Abstract
Small cell lung cancer (SCLC) is an aggressive disease in which, after initial sensitivity to platinum/etoposide chemotherapy, patients frequently relapse with drug-resistant disease. Deregulation of the Bcl-2 pathway is implicated in the pathogenesis of SCLC, and early phase studies of Bcl-2 inhibitors have been initiated in SCLC. Obatoclax is a small-molecule drug designed to target the antiapoptotic Bcl-2 family members to a proapoptotic effect. Preclinical studies were conducted to clarify the kinetics of obatoclax-induced apoptosis in a panel of SCLC cell lines to assist with the interpretation of biomarker data generated during early phase clinical trials. In vitro, obatoclax was synergistic with cisplatin and etoposide, and “priming” cells with obatoclax before the cytotoxics maximized tumor cell death. Peak levels of apoptosis, reflected by cleaved cytokeratin 18 (CK18) levels (M30 ELISA) and caspase activity (SR-DEVD-FMK), occurred 24 hours after obatoclax treatment. A phase 1b-2 trial of obatoclax administered using two infusion regimens in combination with carboplatin and etoposide has been completed in previously untreated patients with extensive-stage SCLC. Circulating pharmacodynamic biomarkers of cell death, full-length and/or cleaved CK18, and oligonucleosomal DNA were studied in the phase 1b trial. All SCLC patients classified as “responders” after two cycles of treatment showed significantly increased levels of full-length and cleaved CK18 (M65 ELISA) on day 3 of study. However, the preclinical data and the absence of a peak in circulating caspase-cleaved CK18 in trial patients suggest suboptimal timing of blood sampling, which will need refinement in future trials incorporating obatoclax.
Introduction
The suppression of apoptosis is recognized as a hallmark of human cancer [1] and is an established mechanism for pleiotropic drug resistance. Consequently, global drug discovery efforts have been marshalled to target those molecules that increase the cellular threshold for apoptosis in tumor cells and resensitize drug-resistant tumors. Small cell lung cancer (SCLC) is initially a chemosensitive tumor responding well to etoposide and platinum-based doublet therapy [2]. However, tumor relapse with drug-resistant disease is common, and novel therapies to combat this aggressive cancer are urgently required. Interactions between antiapoptotic and proapoptotic proteins of the Bcl-2 family determine the threshold for apoptosis (reviewed by Letai [3]). The antiapoptotic family members (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1) are overexpressed in many human tumor types presumably to combat the cellular stress of the tumor microenvironment and inherent cellular damage [4]. Bcl-2 itself is upregulated in at least 75% of SCLC [5–8], and drugs targeting Bcl-2 in SCLC patients have entered early clinical trials [9,10].
Obatoclax (Gemin X Pharmaceuticals) is a novel proapoptotic drug designed to target the BH-3 domain of prosurvival Bcl-2 family members and has been shown to increase expression of BH-3-only proteins Bim and Noxa [11,12]. Obatoclax demonstrated promising preclinical efficacy against non-small cell lung carcinoma (NSCLC), mantle cell lymphoma, and multiple myeloma cells both as a single agent and in combination with clinically relevant cytotoxics in vitro [13–15]. Moreover, in combination with the proteosome inhibitor bortezomib, obatoclax was synergistic in both mantle cell lymphoma and multiple myeloma. This is of particular interest as Mcl-1 is turned over at a steady state due to proteasomal degradation [16], potentially resulting in the accumulation of Mcl-1 and resistance to apoptosis. In multiple myeloma, the sequence of obatoclax and bortezomib treatment was critical, with synergy maximized by obatoclax pretreatment [15]. Obatoclax has also demonstrated enhanced apoptosis in combination with Apo2L/TRAIL in cholangiocarcinoma cells [17] and pancreatic cancer cells [18] and with tyrosine kinase inhibitors in breast cancer [19] and NSCLC [14]. These data provided the stimulus for the clinical development of obatoclax and further preclinical studies in SCLC to optimize the utility of pharmacodynamic biomarkers of cell death.
Obatoclax has displayed single agent antitumor activity in phase 1 trials using a variety of infusion schedules (1, 3, and 24 hours) in patients with advanced hematological malignancies [20,21]. To assess the quantitative effect of obatoclax on apoptosis induction, serial measurements of oligonucleosomal DNA/histone complexes (nDNA; Cell Death Detection ELISAPLUS Roche, Basel, Switzerland) were analyzed. Circulating nDNA concentrations were directly proportional to obatoclax dose, and after an initial increase in response to treatment, elevated nDNA was sustained for several days. In a phase 1 trial of obatoclax plus topotecan in relapsed SCLC and other solid tumors, two patients with SCLC achieved partial responses and four patients stable disease [22]. A phase 1b trial of obatoclax in combination with carboplatin and etoposide, in previously untreated patients with extensive-stage small cell lung cancer (ES-SCLC), has recently concluded. Here, the primary objective was to determine the maximum tolerated doses of obatoclax given in a 21-day cycle (in separate dose escalations as either a 3- or a 24-hour infusion on days 1–3) [23]. High early tumor response rates were observed, and dose-limiting toxicities of somnolence, euphoria, and disorientation were reported in the 3-hour regimen. Dose-limiting toxicities were not observed in the 24-hour infusion regimen, and response rates with this regimen seemed to be lower. Three pharmacodynamic end points of cell death measured in serial peripheral blood samples were incorporated into the trial: the M30 Apoptosome ELISA (M30) that detects caspase-cleaved forms of cytokeratin 18, the M65 ELISA (M65) that detects full-length and caspase-cleaved cytokeratin 18, and the nDNA assay. We have previously reported the utility of these blood-borne biomarkers in SCLC patients treated with conventional etoposide and platinum-based therapy [24] where their levels increased after cytotoxic drug treatment and returned to baseline levels during the following 21 days, mirroring initial tumor response. These data have served as a guide to the interpretation of the biomarker results presented in the phase 1b trial. In parallel, preclinical studies were performed in a panel of SCLC cell lines to evaluate the kinetics of biomarker change in obatoclax-induced apoptosis when the drug is administered as a single agent or in combination with etoposide and cisplatin and to assess the impact of a variety of drug schedules.
The preclinical analysis demonstrated that obatoclax was synergistic with cisplatin and etoposide across a panel of SCLC cell lines with maximum apoptosis occurring 24 hours after pulsed obatoclax treatment. Priming cells with obatoclax before the cytotoxics maximized tumor cell death. The clinical pharmacodynamic biomarker data showed that SCLC patients categorized as “responders” had significant increases in circulating cytokeratin 18 (CK18) during obatoclax exposure indicative of both apoptotic and nonapoptotic cell death. In view of the preclinical data, the absence of a discernable peak in caspase-cleaved CK18 after drug treatment in patients in this phase 1b trial may suggest suboptimal timing of patient blood sampling, which will need to be considered and rectified in future trial designs.
Materials and Methods
Human SCLC Cell Lines, Reagents, and Chemicals
Human SCLC cell lines (H82, DSM114, H146, H196, H345, H524, and H526) were obtained from the American Type Culture Collection (Manassas, VA). Cell lines were authenticated using the Applied Biosystems AmpFlSTR system. All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum. Obatoclax was provided by Gemin X Inc (Malvern, PA) and dissolved in 100% dimethyl sulfoxide for in vitro assays. All cytotoxics were purchased from Sigma-Aldrich (Gillingham, UK).
Cell Proliferation Assay
The MTS assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI) [25] was used to determine cell population growth. SCLC cell lines were plated in exponential growth phase in 96-well plates and treated with varying concentrations of obatoclax. At various times thereafter, cells were stained according to the MTS protocol, and absorbance was measured using a microplate reader (Labsystems Multiskan EX; Thermo Scientific, Milford, MA) at 490 nm.
FACS Array
SCLC cells were treated with obatoclax using the half-maximal inhibitory concentrations (IC50) derived from the MTS assay. Cells were harvested and resuspended in 1 ml of RPMI with 10% FBS; 100 µl of each test sample was added to a 96-well U-bottom plate (Falcon 3077; Becton Dickinson, Franklin Lakes, NJ). SR-DEVD-FMK (10 µl, CaspaTag 3,7; Millipore, Billerica, MA) was incubated for 1 hour at 37°C in the dark before analysis by flow cytometry (BD FACSArray Bioanalyzer). CaspaTag fluorescence was detected at 595 nm after fluorochrome excitation at 532 nm. Electronic gating of live or dead subpopulations was defined by gating using control samples of untreated cells and etoposide (20 µM) cells. A total of 20,000 cells were analyzed per sample.
Immunoblot Assay
Cells were treated with obatoclax or vehicle control for the stated times. Protein lysates were prepared using lysis buffer (Cell Signaling Technology, Danvers, MA) and protease inhibitor cocktail (Sigma-Aldrich). All samples were sonicated at 10 Hz for 10 seconds. Protein lysates were resolved by electrophoresis in appropriate percentage polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Immobilon, Millipore, Watford, UK). Standard immunoblot analysis procedures were followed with overnight incubation at 4°C with the following primary antibodies: A1 1:1000 (Abcam, Cambridge, UK), Bcl-2 1:1000 (Dako UK Ltd, Ely, UK), Bcl-w 1:1000 (Alexis Biochemical, Lausen, Switzerland), Bcl-xL 1:1000 (BD Transduction Laboratories, Oxford, UK), Mcl-1 1:1000 (BD Biosciences, Oxford, UK), cleaved caspase-3 1:500 (Cell Signaling Technology), cleaved poly (ADP-ribose) polymerase (PARP; Cell Signaling Technology), and tubulin (Cancer Research UK, London). Blots were visualized with the enhanced chemiluminescence system (Amersham, Chalfont St. Giles, UK) and analyzed using a Fuji LAS-1000 Plus imaging system with AIDA software (Fuji, Bedford, UK). The proportion of cleaved caspase-3 was measured using the Meso Scale Discovery MULTISPOT Cleaved/Total Caspase-3 Assay (Meso Scale Discovery, Gaithersburg, MD) according to the manufacturer's instructions.
Drug Combination Assays
The combination index (CI) method was applied based on the multiple drug effect equation of Chou and Talaly [26]. Obatoclax was tested in combination with both cisplatin and etoposide (used in the treatment of SCLC patients), considering cisplatin and etoposide as a single entity and obatoclax as the second agent. The IC50 of cisplatin and etoposide combined was determined using the MTS assay before combination studies to account for synergy between the chemotherapeutics. Cells were either treated for 96 hours with obatoclax, cisplatin + etoposide, or with obatoclax and cisplatin + etoposide in 48-hour sequencing experiments. Eight drug concentrations were used covering the concentration effect. Drug additivity is reported in the range CI = 0.9 to 1.1, synergy CI < 0.9, and antagonism CI > 1.1.
Clinical Trial Pharmacodynamic Analysis
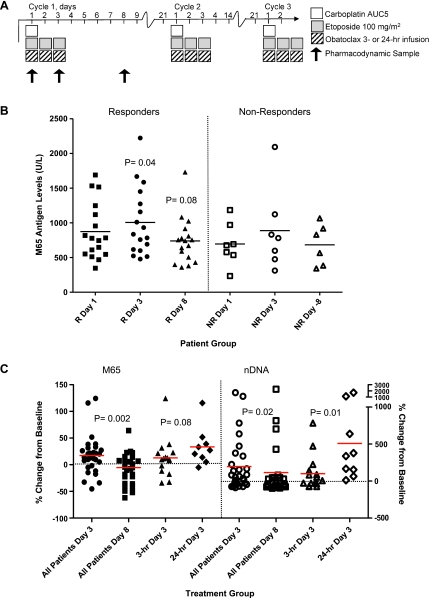
The phase 1b trial of obatoclax was a dose-escalation study of obatoclax administered every 3 weeks in combination with carboplatin and etoposide to patients with previously untreated ES-SCLC [23]. The study was sponsored by Gemin X Pharmaceuticals (NCT00682981). The primary objective was to determine the recommended phase 2 dose of obatoclax administered either as a 3- or 24-hour infusion for three consecutive days in combination with carboplatin (AUC5, day 1) and etoposide (100 mg/m2, days 1–3) in a three-weekly cycle for six cycles (Figure 4A). This was followed by prophylactic cranial irradiation (for complete responders and partial responders) and with three-weekly maintenance of obatoclax. The M30, M65, and nDNA ELISAs were performed according to the manufacturer's instructions and to GCP standards (Standard Operating Procedures: LM/003, LM/004, and LM/016) and were read using a Dynex MRXII Plate Reader (Dynex Technologies, Chantilly, VA). Patient serum samples were drawn for analysis on days 1, 3, and 8 of cycle 1. Samples were analyzed in duplicate. The dynamic ranges of these ELISAs are 0 to 1000 U/L (M30), 0 to 2000 U/L (M65), and 0.1 to 3.5 o.d. (nDNA).
Figure 4.
Pharmacodynamic biomarker analysis from the phase 1 trial of obatoclax in combination with cisplatin and etoposide in SCLC. (A) Schematic for clinical trial drug administration for obatoclax, cisplatin, and etoposide and timing of blood samples for pharmacodynamic biomarker analysis. Patients could receive up to six cycles of chemotherapy. (B) Circulating caspase cleaved plus full-length CK18 levels (M65 assay) in “responders” (n = 17) and “nonresponders” (n = 7). Statistical analysis by Wilcoxon matched-pairs signed rank test. (C) Percentage change from pretreatment baseline values in full-length plus caspase cleaved CK18 (M65 assay) and nucleosomal DNA (nDNA assay) between day 3 circulating biomarker increases and day 8 decreases (P = .002 and P = .02, respectively), and group comparisons between patients who received the 3-hour infusion and those who received the 24-hour infusion.
Statistics
Statistical significance (P ≤ .05) for a change in M30 concentration was determined by two-tailed paired t tests (assuming equal variance) between the drug combination counts and the single agents. For the biomarker data, the Mann-Whitney test was used to compare differences between the two unmatched infusion protocols. Intrapatient comparisons were analyzed using Wilcoxon matched-pairs signed rank test to compare medians.
Results
Obatoclax Reduced Cell Viability across the SCLC Cell Line Panel
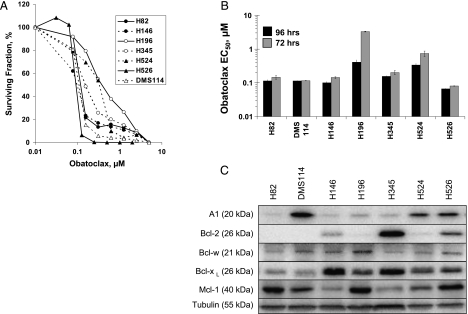
Obatoclax caused concentration-dependent reduction in viable cells in all tested SCLC cell lines, as measured by the MTS assay at 96 hours (Figure 1, A and B). The range of IC50 concentrations was 0.08 to 1.04 µM, and in most instances, the IC50 was less than 0.2 µM. The order of increasing resistance (judged by IC50 concentration) after a 96-hour constant drug challenge was as follows: H526 > H146 > DMS 114 > H82 > H345 > H524 > H196. Cell lines were also treated for 24, 48, and 72 hours with obatoclax concentrations ranging from 0.01 to 5 µM. Maximal effect on cell viability was observed after 96 hours of obatoclax exposure, with near-maximal effect at 72 hours of exposure. In most cell lines, IC50 values after 24 and 48 hours of treatment were several fold higher, with only two SCLC cell lines (H526 and DMS114) sensitive to a 48-hour obatoclax treatment, suggesting that a prolonged exposure was generally required for cytotoxicity in vitro.
Figure 1.
Obatoclax decreased the viability of SCLC cells with no clear correlation with antiapoptotic Bcl-2 family protein expression levels. (A) Concentration-effect curves for a panel of SCLC cell lines treated at varying concentrations of obatoclax for 96 hours, using the MTS assay. (B) IC50 values for log phase SCLC cells, treated with obatoclax for 96 and 72 hours. (C) Immunoblot analysis of antiapoptotic Bcl-2 family members in SCLC cell lines. Untreated log-phase SCLC cells were lysed, and lysates were normalized for total protein content (15 µg per lane) and analyzed by SDS-PAGE using antibodies recognizing A1, Bcl-2, Bcl-w, Bcl-xL, and Mcl-1. Tubulin was used as a protein loading control. Data are representative of n = 3 repeat experiments ± SEM.
Obatoclax Induced Time- and Concentration-Dependent Apoptosis in SCLC Cell Lines
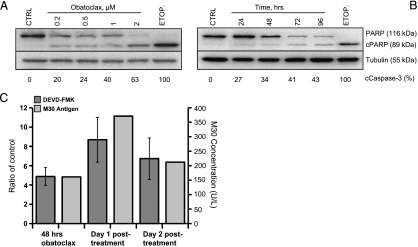
Analysis of cleaved PARP and cleaved caspase-3 levels in obatoclax-treated SCLC cell lines confirmed time- and concentration-dependent effects on cell death (Figure 2, A and B (representative data for H526 shown)), suggesting that the observed reduction in viable cells occurred by apoptosis. To provide further confirmatory evidence of apoptotic cell fate, H526 cells treated for 48 hours with obatoclax were analyzed using SR-DEVD-FMK (to report caspase-3 and -7 activity) and caspase-cleaved CK18 (M30) immediately after pulsed treatment, as well as 1 day and 2 days after the drug had been removed and replaced with drug-free medium (Figure 2C). Caspase-3/7 activity was highest 24 hours after obatoclax removal, consistent with significantly high M30 values at this time point (Figure 2C; P < .01), which accumulated in the supernatant after drug removal.
Figure 2.
Obatoclax induced apoptosis in SCLC cells. (A and B) Immunoblot analysis of H526 cells treated with 48 hours of obatoclax at the indicated concentrations (A) or with a fixed dose of 0.2 µM for the indicated times (B). Membranes were probed with antibodies specific for cleaved (“c” as indicated) and full-length PARP. Cleaved caspase-3 as a percentage of total caspase-3 measured using the Mesoscale Discovery assay are reported below. (C) Ratio of drug-treated and vehicle-treated H526 cells with respect to staining for active caspase-3/7 by SR-DEVD-FMK by flow cytometry after cells were treated with obatoclax at the IC50 concentration for 48 hours, then 1 day and 2 days after the drug was removed and replaced by medium. Levels of cleaved CK18 (by M30 assay) in the cell culture medium after 48 hours of obatoclax at the IC50 concentration at the same time points. CTRL indicates control; ETOP, etoposide 20 µM. Results are representative of n = 3 ± SEM.
Protein Expression Levels of Antiapoptotic Bcl Family Members in SCLC
The expression levels of antiapoptotic Bcl-2 family members varied across the SCLC panel (Figure 1C) and were consistent with previously reported data [27,28]. The relative affinity of obatoclax for Bcl-2 is believed to be fourfold higher than for Bcl-xL [12], leading to the hypothesis that obatoclax sensitivity would correlate better with high Bcl-2 expression than with high Bcl-xL expression. Up-regulation of both of these proteins has been shown to have prognostic significance in a variety of tumor types in immunohistochemical studies [29,30], but none of these studies have examined the relative abundance of all antiapoptotic Bcl-2 family members. However, overall, there is no clear relationship between Bcl-2 family protein expression and sensitivity to obatoclax across the SCLC cell line panel.
Obatoclax, Cisplatin, and Etoposide Are a Synergistic Drug Regimen
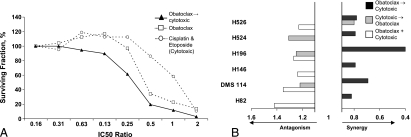
The observations that conventional cytotoxics induce apoptosis through the intrinsic pathway and that apoptosis is suppressed by upregulated levels of antiapoptotic Bcl-2 family proteins led to the prediction that Bcl-2 inhibition should increase antitumor efficacy of standard cytotoxic chemotherapy [31,32]. SCLC is considered to be a chemosensitive tumor and combination chemotherapy with platinum and etoposide is the standard regimen for ES-SCLC with four to six cycles considered optimal [2]. Therefore, the effects of obatoclax in combination with cisplatin and etoposide were explored using the CI method. Table 1 shows that obatoclax plus cisplatin and etoposide was a synergistic drug regimen with CI values less than 0.9 (shaded cells). Synergy was also observed in the combination effect graph (Figure 3A). The surviving fraction was less for the combination compared with either chemotherapy or obatoclax alone, demonstrated by a left shift in the curve. Priming the cells with obatoclax for 48 hours resulted in the greatest synergy, enhancing the effect of cisplatin and etoposide (Figure 3B). This was observed in all SCLC cell lines tested, consistent with the hypothesis that obatoclax reduces the cellular threshold for cytotoxic induced apoptosis. In only one of the cell lines, H526, both sequences of treatment were synergistic, implying that, for this cell line, synergy occurred independently of sequencing.
Table 1.
Priming Cells with Obatoclax Enhances the Effect of Cisplatin and Etoposide for All Tested Cell Lines.
| Cell Line | Sequence | ||
| (A) Concurrent | (B) Cytotoxics → Obatoclax | (C) Obatoclax → Cytotoxics | |
| H82 | 1.42 (-) | 1.07 (±) | 0.82 (++) |
| DMS114 | 1.35 (-) | 1.22 (-) | 0.69 (+++) |
| H146 | 1.24 (-) | 0.92 (±) | 0.79 (++) |
| H196 | 1.27 (-) | 1.25 (-) | 0.4 (+++) |
| H524 | 1.05 (±) | 1.31 (-) | 0.79 (++) |
| H526 | 1.23 (-) | 0.80 (++) | 0.78 (++) |
Cells were treated with schedule A, B, or C for 96 or 48 hours in sequence using fixed ratios of the IC50 values. Cisplatin and etoposide were considered a single entity, and obatoclax was considered as the second agent. Cell viability was determined using the MTS assay, and synergy was evaluated using CalcuSyn (BioSoft, Cambridge, England) where additivity is CI = 0.9 to 1.1 (±), antagonism >1.1 (-), and synergy <0.9 (+). Results represent n = 3 independent experiments.
Shaded values represent synergistic combinations.
Figure 3.
Synergistic combination of obatoclax, cisplatin and etoposide in SCLC cells. (A) H526 cells were treated with obatoclax for 48 hours followed by the addition of cisplatin and etoposide for 48 hours. Equipotent concentrations of the single agents were selected to cover the concentration-effect curve; the x axis shows the fixed ratio. Data are representative of six SCLC cell lines. (B) SCLC cells were treated either concurrently with the three drugs for 96 hours or 48 hours with the first agent (termed cytotoxics) followed by the addition of obatoclax for 48 hours, covering the concentration-effect curve. After 96 hours, surviving fraction was determined using the MTS assay, and synergy was evaluated using CalcuSyn software.
Evaluation of Circulating Biomarkers of Cell Death in the Phase 1 Trial of Obatoclax
The phase 1b trial of obatoclax was a dose-escalation study of obatoclax administered every 3 weeks to patients with ES-SCLC in combination with carboplatin and etoposide [23] (Figure 4A). Patient serum samples were drawn for analysis on days 1, 3, and 8 of cycle 1, and in total, 24 sets of samples were received. Seventeen patients were classified as “responders” and seven patients were classified as “nonresponders” according to RECIST criteria [33] at an early time point after cycle 2 of treatment and compared with baseline scans. Responders were considered to have radiologic unconfirmed complete or partial responses after two cycles of treatment, whereas nonresponders were patients with stable disease or disease progression. Responders, but not nonresponders, demonstrated significant increases in circulating full-length and/or cleaved CK18 values (M65) only between days 1 and 3 (P = .037) and a trend toward decreased M65 values at day 8 (that did not reach significance, P = .08; Figure 4B).
The biomarker profiles for each infusion regimen were compared to identify which regimen was associated with the largest change in cell death biomarker concentration. In all patients, there was a significant percentage change from baseline between day 3 biomarker increases and day 8 decreases in M65 and nDNA (P = .002 and P = .02, respectively; Figure 4C). Group comparisons between patients who received the 3-hour infusion (n = 16) and those who received the 24-hour infusion (n = 8) showed that the 24-hour infusion regimen had significantly higher day 3 nDNA values (P = .01) compared with the 3-hour infusion regimen, a trend that was also observed in the M65 results (P = .08; Figure 4C). No significant differences between the 3 and 24-hour infusion regimens were observed at day 8 or in the cleaved CK18 (M30) results. Within the 24-hour infusion group, longitudinal data for individual patients (intrapatient comparisons) showed significant increases in both M65 and nDNA at day 3 (P = .01 and P = .004, respectively; data not shown).
Discussion
After the discovery that antiapoptotic Bcl-2 family proteins can suppress drug-induced apoptosis [34,35], one approach to restore a functioning cell death program in drug-resistant cancer cells is to combine Bcl-2-targeted drugs with standard of care chemotherapy. However, attempts to target Bcl-2 in SCLC using Bcl-2 antisense oligonucleotides, such as oblimersen in combination with carboplatin and etoposide in chemotherapy-naive patients, failed to show improvements in response rates or survival [10]. Possible explanations for the failure of this antisense approach are that oblimersen did not sufficiently suppress intratumoral levels of Bcl-2 or that functional redundancy exists between Bcl-2 and other antiapoptotic family members such as Bcl-xL and Mcl-1. The development of novel compounds with selective activity for Bcl-2 and Bcl-xL has revealed the ability of cancer cells to adapt [36] and express Mcl-1 as a resistance mechanism [37]. This led to the proposition that small-molecule drugs that target the Bcl-2 family members less selectively may meet with greater therapeutic success [38].
Here, the effect of combining obatoclax with platinum and etoposide chemotherapy in SCLC cell lines was evaluated, in parallel with the same strategy in patients receiving treatment in the context of a phase 1b trial. The rationale for this approach was to optimize drug scheduling and the timing of pharmacodynamic sampling to inform future trial design and test the validity of the combination treatment as a therapeutic strategy. The preclinical studies demonstrated that obatoclax induced apoptosis in a time- and concentration-dependent manner across multiple SCLC cell lines and that it is synergistic with clinically relevant chemotherapeutics. Similar to studies in NSCLC, no obvious relationship between Bcl-2 family protein expression profiles and sensitivity to obatoclax was observed [14]. Our preclinical results suggest that the optimal drug schedule is to “prime” cells with obatoclax before chemotherapy, an observation consistent with sequencing experiments with obatoclax and bortezomib in multiple myeloma [15], and that peak apoptosis end points occur 24 hours after obatoclax treatment.
The precise mechanism of action of several novel drugs designed to interrupt the BH-3-mediated interactions between antiapoptotic and proapoptotic Bcl-2 family is often tested by comparing their ability to induce apoptosis in Bak/Bax-deficient cells, the premise being that pure BH-3 mimetics will be innocuous in this cellular context. A comparison of six putative BH-3 mimetics (but not obatoclax) showed that, in both short-term and clonogenic survival assays, cells deficient in both Bax/Bak were killed at the same efficiency as wild-type cells, strongly implying that none of these compounds function as a pure BH-3 mimetic [37]. The mechanism of obatoclax-induced apoptosis was initially reported to be Bax and/or Bak dependent, based on studies using mouse kidney epithelial cells lacking Bax and Bak that showed resistance to obatoclax induced DNA fragmentation and caspase-3 cleavage [11]. However, using mouse embryonic fibroblasts, obatoclax-mediated cell killing was shown to be at least in part dependent on Bax/Bak; mouse embryonic fibroblasts deficient in Bax/Bak exhibited partial resistance and decreased colony-forming ability [17]. These data suggest the existence of mechanisms of obatoclax-induced cell death alternative to the established BH-3 sensitizer or effector models that modulate Bcl-2 family interactions to drive apoptosis [39–41].
The phase 1b pharmacodynamic biomarker data showed a clear and significant biomarker increase by day 3 in M65 and nDNA levels followed by a decrease below pretreatment levels by day 8, consistent with a biomarker profile indicative of treatment response in SCLC patients and reflecting decreased tumor load due to treatment-induced tumor cell death [42]. The lack of significant drug-induced M30 effects, which measures apoptosis specifically, may have been confounded by the combination with chemotherapeutics and cell loss occurring by more than one mechanism. Chemonaive SCLC patients typically respond well to carboplatin and etoposide in the first cycle, making it difficult to dissect out the biomarker effects of obatoclax alone. Comparing the 24- and 3-hour infusion regimens, the greatest increase in biomarker levels on day 3 was observed for patients receiving the 24-hour infusion regimen. However, the day 8 biomarker data showed decreases below the pretreatment baseline only in the 3-hour infusion regimen, which is typical of a biomarker profile in a patient with SCLC responding to treatment [24,43]. In support of the 3-hour regimen demonstrating superior efficacy, overall (after six cycles of chemotherapy), the clinical data demonstrated a higher number of responders in the 3-hour schedule (12 vs 5). The reason the day 3 biomarker levels were noninformative in the 3-hour infusion arm may be that the biomarker peak occurred earlier and that the timing of blood sampling for was suboptimal. In the phase 1 trial of obatoclax in CLL [21], activated Bax/Bak complexes in peripheral mononuclear cells, detected by immunoprecipitation, peaked 1 to 3 hours after drug infusion in a dose-dependent manner. Furthermore, our analyses of serological cell death biomarkers in chemonaive SCLC patients undergoing platinum and etoposide chemotherapy indicated that the optimal sampling time for the cell death assays was just 24 to 48 hours after chemotherapy [24]. Detection of circulating cell death biomarkers is dependent on many factors including tissue/tumor perfusion, tumor turnover rate, susceptibility/“priming” of the cancer cell, and efficiency of elimination processes [44]. Although decisions on the timing of sampling for a new agent may be determined by preclinical data a priori, it would be prudent to perform pilot studies during early clinical trials of novel agents to optimize the timing of pharmacodynamic sampling. We have analyzed circulating cell death biomarkers in mice bearing human SCLC xenografts treated with a proapoptotic dose of the BH-3 mimetic ABT-737 [45] and showed significant increases in circulating cleaved CK18 as early as 6 hours after treatment. However, optimal blood sampling in similar studies of mice bearing human colon cancer xenografts treated with the aurora kinase inhibitor AZD1152 was 24 hours after drug administration [46]. The biomarker analyses suggest that early M65 changes (within the first week of therapy) may predict likelihood of radiologic response to combination treatment. However, this only occurred when the statistical analysis focused on individual patient profiles. No significant differences were detected when biomarkers were compared between infusion groups. As with other biomarker studies in phase 1 trials, their value is best seen with longitudinal patient data because small heterogenous patient groups with inherent interpatient differences and baseline variability make group comparisons less sensitive. A phase 1 trial of obatoclax in combination with topotecan has recently been published [22], demonstrating that the combination was well tolerated with no apparent elevation in circulating caspase cleaved CK18 (M30). The lack of rise in M30 is pertinent to chemotherapy tolerability because a rise in M30 has previously been correlated with severe toxicity requiring hospitalization in patients with SCLC receiving platinum-based chemotherapy [24]. Obatoclax was also safely administered in combination with full doses of carboplatin and etoposide, but because of practical issues with the 24-hour infusion arm, the 3-hour 30-mg maximum tolerated dose was selected for a randomized phase 2 versus carboplatin and etoposide alone; the results of which are eagerly awaited.
These data extend our knowledge showing that obatoclax promotes cell death synergistically in combination with platinum/etoposide in SCLC and is optimized by drug scheduling. Applied clinically, this raises the possibility of chemotherapy dose reduction with maintained efficacy. The data presented here suggest that blood samples drawn 24 hours after completion of therapy for pharmacodynamic cell death biomarker analysis are likely to be most informative in future trials incorporating obatoclax.
Acknowledgments
The authors thank A. Chiappori (H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL) for his contribution to the collection of samples during the clinical trial.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R, Boye N, Wang M, Vigander T, Vilsvik J, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20(24):4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 3.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8(2):121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 4.Buolamwini JK. Novel anticancer drug discovery. Curr Opin Chem Biol. 1999;3(4):500–509. doi: 10.1016/S1367-5931(99)80073-8. [DOI] [PubMed] [Google Scholar]
- 5.Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of Bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54(1):6–8. [PubMed] [Google Scholar]
- 6.Jiang SX, Sato Y, Kuwao S, Kameya T. Expression of Bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol. 1995;177(2):135–138. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 7.Stefanaki K, Rontogiannis D, Vamvouka C, Bolioti S, Chaniotis V, Sotsiou F, Vlychou M, Delidis G, Kakolyris S, Georgoulias V, et al. Immunohistochemical detection of bcl2, p53, mdm2 and p21/waf1 proteins in small-cell lung carcinomas. Anticancer Res. 1998;18(3A):1689–1695. [PubMed] [Google Scholar]
- 8.Kaiser U, Schilli M, Haag U, Neumann K, Kreipe H, Kogan E, Havemann K. Expression of Bcl-2-protein in small cell lung cancer. Lung Cancer. 1996;15(1):31–40. doi: 10.1016/0169-5002(96)00568-5. [DOI] [PubMed] [Google Scholar]
- 9.Rudin CM, Kozloff M, Hoffman PC, Edelman MJ, Karnauskas R, Tomek R, Szeto L, Vokes EE. Phase I study of G3139, a Bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with small-cell lung cancer. J Clin Oncol. 2004;22(6):1110–1117. doi: 10.1200/JCO.2004.10.148. [DOI] [PubMed] [Google Scholar]
- 10.Rudin CM, Salgia R, Wang X, Hodgson LD, Masters GA, Green M, Vokes EE. Randomized phase II study of carboplatin and etoposide with or without the Bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26(6):870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Belec L, Billot X, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104(49):19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13(8):1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109(10):4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61(3):525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 15.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109(12):5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 16.Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17(23):2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther. 2008;7(8):2339–2347. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15(1):150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17(2):465–469. [PubMed] [Google Scholar]
- 20.Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, Yee K, Ravandi F, Giles F, Schuh A, et al. A phase I study of the pan Bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(24):8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, Viallet J, Cheson BD. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik PK, Rudin CM, Brown A, Rizvi NA, Takebe N, Travis W, James L, Ginsberg MS, Juergens R, Markus S, et al. A phase I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in solid tumor malignancies. Cancer Chemother Pharmacol. 2010;66(6):1079–1085. doi: 10.1007/s00280-010-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiappori A, Schreeder MT, Moezi MM, Stephenson J, Blakely JL, Salgia R, Chu QS, Malik SM, Modiano MM, Berger MS. A phase Ib trial of Bcl-2 inhibitor obatoclax in combination with carboplatin and etoposide for previously untreated patients with extensive-stage small cell lung cancer (ES-SCLC) J Clin Oncol. 2009;27(15s) Abstr 3576. [Google Scholar]
- 24.Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009;175(2):808–816. doi: 10.2353/ajpath.2009.090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riss TL, Moravec RA. Comparison of MTT, XTT, and a novel tetrazolium compound MTS for in vitro proliferation and chemosensitivity assays. Mol Biol Cell. 1992;3(suppl)(184a) [Google Scholar]
- 26.Chou TC, Talaly P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem. 1977;252(18):6438–6442. [PubMed] [Google Scholar]
- 27.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67(3):1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 28.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, Lin X, Hierman JS, Wilburn DL, Watkins DN, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68(7):2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochaix P, Krajewski S, Reed JC, Bonnet F, Voigt JJ, Brousset P. In vivo patterns of Bcl-2 family protein expression in breast carcinomas in relation to apoptosis. J Pathol. 1999;187(4):410–415. doi: 10.1002/(SICI)1096-9896(199903)187:4<410::AID-PATH266>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Selzer E, Schlagbauer-Wadl H, Okamoto I, Pehamberger H, Potter R, Jansen B. Expression of Bcl-2 family members in human melanocytes, in melanoma metastases and in melanoma cell lines. Melanoma Res. 1998;8(3):197–203. doi: 10.1097/00008390-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, Archimbaud E, Magaud JP, Guyotat D. High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091–3096. [PubMed] [Google Scholar]
- 32.Dole M, Nunez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994;54(12):3253–3259. [PubMed] [Google Scholar]
- 33.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimoto Y. Stress-resistance conferred by high level of Bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989;4(11):1331–1336. [PubMed] [Google Scholar]
- 35.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60(21):6101–6110. [PubMed] [Google Scholar]
- 36.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, Cohen GM. Concurrent up-regulation of Bcl-xL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 37.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Placzek WJ, Wei J, Kitad S, Zha D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death and Disease. 2010;1(e40):1–9. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, Schafer BW, Schrappe M, Stanulla M, Bourquin JP. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120(4):1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Ilizaliturri FJ, Khubchandani S, Olejniczak SH, Hoskin P, Czuczman MS. Strategies to overcoming rituximab-chemotherapy resistance by targeting the autophagy pathwya using bortezomib in combination with the Bcl-2 inhibitor obatoclax in non-Hodgkin's lymphomas (NHL) J Clin Oncol. 2009;27(15s) abstr 8543. [Google Scholar]
- 41.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68(9):3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Haas EC, di Pietro A, Simpson KL, Meijer C, Suurmeijer AJ, Lancashire LJ, Cummings J, de Jong S, de Vries EG, Dive C, et al. Clinical evaluation of M30 and M65 ELISA cell death assays as circulating biomarkers in a drugsensitive tumor, testicular cancer. Neoplasia. 2008;10(10):1041–1048. doi: 10.1593/neo.08620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holdenrieder S, von Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K, Hoffmann H, et al. Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in monitoring first-line chemotherapy of small cell lung cancer. Clin Cancer Res. 2008;14(23):7813–7821. doi: 10.1158/1078-0432.CCR-08-0678. [DOI] [PubMed] [Google Scholar]
- 44.Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J, Raith H, Feldmann K, Kremer AE, Muller S, et al. Clinical relevance of circulating nucleosomes in cancer. Ann N Y Acad Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- 45.Micha D, Cummings J, Shoemaker A, Elmore S, Foster K, Greaves M, Ward T, Rosenberg S, Dive C, Simpson K. Circulating biomarkers of cell death after treatment with the BH-3 mimetic ABT-737 in a preclinical model of small-cell lung cancer. Clin Cancer Res. 2008;14(22):7304–7310. doi: 10.1158/1078-0432.CCR-08-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummings J, Hodgkinson C, Odedra R, Sini P, Heaton SP, Mundt KE, Ward TH, Wilkinson RW, Growcott J, Hughes A, et al. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol Cancer Ther. 2008;7(3):455–463. doi: 10.1158/1535-7163.MCT-07-2136. [DOI] [PubMed] [Google Scholar]