Abstract
The activated mutants of the α-subunits of G proteins G12 and G13 have been designated as the gep oncogenes owing to their ability to stimulate diverse oncogenic signaling pathways that lead to neoplastic transformation of fibroblast cell lines and tumorigenesis in nude mice models. Studies from our laboratory as well as others have shown that the growth-promoting activities of Gα12 and Gα13 involve potent activation of c-Jun N-terminal kinases (JNKs). Our previous studies have indicated that the JNK-interacting leucine zipper protein (JLP), a scaffold protein involved in the structural and functional organization of the JNK/p38 mitogen-activated protein kinase module, tethers Gα12 and Gα13 to the JNK signaling module. In the present study, in addition to demonstrating the physical association between JLP and Gα12, we show that this interaction is enhanced by the receptor- or mutation-mediated activation of Gα12. We also establish that JLP interacts with Gα12 through the C-terminal domain that has been previously identified to be involved in binding to Gα13. Furthermore, using this C-terminal domain as a competitively inhibitor of JLP that can disrupt Gα12-JLP interaction, we demonstrate that JLP is required for the stimulation of JNK by Gα12. Our results also indicate that such JLP interaction is required for Gα12 as well as Gα13-mediated neoplastic transformation of JLP. These studies demonstrate for the first time a functional role for JLP in the gep oncogene-regulated neoplastic signaling pathway.
Introduction
The gep oncogenes, defined by the activated mutants of Gα12 and Gα13 [1,2], stimulate critical signaling pathways involved in cell growth, differentiation, and apoptosis [3–10]. Analyses of Gα12/13-mediated cell proliferation, differentiation, and apoptosis have identified that the activation of c-Jun N-terminal kinase (JNK) plays a central role in all of the critical pathways activated by Gα12/13 [2,3,7,11]. Our previous studies have shown that JNK-interacting leucine zipper protein (JLP) acts as a scaffold in linking Gα12 and Gα13 to the JNK signaling module [12]. Although these initial studies indicated the association between JLP and Gα12 or Gα13, the functional consequence of the interaction remains far from clear. Considering the observations that JNK plays a critical role in Gα12-mediated cell proliferation [6,11,13,14], we sought to investigate the role of JLP in Gα12-mediated activation of JNK and neoplastic transformation. In this report, we present our findings that JLP interacts with Gα12 through a specific C-terminal domain-similar to Gα13—and this interaction promotes Gα12-mediated activation of JNK. Furthermore, we demonstrate that the coexpression of JLP along with Gα12 greatly enhances the ability of Gα12 to induce neoplastic transformation of NIH3T3 cells and the expression of the dominant negative inhibitory mutant of JLP drastically decreases the transforming ability of activated Gα12 as well as Gα13. Thus, our findings presented here for the first time demonstrate a unique role for JLP in the oncogenic pathway(s) activated by the gep oncogenes.
Materials and Methods
Cells, Plasmids, and Transfections
NIH3T3 and COS-7 cells were maintained in Dulbecco modified Eagle medium (Invitrogen Life Technologies, Inc, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen Life Technologies) and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator. Expression vectors encoding S-epitope-tagged JLP, its mutant derivatives, Gα12, and Gα13 constructs have been previously described [3,10]. Transfection of COS-7 cells was carried out using FuGENE-6 reagent (Roche Applied Sciences, Indianapolis, IN) according to the manufacturer's protocol. Transfection of NIH3T3 cells was carried out using calcium phosphate precipitation method following the previously published procedures [9,12].
Coprecipitation and Immunoblot Analysis
Coprecipitation studies were carried out with COS-7 cells using S-protein agarose (Novagen, EMD Biosciences, Inc, Madison, WI) or antibodies specific to proteins of interest followed by protein A Sepharose beads (Amersham Biosciences Corp, Piscataway, NJ) as follows. For coprecipitation analyses with S-tagged JLP constructs, cells were lysed at 24 hours after transfection, and the lysate protein (500 µg each) was incubated with 30 µl of S-protein agarose for 4 hours at 4°C. After repeated washes with lysis buffer, the S-protein agarose-bound proteins were separated by SDS-PAGE and electroblotted on to polyvinylidene difluoride membranes for immunoblot analysis. Immunoblot analyses of lysates or immunoprecipitated proteins were carried out according to previously published procedures [9,12]. For immunoprecipitation analyses, cell lysate protein (500 µg each) was incubated with 1 µg of respective antibodies for 4 hours at 4°C followed by the addition of 20 µl of 50% slurry of protein A Sepharose. After repeated washes with cell lysis buffer, the immunoprecipitated proteins were separated by SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes for immunoblot analysis. Antibodies to HA epitope (2362) and phosphorylated JNK (9251) were obtained from Cell Signaling Solutions (Beverly, MA), whereas antibodies to S-epitope (sc-802), JNK-1 (sc-474), and Gα12 (sc-409) were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). For immunoblot analyses with Gα13, antibodies raised against the C-terminus of Gα13 (AS1-89-2) that has been previously described were used [15]. After washing the immunoprecipitates twice with lysis buffer, the immunoprecipitated proteins were separated by SDS-PAGE and electroblotted on to PVDF membranes. Immunoblot analyses with specific antibodies were carried out following the previously published procedures [12].
Focus Formation Assay
NIH3T3 cell focus formation assay was carried out as previously described [9]. Parental NIH3T3 cells were transfected with pcDNA3 vectors encoding Gα12QL or Gα13QL (5 µg) along with or without pSG5 vector encoding JLP and different domain constructs of JLP including GID-JLP (5 µg) using the calcium phosphate transfection method. Control group included the transfections with empty pcDNA3 vector (10 µg). Transfected NIH3T3 cells were cultured in the presence of 5% serum, and transformed foci were stained and scored after 14 days.
Results
Interaction between the gep Oncogene Gα12 and JLP
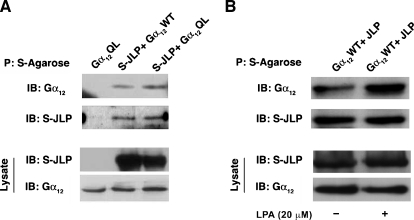
Our previous findings have shown that both Gα12 and Gα13 physically associate with JLP [12]. To analyze this further, especially the interaction between JLP and Gα12, we investigated whether the interaction is dependent on the activated status of Gα12. To test, COS-7 cells were cotransfected with pSG5-vector encoding S-protein epitope-tagged JLP (S-JLP) and pcDNA3 vector encoding Gα12WT or constitutively activated, GTPase-deficient mutant of Gα12QL. Transfectants were lysed at 48 hours, and the cell lysates were subjected to an S-protein agarose pull-down assay followed by immunoblot analysis with antibodies-specific to Gα12. Results from such analysis indicated the presence of Gα12 in JLP precipitates, thereby confirming our previous findings that Gα12 physically associates with JLP (Figure 1A). More interestingly, the levels of JLP pulled down from cells expressing Gα12QL showed an increase—although modest—in the associated Gα12 levels compared to that of wild-type Gα12 (lane 2 vs lane 3), indicating that the activated Gα12 more preferentially interacts with JLP.
Figure 1.
Gα12 interacts with JLP. (A) COS-7 cells were cotransfected with vectors encoding S-JLP along with wild-type form (Gα12WT) or constitutively active form (Gα12QL) of Gα12 expression constructs. Lysates were collected after 48 hours, and the pull-down assay was carried out using S-agarose beads. Immunoblot analysis was carried out to assess the presence of Gα12 in the S-JLP precipitates, using an antibody specific to Gα12. Expression levels of JLP and Gα12 were monitored by immunoblot analysis with Gα12 or S-protein antibody. Results form a typical experiment presented here (n = 3). (B) COS-7 cells were cotransfected with vectors encoding S-JLP along with wild-type form (Gα12WT). Transfectants were serum-starved for 24 hours after which the cells were stimulated with 20 µM of LPA for 10 minutes. After LPA stimulation, cells were lysed, and the lysates were subjected to S-agarose pull-down assay. Immunoblot analysis was carried out to assess the presence of Gα12 in the S-JLP precipitates, using an antibody specific to Gα12. Expression levels of JLP and Gα12 were monitored by immunoblot analysis with Gα12 or S-protein antibody. Results form a typical experiment from a set of three repeats.
To establish further that the activated conformation of Gα12 interacts more efficiently with JLP, we investigated whether receptor-mediated activation of Gα12 promotes an increase in its association with JLP. Therefore, we tested whether lysophosphatidic acid (LPA), which activates Gα12/13 through specific LPA receptors, can stimulate the association of Gα12 and JLP. Because COS-7 cells that are known to express LPARs [16], these cells were transfected with vectors encoding S-JLP and Gα12WT. At 24 hours after transfection, the transfectants were serum starved for 24 hours after which they were stimulated with 20 µM LPA for 10 minutes. The lysates from these transfectants were subjected to S-protein agarose precipitation. The S-protein agarose precipitates were analyzed for the presence of Gα12 by immunoblot analyses using Gα12-specific antibodies. As shown in Figure 1B, stimulating the transfectants with LPA increased the levels of Gα12 that get co-precipitated with S-JLP. Together with the increased association seen with mutationally activated Gα12 (Figure 1A), these results suggest that both the mutational and receptor-mediated activation of Gα12 can enhance its interaction with JLP.
Mapping the Gα12-Interacting Domain of JLP
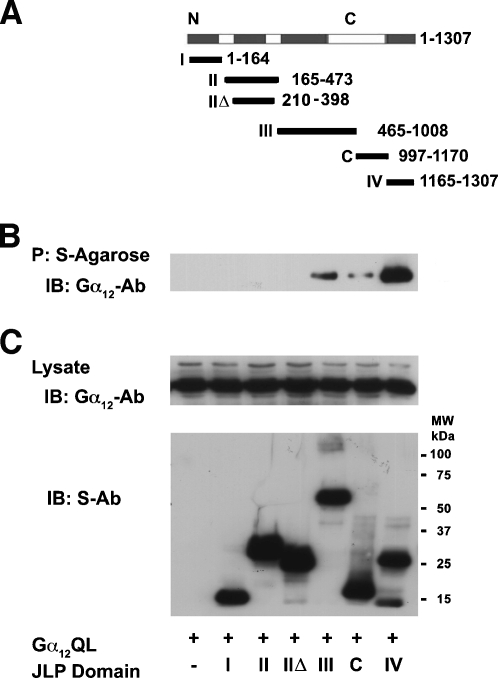
As a scaffold protein involved in JNK/p38 mitogen-activated protein kinase (MAPK) signaling, JLP contain specific motifs such as SH2, SH3, and leucine zipper domain to tether the functional constituents of the JNK module [17]. Previously, we have shown that the JNK-, KLC-1-, Gα13-, and SCG10-interacting domains of JLP can be mapped to distinct nonoverlapping regions of JLP [12,17–19]. Therefore, we were interested in determining whether JLP interacts with Gα12 through any of these known domains or a distinctly different one. To test, we analyzed its interactions with Gα12 using S-tagged constructs encoding distinct domains of JLP (Figure 2A). COS-7 cells were transfected with vector encoding the constitutively active Gα12QL along with S-tagged mutant constructs with different JLP domains for 48 hours. The transfectants were lysed and the lysates were precipitated with S-protein agarose beads,. and the presence of Gα12 in the precipitates was examined by immunoblot analysis using an antibody specific to Gα12. Results from this analysis indicated that Gα12 could be coprecipitated with S-JLP only from the transfectants expressing the extreme C-terminal domain of JLP spanning amino acids 1165–1307 (Figure 2B). Thus, these results demonstrate that JLP interacts with Gα12 through its extreme C-terminal domain, spanning 1165 to 1307 amino acids (domain IV). Interestingly, this is the same domain that was previously identified to be involved in JLP's interaction with Gα13 [12]. Thus, the C-terminal domain encompassing 1165–1307 amino acids of JLP that was previously referred as Gα13-interacting domain of JLP (GID-JLP) turns out to be an interacting domain for both Gα12 and Gα13.
Figure 2.
Gα12 interacts with the C-terminal domain of JLP. (A) Schematic diagram representing JLP deletion mutant constructs encoding different domains. These constructs encode S-tagged JLP-domain I (amino acids 1–164), II (amino acids 165–473), IIΔ (amino acids 210–398), III (amino acids 465–1008), C (amino acids 997–1170), and IV (amino acids 1165–1307). (B) Vector constructs expressing Gα12QL were coexpressed with the different domain mutants of S-JLP in COS-7 cells. After 48 hours, the lysates were subjected to the pulldown assay with the S-protein agarose beads. The coprecipitated Gα12 was identified by immunoblot analysis using antibodies to Gα12. (C) The expression levels of transfected Gα12QL and JLP constructs were monitored by immunoblot analyses using antibodies to Gα12 (upper panel) or S-tag (lower panel).
JLP Enhances Gα12 Activation of JNK
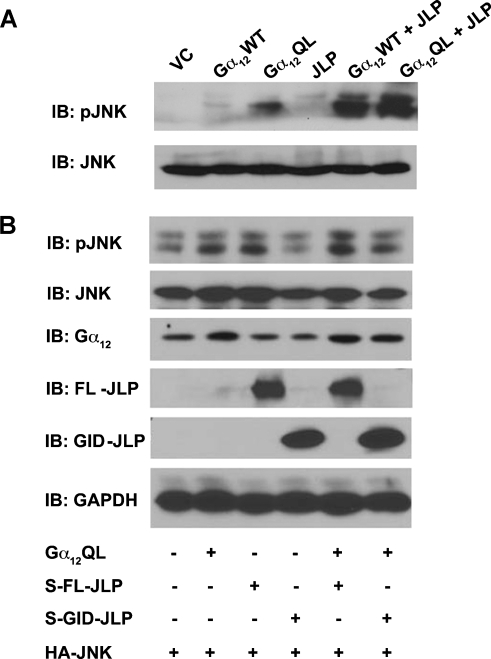
Based on the findings that 1) Gα12 interacts with JLP through the extreme C-terminal GID domain and 2) JLP interacts with JNK through the N-terminal domain [17], it can be hypothesized that JLP tethers Gα12 to the JNK module, thereby promoting its ability to stimulate the JNK module. If this hypothesis is true, cotransfection of JLP should enhance Gα12-mediated JNK response, whereas the inhibition of such interaction should attenuate Gα12-mediated activation of JNK. In fact, our previous studies have shown that the coexpression of JLP stimulates LPA as well as Gα13-mediated activation of JNK, whereas the coexpression of GID-JLP inhibits such activation [12]. To test whether the same holds true for Gα12, COS-7 cells were transfected with the activated mutant of Gα12 (Gα12QL) in the absence or presence of JLP for 48 hours. The lysates from these transfectants were resolved by SDS-PAGE and subjected to immunoblot analysis using antibodies specific to the phosphorylated forms of JNK to monitor their activation profile. Results indicated that Gα12 stimulated an increase in the activation of JNK (Figure 3, lane 3), and this could be further enhanced by the coexpression of JLP (Figure 3A, lanes 5 and 6), thus suggesting that JLP plays a potentiating role in the Gα12-mediated activation of JNK signaling module. In contrast, when COS-7 cells were transiently transfected with vectors encoding Gα12QL along with S-epitope-tagged GID-JLP for 48 hours, Gα12-stimulated increase in the activation of JNK (Figure 3B, lane 2) was attenuated by the coexpression of GID-JLP (Figure 3B, lane 6). Taken together, these results establish a cardinal role for JLP in Gα12-mediated stimulation of JNK.
Figure 3.
JLP enhances Gα12-mediated activation of JNK. (A) COS-7 cells were transiently transfected with pcDNA3 vector control, wild-type (Gα12WT), or constitutively active (Gα12QL) forms of Gα12 alone or along with JLP. Cells were collected after 48 hours of transfection. These lysates were subjected to immunoblot analysis with antibodies to phospho-specific JNK to assess its activation profile. After stripping, the blot was reprobed with JNK antibody to monitor equal expression levels of JNK. (B) COS-7 cells were cotransfected with constructs expressing JNK, Gα12QL, and full-length S-JLP or mutant GID-JLP (bearing amino acids 1165–1307). Total cell lysates were subjected to immunoblot analysis and assessed for JNK phosphorylation using phospho-JNK antibody. Cell lysates were also analyzed for the expression of HA-JNK, Gα12, full-length S-JLP, and mutant GID-JLP with their respective antibodies.
JLP Is Involved in gep Oncogene-Mediated Neoplastic Transformation
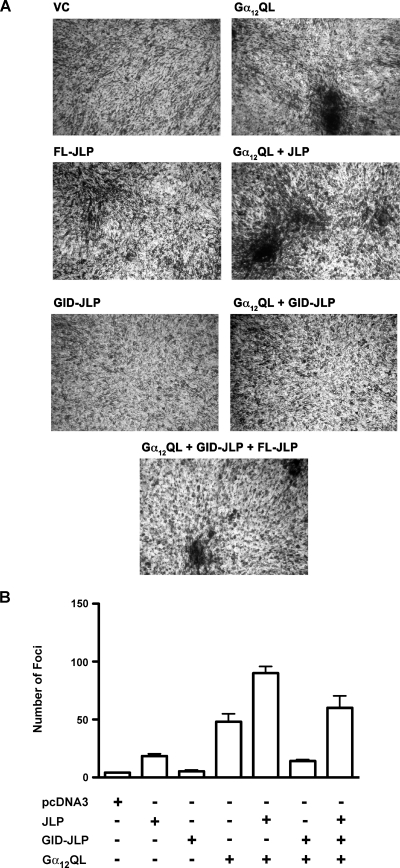
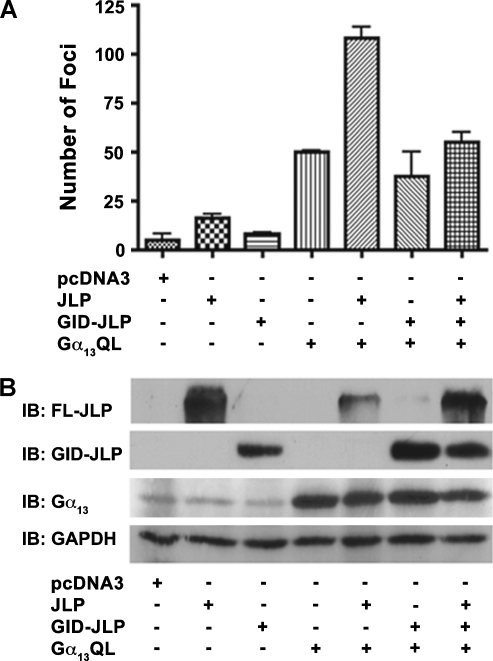
Our findings that JLP potentiates Gα12-mediated activation of JNK gains greater significance in light of the previous observation from our laboratory as well as others that JNK plays a critical role in Gα12-mediated mitogenic signaling pathways [3,5,6,10]. Therefore, we focused on evaluating whether the inhibition of Gα12-mediated JNK response by the coexpression of GID-JLP would attenuate the neoplastic transformation mediated by the either of the gep oncogenes. Neoplastic transformation of NIH3T3 fibroblast cells and subsequent foci formation have been widely used to monitor the oncogenic potential of different oncogenes including Gα12/13 [20–24]. To carry out this analysis, NIH3T3 cells were transfected with vectors encoding Gα12QL, full-length JLP, and GID-JLP alone or in combinations. At 14 days after transfection, the number of foci formed was scored by fixing the cells using glutaraldehyde followed by crystal violet staining. Our results indicated that the expression of Gα12QL induces foci formation in NIH3T3 cells confirming the previous results from several laboratories including ours [22–24]. The foci formation was greatly increased by the coexpression of JLP. Quite significantly, the coexpression of GID-JLP drastically inhibited the number of foci formed compared with Gα12QL alone (Figure 4). More interestingly, the inhibitory effect of GID-JLP can be neutralized by the coexpression of full-length JLP (Figure 4), thereby establishing the critical role of JLP inGα12-mediated neoplastic transformation. Quantification of the foci from repeat experiments indicated that the number of foci induced by Gα12QL was increased by the coexpression of by 90%. In contrast, the coexpression of the dominant negative GID-JLP reduced Gα12QL-induced foci formation by 70%. Interestingly, the coexpression of JLP along with GID-JLP rescued such GID-JLP mediated inhibition. Similar results were obtained using Gα13QL, which is known to involve JNK signaling interacting with JLP (Figure 5A). Taken together, these results suggest that a functional interaction between Gα12/13 and JLP is involved in neoplastic transformation mediated by the gep oncogenes. These results for the first time identify a novel role for JLP in the oncogenic pathways induced by the gep oncogenes Gα12QL and Gα13QL.
Figure 4.
Effect of JLP and GID-JLP on Gα12QL-mediated foci formation. Parental NIH3T3 cells were transfected with empty vector pcDNA3 (VC), Gα12QL, full-length JLP (FL-JLP), Gα12QL + FL-JLP, GID-JLP, Gα12QL + GID-JLP, or Gα12QL + GID-JLP + FL-JLP using the calcium phosphate transfection method. (A) The representative foci formed at 14 days were stained photographed (4x). Results presented are from a typical experiment (n = 4). (B) The foci formed from four independent experiments were scored and plotted (mean ± SD).
Figure 5.
Effect of JLP and GID-JLP on Gα13QL-mediated foci formation. (A) NIH3T3 cells were transfected with empty vector pcDNA3 (vector), full-length JLP (FL-JLP), GID-JLP, Gα13QL, Gα13QL + FL-JLP, Gα13QL + GID-JLP, or Gα13QL + GID-JLP + FL-JLP using the calcium phosphate transfection method. After 14 days, the number of foci from three independent experiments was scored and plotted (mean ± SD). (B) At 14 days after transfection, expressions of the encoded proteins by the transfected complementary DNA constructs were verified by immunoblot analysis using a parallel set of transfectants.
Discussion
Members of the gep oncogene family, defined by the activated mutants of Gα12 and Gα13, are the most potent G protein subunits that have been observed to promote oncogenic signaling [2]. It has been demonstrated that the activation JNK is critically required for the G1-S transition stimulated by Gα12/13 [6,10]. It has also been observed that Gα12 differentially regulates JNK, extracellular signal-regulated kinase, and p38 MAPK modules [25]. Whereas these findings suggested the role for MAPKs, specifically JNK in the oncogenic signaling by the gep oncogenes, the possible role of a specific scaffold protein that can tether Gα12 or Gα13 to MAPK signaling is largely not understood until now. Our observation presented here demonstrating a novel interaction between Gα12 and the JNK scaffold protein JLP and (Figures 1–3) as well as the critical role of JLP in Gα12/13-mediated oncogenic signaling pathway (Figure 4) confirms and identifies JLP as a scaffold protein involved in G12/13-mediated oncogenic signaling.
Our results also identify the C-terminal region of JLP, spanning amino acids 1165–1307, as the critical domain of interaction with Gα12 (Figure 2). It is significant to note here that we have previously established this region as the domain that interacts with Gα13 and hence referred to it as the Gα13-interacting domain of JLP. Our present finding redefines this region as the domain that can interact with both Gα12 and Gα13. Similar to our previous results obtained with Gα13, we have shown here that the coexpression of JLP enhances Gα12-mediated JNK activation, whereas the overexpression of GID-JLP inhibits JNK activation. The fact that JLP interacts with both Gα12 and Gα13 through a single domain may be indicative that JLP binding to these α-subunits is mutually exclusive. If so, this would mean that the JLP interaction with Gα12 or Gα13 might be cell type- and/or context-specific. In this context, it should be noted here that JLP has been shown to interact with kinesin light chain 1 and this interaction has been ascribed to play a role in spatial regulation of JLP-associated signaling molecules [18]. This raises an interesting possibility that the spatiotemporal context involving JLP and its interacting proteins may determine whether JLP interacts with Gα12 or Gα13. Further studies should clarify whether such context-specific events determine which member of the gep oncogenes interacts JLP in vivo. Another set of observations that can support such context-specific signaling is our present observation that JLP is involved in Gα12/13-mediated neoplastic transformation of NIH3T3 cells (Figures 4 and 5) in conjunction with our previous findings demonstrating that JLP is involved in Gα13-specific retinoic acid-mediated endodermal differentiation of P19 embryonic carcinoma cells [26]. In this regard, the scaffolding role of JLP can be equated to the context-specific signaling role observed with the yeast MAPK scaffold protein Ste5 [27–29]. In yeast, upon stimulated by the mating pheromone, the scaffold protein Ste5 nucleates the assembly of the β-subunit of heterotrimeric G protein GPA (Gβ) to a kinase module consisting of MAP4K, MAP3K, MAP2K, defined by Ste20, Ste 11, Ste7 to activate Fus3, a MAPK [30,31]. Interestingly, it has been shown that, in the absence of any pheromone, Ste5 can also facilitate the assembly of the same Gβ-Ste20-Ste11-Ste7 complex to activate Kss1, a different MAPK, to elicit invasive growth response of haploid cells or vegetative growth of diploid cells [27]. Thus, in this model system, the differential context-specific recruitment of the downstream MAPKs, namely, Fus3 and Kss1 can drastically alter the final signaling outcome of the Gprotein-MAPK scaffold [30]. It is likely that JLP provide such context-specific scaffolding role for both oncogenic and differentiation by effectively tethering different downstream signaling components.
In addition to these observations, careful analysis of JNK activation by Gα12 in the presence of JLP provides novel insight into the dynamic manner in which the scaffold protein such as JLP modulates the activity of the associated kinase. Whereas the expression of Gα12 or Gα12QL along with JLP leads to the maximal activation of JNK (Figure 3A, lanes 5 and 6), coexpression of epitope-tagged JNK seems to elicit only a moderate activation of JNK by Gα12QL (Figure 3B, lane 2 vs lane 5). It is quite possible that the overexpression of JNK and the resultant increase in the basal levels of activated JNK (Figure 3B, lane 1)— possibly forming endogenous competing signaling complexes—leads to the squelching of Gα12QL-JLP signaling. Further characterization of JLP and its interacting proteins should provide finer details on the dynamics of JLP and Gα12 interaction in the activation of JNK. Nevertheless, the observation that the coexpression of GID-JLP attenuates Gα12QL-stimulated JNK activity to basal levels (Figure 3B, lanes 3 vs 6) further confirms the crucial role played by JLP in Gα12-mediated activation of JNK.
Finally, our results presented here point to a critical pathobiological role JLP can play in the oncogenic pathway stimulated by LPA. It has been well established that LPA plays a critical role in the genesis and progression of many different cancers [32–35]. It has also been shown that LPAR-deficient mouse embryonal fibroblasts show deficiency in the activation of JNKs. In this context, our observation that LPA stimulates the association of JLP to Gα12 (Figure 1B) and our previous findings that LPA-stimulated JNK activity can be inhibited by the expression of GID-JLP that can inhibit JLP-Gα12/13 interaction [12] gain greater significance. The observations that 1) LPA signaling is involved in the pathophysiology of many cancers [32–35], 2) LPA potently stimulates the gep oncogenes Gα12/13 [13], 3) LPA stimulates the association between Gα12 and JLP (Figures 1 and 2), and 4) both LPA- [12] and Gα12-mediated stimulation of JNK is inhibited by GID-JLP (Figures 3 and 4) suggest a key role JLP in LPA-Gα12/13-mediated oncogenic pathway. The observation that JLP plays a critical role in Gα12/13-mediated neoplastic transformation (Figures 4 and 5) provides additional support for this view. Now that such basic paradigm is established, further studies should clarify the critical role of Gα12/13 and JLP in LPA-promoted mediated tumorigenesis and tumor progression.
Abbreviations
- JNK
c-Jun N-terminal kinase
- JLP
JNK-interacting leucine zipper protein
- MAPK
mitogen-activated protein kinase
Footnotes
This work was supported by grants from the National Institutes of Health (CA123233) and the World Class University project funded by Ministry of Education, Science and Technology Development, Republic of Korea (no. R32-2008-000-10098-0).
References
- 1.Gutkind JS, Coso OA, Xu N. Gα12- and Gα13-subunits of heterotrimeric G proteins: a novel family of oncogenes. In: Spiegel S, editor. G Proteins, Receptors, and Diseases. Totowa, NJ: Humana Press; 1998. pp. 101–117. [Google Scholar]
- 2.Radhika V, Dhanasekaran N. Transforming G proteins. Oncogene. 2001;20:1607–1614. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- 3.Prasad MV, Dermott JM, Heasley LE, Johnson GL, Dhanasekaran N. Activation of Jun kinase/stress-activated protein kinase by GTPase-deficient mutants of Gα12 and Gα13. J Biol Chem. 1995;270:18655–18659. doi: 10.1074/jbc.270.31.18655. [DOI] [PubMed] [Google Scholar]
- 4.Voyno-Yasenetskaya TA, Faure MP, Ahn NG, Bourne HR. Gα12 and Gα13 regulate extracellular signal-regulated kinase and c-Jun kinase pathways by different mechanisms in COS-7 cells. J Biol Chem. 1996;271:21081–21087. doi: 10.1074/jbc.271.35.21081. [DOI] [PubMed] [Google Scholar]
- 5.Collins LR, Minden A, Karin M, Brown JH. Gα12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J Biol Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- 6.Mitsui H, Takuwa N, Kurokawa K, Exton JH, Takuwa Y. Dependence of activated Gα12-induced G1 to S phase cell cycle progression on both Ras/mitogen-activated protein kinase and Ras/Rac1/Jun N-terminal kinase cascades in NIH3T3 fibroblasts. J Biol Chem. 1997;272:4904–4910. doi: 10.1074/jbc.272.8.4904. [DOI] [PubMed] [Google Scholar]
- 7.Berestetskaya YV, Faure MP, Ichijo H, Voyno-Yasenetskaya TA. Regulation of apoptosis by α-subunits of G12 and G13 proteins via apoptosis signalregulating kinase 1. J Biol Chem. 1998;273:27816–28723. doi: 10.1074/jbc.273.43.27816. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RN, Radhakrishnan R, Ha JH, Dhanasekaran N. Proteome analysis of NIH3T3 cells transformed by activated Gα12: regulation of leukemia-associated protein SET. J Proteome Res. 2004;3:1177–1183. doi: 10.1021/pr049896n. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RN, Ha JH, Radhakrishnan R, Dhanasekaran DN. Transactivation of platelet-derived growth factor receptor alpha by the GTPase-deficient activated mutant of Gα12. Mol Cell Biol. 2006;26:50–62. doi: 10.1128/MCB.26.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radhakrishnan R, Ha JH, Dhanasekaran DN. Mitogenic signaling by the gep oncogene involves the upregulation of S-phase kinase-associated protein 2. Genes Cancer. 2010;1:1033–1043. doi: 10.1177/1947601910390516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jho EH, Davis RJ, Malbon CC. c-Jun amino-terminal kinase is regulated by Gα12/Gα13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J Biol Chem. 1997;272:24468–24474. doi: 10.1074/jbc.272.39.24468. [DOI] [PubMed] [Google Scholar]
- 12.Kashef K, Lee CM, Ha JH, Reddy EP, Dhanasekaran DN. JNK-interacting leucine zipper protein is a novel scaffolding protein in the Gα13 signaling pathway. Biochem. 2005;44:14090–14096. doi: 10.1021/bi050604l. [DOI] [PubMed] [Google Scholar]
- 13.Radhika V, Ha JH, Jayaraman M, Tsim ST, Dhanasekaran N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Gα12. Oncogene. 2005;24:4597–4603. doi: 10.1038/sj.onc.1208665. [DOI] [PubMed] [Google Scholar]
- 14.Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease activated receptor-1 signals though Gq and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J Biol Chem. 2003;278:46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- 15.Radhika V, Onesime D, Ha JH, Dhanasekaran N. Gα13 stimulates cell migration through cortactin-interacting protein Hax-1. J Biol Chem. 2004;279:49406–49413. doi: 10.1074/jbc.M408836200. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J, Slice LW, Rozengurt E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J Biol Chem. 2003;278:4882–4891. doi: 10.1074/jbc.M211175200. [DOI] [PubMed] [Google Scholar]
- 17.Lee CL, Onesime D, Reddy CD, Dhanasekaran N, Reddy EP. JLP: a scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors. Proc Natl Acad Sci USA. 2000;99:14189–14194. doi: 10.1073/pnas.232310199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen Q, Lee CM, Reddy EP. JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J Biol Chem. 2005;280:30185–30191. doi: 10.1074/jbc.M505499200. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Dhanasekaran DN, Lee CM, Reddy EP. Regulation of neurite outgrowth by interactions between the scaffolding protein, JNK-associated leucine zipper protein, and neuronal growth-associated protein superior cervical ganglia clone 10. J Biol Chem. 2010;285:3548–3553. doi: 10.1074/jbc.M109.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox AD, Der CJ. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. doi: 10.1016/0076-6879(94)38026-0. [DOI] [PubMed] [Google Scholar]
- 21.Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA. Expression cDNA cloning of a transforming gene encoding the wild-type Gα12 gene product. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu N, Ambudkar I, Gutkind JS. A mutant α subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH3T3 cells. Proc Natl Acad Sci USA. 1994;90:6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vara Prasad MV, Shore SK, Dhanasekaran N. Activated mutant of Gα13 induces Egr-1, c-fos, and transformation in NIH 3T3 cells. Oncogene. 1994;9:2425–2429. [PubMed] [Google Scholar]
- 24.Voyno-Yasenetskaya TA, Pace AM, Bourne HR. Mutant a subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene. 1994;9:2559–2565. [PubMed] [Google Scholar]
- 25.Dermott JM, Ha J, Lee C, Dhanasekaran N. Differential regulation of Jun N-terminal kinase and p38MAP kinase by Gα12. Oncogene. 2004;23:226–232. doi: 10.1038/sj.onc.1207009. [DOI] [PubMed] [Google Scholar]
- 26.Kashef K, Xu H, Reddy EP, Dhanasekaran DN. Endodermal differentiation of murine embryonic carcinoma cells by retinoic acid requires JLP, a JNK-scaffolding protein. J Cell Biochem. 2006;98:715–722. doi: 10.1002/jcb.20930. [DOI] [PubMed] [Google Scholar]
- 27.Andersson J, Simpson DM, Qi M, Wang Y, Elion EA. Differential input by Ste5 scaffold and Msg5 phosphatase route a MAPK cascade to multiple outcomes. EMBO J. 2004;23:2564–2576. doi: 10.1038/sj.emboj.7600250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elion EA, Qi M, Chen W. Signal transduction. Signaling specificity in yeast. Science. 2005;307:687–688. doi: 10.1126/science.1109500. [DOI] [PubMed] [Google Scholar]
- 29.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 30.Elion EA. Ste5: a meeting place for MAP kinases and their associates. J Cell Sci. 2001;114:3967–3978. doi: 10.1016/s0962-8924(00)89055-8. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MA, Madhani HD. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- 32.Daaka Y. Mitogenic action of LPA in prostate. Biochim Biophys Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, Kimura T, Kuwabara A, Yanagita Y, Ikeya T, Tanahashi Y, et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem. 2004;279:6595–6605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- 34.Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, Yamori T, Aoki J, Fujimaki T, Arai H. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J Biol Chem. 2006;281:17492–17500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- 35.Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010;102:941–946. doi: 10.1038/sj.bjc.6605588. [DOI] [PMC free article] [PubMed] [Google Scholar]