WT-GALNT6, although it was not observed in MCF10A-H271D-GALNT6 stable cells (Figure 2B). In addition, we performed immunocytochemical staining and detected reduction of E-cadherin protein in the cell-cell contact region in MCF10A-WT-GALNT6 stable cells (Figure 2C), but E-cadherin was augmented by H271D-GALNT6 in concordance with the results of Western analysis as shown in Figure 1D.
Figure 2.
Three-dimensional cell culture of MCF10A-GALNT6 stable transfectants. (A) Disruptive and invasive proliferation of MCF10A cells was observed from WT-GALNT6 stable transfectants cultured in the Matrigel matrix after incubating for 10 days. (B) Quantification and statistical analysis for acinar sizes (n > 50). *P = 9.6 x 10-5. **P = 4.4 x 10-5. (C) Reduction of E-cadherin by overexpression of WT-GALNT6. (D) Staining of integrin α6 around the outer region of the acinar was abrogated (arrow) by the overexpression of WT-GALNT6, which indicated loss of cell polarity. Scale bars, 100 µm (A); 20 µm (C, D).
It has been suggested that disruption of the acinar morphogenesis usually coincides with loss of cell polarity and antiapoptotic activity of inner cells [6,16]. Therefore, we examined a cell polarity marker (integrin α6) and a cell apoptosis marker (active caspase-3) by immunocytochemistry. Expectedly, we confirmed that WT-GALNT6 induced loss of the cell polarity (Figure 2D) and facilitated the survival of inner cells (Figure W2). Because these morphologic alterations were abrogated by the enzyme-dead mutant (H271D), our findings strongly suggested that transformational potentials of GALNT6 should be due to its O-glycosyltransferase activity.
Fibronectin Is O-glycosylated and Stabilized by GALNT6
To further understand the biologic function of GALNT6, we focused on fibronectin as a substrate because it contained candidate sites of glycosylation by GALNT6 [17] and was reported to promote MCF10A cell proliferation in three-dimensional culture [18]. To examine in vivo glycosylation of fibronectin by GALNT6, we immunoprecipitated the endogenous fibronectin protein from MCF10A-GALNT6 stable cells and detected a glycosylated band of the fibronectin protein by VVA-lectin Western blot (Figure 3A). Because the O-glycosylation was considered to be important to maintain protein stability [4,19,20], we next examined the expression of fibronectin at the transcript and protein levels. The result revealed that there was no difference in the transcript levels among WT-GALNT6 cells, H271D-GALNT6 cells, and mock cells but that the fibronectin protein was significantly more abundant in WT-GALNT6 cells than mock and H271D-GALNT6 cells (Figure 3B). This suggested that GALNT6 might contribute to maintain protein stability of fibronectin through O-glycosylation. In addition, this speculation was further validated by the reduction of the fibronectin protein in the GALNT6-depleted T47D cells (Figure 3C).
Figure 3.
GALNT6 O-glycosylates and stabilizes fibronectin. (A) In vivo O-glycosylation of fibronectin by WT-GALNT6. From each of the MCF10A-GALNT6 stable transfectants, the endogenous fibronectin protein (arrow) was immunoprecipitated and blotted with VVA-lectin. Then, the membrane was stripped and immunoblotted with an anti-fibronectin monoclonal antibody. A posttranscriptional regulation of fibronectin protein was validated by Western blot analysis (upper) and reverse transcription-polymerase chain reaction (lower) in MCF10A cells overexpressing GALNT6 (B) or in T47D cells depleted with GALNT6 (C).
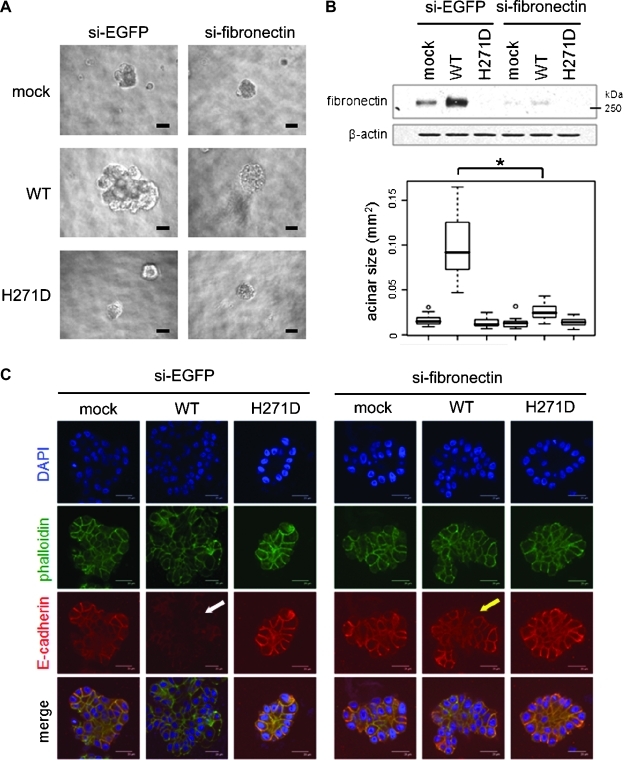
Fibronectin Mediates the Acinar Disruption That Was Induced by GALNT6
According to previous findings for fibronectin in three-dimensional cultured MCF10A cells [18], we speculated that fibronectin could be a critical downstream molecule of GALNT6 and be indispensable for the disruptive proliferation of the MCF10A stable cells. When the fibronectin was depleted by transfections with siRNA, the irregular shape morphology and aberrant proliferation of MCF10A cells were diminished as observed in 3D-cell culture (Figure 4A). After 3 weeks of incubation in three-dimensional culture, we quantified each acinar size and found that the knockdown of fibronectin significantly abolished the effect of WT-GALNT6 overexpression that had led to disruption of acinar structure (Student's t test, P = 2.1 x 10-7; Figure 4B). Moreover, knockdown of fibronectin restored E-cadherin protein (Figure 4C) that had been reduced by WT-GALNT6 (Figure 2C). These findings supported our speculation that fibronectin, as one of the O-glycan substrates of GALNT6, would be a critical mediator that conferred transformational potentials to MCF10A cells.
Figure 4.
GALNT6-induced disruptive proliferation of MCF10A cell is dependent on fibronectin. (A) Knockdown of fibronectin abolished the disruptive and invasive proliferation of MCF10A-GALNT6 stable transfectants. The cells were incubated for 3 weeks with repeated transfections with siRNA. (B) Knockdown of fibronectin was validated in the MCF10A-GALNT6 stable cells transfected with siRNA for 1 week. Quantification and statistical analysis were conducted for acinar sizes (n > 100). *P = 2.1 x 10-7. (C) Reduction of E-cadherin by overexpressed WT-GALNT6 (white arrow) was restored in the fibronectin-depleted WT-GALNT6 stable transfectants (yellow arrow). Scale bars, 100 µm (A); 20 µm (C).
Discussion
Despite that the short and immature O-glycans of breast cancer cells have been known for a long time, the molecular characteristics of O-type glycosylation make it difficult to be analyzed. For instance, neither amino acid consensus sequence nor universal enzyme for the release of O-glycans has been identified [21]. Because O-type glycosylation is initiated by glycosyltransferases belonging to the GALNT family [22], mutual examination of both GALNT enzyme and its direct substrate(s) may contribute to our understanding of aberrant O-glycosylations in carcinogenesis. Indeed, accumulating studies have elucidated cellular functions of O-glycosylation throughout identification of deregulated GALNT enzymes and their O-glycan substrates [23,24].
We previously identified GALNT6 as a novel therapeutic target for breast cancer. We also characterized GALNT6's roles in breast cancer cell by identification of a direct O-glycan substrate, MUC1 [4]. Because the enzyme activity of GALNT6 was critical to modulate oncogenic pathways of MUC1, high-throughput screening of GALNT6 inhibitor was expected to lead to the development of novel anticancer drugs. This idea is in concordance with recent approaches for cancer therapy by means of inhibiting cancer-specific glycosylations [25]. However, because different isoforms of GALNT family glycosylate diverse O-glycan substrates [21], intensive characterization in both glycosyltransferase and downstream should be preceded before screening of therapeutic chemicals.
In this study, we identified fibronectin, which is known to be degraded intracellularly after endocytosis [26], as an important in vivo substrate of GALNT6. Our data reported here imply that GALNT6-induced O-glycosylation may interfere with this degradation process and stabilize fibronectin. Although the molecular mechanism that links between stabilization of fibronectin and reduction of E-cadherin is still unclear, we suggest that the upregulated expression of GALNT6 enhances transformational potentials of mammary epithelial cells through O-glycosylation of fibronectin that might facilitate disruptive and invasive cell proliferation in vivo. However, because breast cancer cells may have another O-glycan substrates of GALNT6 (Figure 1A), identifying more substrates and related downstream molecules for the comprehensive understanding of GALNT6's role in breast cancer is warranted.
Supplementary Material
Acknowledgments
The authors thank Kie Naito, Yoshiko Fujisawa, and Aya Sasaki for excellent technical assistance and Chikako Fukukawa and Yosuke Harada for helpful discussions.
Abbreviations
- EMT
epithelial-to-mesenchymal transition
- GALNT6
polypeptide N-acetylgalactosaminyltransferase 6
- VVA
Vicia villosa
- WT
wild-type
Footnotes
No potential conflict of interest was disclosed.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Burchell JM, Mungul A, Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001;6:355–364. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- 2.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 3.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, Hirata K, Nakamura Y, Katagiri T. Critical roles ofmucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 5.Freire T, Berois N, Sonora C, Varangot M, Barrios E, Osinaga E. UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer. 2006;119:1383–1388. doi: 10.1002/ijc.21959. [DOI] [PubMed] [Google Scholar]
- 6.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 7.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 8.Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, Zhou J, Turner J, Lisanti MP, Russell RG, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 10.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 11.Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Lin ML, Nishidate T, Nakamura Y, Katagiri T. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res. 2006;66:9186–9195. doi: 10.1158/0008-5472.CAN-06-1601. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, Tsunoda T, Kamatani N, Kubo M, Nakamura Y, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 16.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 17.Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H. Cloning and characterization of a close homologue of human UDP-N-acetyl-α-d-galactosamine: polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem. 1999;274:25362–25370. doi: 10.1074/jbc.274.36.25362. [DOI] [PubMed] [Google Scholar]
- 18.Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschuler Y, Kinlough CL, Poland PA, Bruns JB, Apodaca G, Weisz OA, Hughey RP. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell. 2000;11:819–831. doi: 10.1091/mbc.11.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu P, Berkowitz P, Madden VJ, Rubenstein DS. Stabilization of plakoglobin and enhanced keratinocyte cell-cell adhesion by intracellular O-glycosylation. J Biol Chem. 2006;281:12786–12791. doi: 10.1074/jbc.M511702200. [DOI] [PubMed] [Google Scholar]
- 21.Jensen PH, Kolarich D, Packer NH. Mucin-type O-glycosylation—putting the pieces together. FEBS J. 2010;277:81–94. doi: 10.1111/j.1742-4658.2009.07429.x. [DOI] [PubMed] [Google Scholar]
- 22.Ten Hagen KG, Fritz TA, Tabak LA. All in the family: the UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R–16R. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agard NJ, Bertozzi CR. Chemical approaches to perturb, profile, and perceive glycans. Acc Chem Res. 2009;42:788–797. doi: 10.1021/ar800267j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.