Abstract
There is an increasing incidence of esophageal adenocarcinoma (EA) among younger people in the western populations. However, the association between genetic polymorphisms and the age of EA onset is unclear. In this study, 1330 functional/tagging single-nucleotide polymorphisms (SNPs) from 354 cancer-related genes were genotyped in 335 white EA patients. Twenty important SNPs that have the highest importance scores and lowest classification error rate were identified by the random forest algorithm to be associated with early onset of EA (age ≤ 55 years). Subsequent logistic regression analysis indicated that 10 SNPs (rs2070744 of NOS3, rs720321 of BCL2, rs17757541 of BCL2, rs11775256 of TNFRSF10A, rs1035142 of CASP8, rs2236302 of MMP14, rs4740363 of ABL1, rs696217 of GHRL, rs2445762 of CYP19A1, and rs11941492 of VEGFR2/KDR) were significantly associated with early onset of EA (≤55 vs >55 years, all P < .05 after adjusting for co-variates and false discovery rate). Among them, five SNPs in the NOS3, BCL2, TNFRSF10A, and CASP8 genes were known to be involved in apoptosis processes. In Kaplan-Meier analyses, rs2070744 of NOS3, rs720321 of BCL2, and rs1035142 of CASP8 were also significantly associated with early onset of EA. Moreover, there was a higher risk of developing EA at a younger age when one had more risk genotypes. In conclusion, polymorphisms in cancer-related genes, especially those in the apoptotic pathway, play an important role in the development of younger-aged EA in a dose-response manner.
Introduction
The incidence of esophageal adenocarcinoma (EA) has increased rapidly in the past few decades and surpassed that of squamous cell carcinoma in many western countries [1]. There is a concomitant rise in the numbers of young EA patients. Previous reviews showed that such patients had more advanced disease at presentation compared with those diagnosed at an older age [2,3]. This is probably because current guidelines do not suggest urgent endoscopic examination in dyspeptic patients younger than 55 years without alarm symptoms such as chronic gastrointestinal bleeding, progressive weight loss, or dysphagia [4,5]. However, once such symptoms develop, the disease is usually in the advanced stages and inoperable. Factors contributing to early onset of EA are still poorly understood.
The cause of early and late onset of esophageal cancer may be both different and complex. Nevertheless, genetic variation may be an important variable for early onset cancers. An esophageal squamous cell carcinoma (ESCC) study indicated that polymorphisms in ADH1B and ALDH2 genes that are implicated in acetaldehyde elimination can modify the risk of alcohol drinking in ESCC and can contribute substantially to the early onset of ESCC [6]. A study of EA risk showed that patients carrying the A870 allele in cyclin D1 had higher risk for younger age of EA development [7]. However, investigation on polymorphisms of p53 Arg72Pro, MDM2 T309G, and CCND1 G870A genes did not find any association between polymorphisms and age of EA onset [8]. These observations suggest that a few number of SNPs or genes may not be sufficient enough to define the contribution of genetic factors to EA development because EA is a complex disease likely involved in multiple genetic factors [9]. In this study, we systematically investigated the associations of genetic polymorphisms with early onset of EA using a pathway-based approach that included 1330 functional/tagging SNPs in 354 cancer-related genes. Our hypothesis is that patients who develop early onset of EA carry different alleles in cancer-related genes compared with those who develop late onset of EA.
Patients and Methods
Study Population and Interview
Three hundred thirty-five incident white patients were recruited into this study. They were older than 18 years and histologically confirmed to have EA at the Massachusetts General Hospital between 1999 and 2005 and at Dana-Farber Cancer Institute between 2004 and 2005. All of them had a tumor center located at or above the gastroesophageal junction and had at least two-thirds of the bulk tumor located in the esophagus. Patients with secondary or recurrent cancers were excluded. The recruitment rate was 86%. Details of patient recruitment were described in our previous article [10,11]. Patients underwent upper gastrointestinal endoscopy, esophagogram, and computed tomography of chest and abdomen for staging workup. Clinicopathologic stage was determined based on the TNM classification according to the International Union Against Cancer [12].
All participants were interviewed using a modified questionnaire [13] immediately after enrollment to collect information on their demographic characteristics and history about gastroesophageal reflux disease (GERD) symptoms and smoking. Lifetime GERD-related symptoms were collected based on the questions described previously [14]. The presence of GERD was defined as having heartburn or regurgitation symptoms at least once per month for more than six continuous months in one's lifetime [10,14]. Smoking status was defined based on whether the patient smoked 1 year before diagnosis. Body weight and height at age 18 were obtained from each subject in a self-reported manner, and the body mass index (BMI) was calculated accordingly.
Definition of Younger Age of Onset
Current guidelines use 55 years as the cutoff for recommendation of urgent endoscopy referral [4,5]. This is based on the reports that cancers of the upper gastrointestinal tract is detected in less than 1% of western populations undergoing endoscopy for dyspepsia [15]. The incidence of EA in males younger than 55 years was less than 5 per 100,000, which doubled at age 65 years and peaked at the age of 75 to 79 years (15/100,000) during 1973 to 2002 [1]. Here, we use 55 years or younger as the definition of early onset of EA, as suggested in previous reports [3].
DNA Preparation
Blood samples were collected at the time of recruitment, and DNA was extracted using the Puregene DNA Isolation Kit (Gentra Systems/Qiagen, Valencia, CA). The success rate in extracting DNA was 100%. This study was approved by the human subjects committees of Massachusetts General Hospital, Dana-Farber Cancer Institute, and the Harvard School of Public Health (Boston, MA). All subjects signed the informed consent before study participation.
SNP Selection, Pathway Categorization, and Genotyping
A custom-designed Illumina GoldenGate platform was used. The candidate SNPs in this study were common SNPs with minor allele frequency ≥ 5% selected from the HapMap database of white population (CEU). They were either missense/exonic SNPs, SNPs within UTR regions, and 2 kb of the 5′ region of the gene, or tagSNPs. The common nonsynonymous SNPs were selected from the databases of the SNP500Cancer Project and International HapMap Project. Potential functional nonsynonymous SNPs were selected from the PICS (Predicted Impact of Coding SNPs) database and FASTSNP. SNPs on the Illumina Cancer Panel were chosen with priority. TagSNPs were selected using the r2-based Tagger program with pairwise r2 ≥ 0.80 and minor allele frequency ≥ 5% among whites. The details for SNP selection in the GoldenGate assay was described previously [11]. Genotyping was finished by February 2008 at the Broad Institute of Massachusetts Institute of Technology and Harvard University. Of all cases, 97% were successfully genotyped. Ninety-six DNA samples from patients with early and later onset of EA were analyzed in each platform.
Genes in cancer-related pathway were chosen based on the literature reports and information in the National Center for Biotechnology Information Genetic Association Database, National Institute for Environmental Health Science GeneSNPs, and Kyoto Encyclopedia of Genes and Genomes. More frequently cited genes were given greater priority. Genotyping was performed on the Illumina GoldenGate assay at the Broad Institute (Cambridge, MA) by laboratory personnel blinded to the subjects' clinical information. Forty-eight duplicate samples were randomly selected for quality control. In total, 1330 SNPs (354 genes) categorized into 14 cancer-related pathways were successfully genotyped. The concordance rate of the replicate samples for all assays was greater than 99%.
Statistical Analysis
Mean and SD were used to describe quantitative data, and proportions are used to describe categorical data. The Student's t and χ2 tests were used to compare the means and proportions between early and later onset groups.
To test the joint effects of SNPs in the same pathway, we used a logistic kernel machine model. This method tests the association between outcome and a kernel function combining a set of SNPs, which allows for flexible modeling of SNP effects [11]. An Identity-by-State kernel was used to account for the relationship among SNPs.
For SNP-based analysis, we applied a three-step approach. First, we used a random forest algorithm [16], using early or later onset as the outcome and SNPs as the predictors, to impute the missing values (<4% in each variable) and then obtained the mean decrease of accuracy (MDA) for each SNP. The MDA measures the degree of loss if the corresponding SNP was “removed” from the model and could be regarded as an “importance” score for the SNP. Second, the sliding windows sequential forward feature selection method (SWSFS) was used to identify the top important SNPs [17]. The SWSFS method includes the SNPs one by one to the random forest model by the order of MDA scores in step one. To minimize random bias, 100,000 trees were constructed, allowing each variable to have approximately 100% of the probability tested 500 times. Then we plotted the “out of bagging” error, which measured the performance of each model consisting of different number of SNPs to identify a SNP set with the lowest error rate for further analysis. Third, we performed a case-control univariate logistic regression analysis using additive genetic model and multiple logistic regressions for covariate adjustment. False discovery rates (FDRs) were used to correct for multiple comparisons [18]. To estimate the cumulative risk effects of the significant SNPs, we counted the number of risk genotypes representing a genetic risk “score” and used logistic models to find the contribution of the score to the risk of early onset.
We also used Kaplan-Meier curves to describe the relationships between risks of onset on different ages and genotypes in dominant model (mutant carriers vs wild-type homozygotes). Statistical significance was tested using Cox proportional hazard models. The goodness of fit for each model was tested using Hosmer-Lemeshow lack of fit test for logistic regression. P > .10 was considered an acceptable goodness of fit. All data analyses were performed using the SAS statistical package, version 9.1 (SAS Institute, Cary, NC). Random forest analysis was performed using rpart package in R software, version 2.9.1.
Results
Compared with those older than 55 years when diagnosed with cancer, patients with younger age of EA onset had significantly higher BMI (24.6 ± 4.4 vs 23.3 ± 3.5 kg/m2, P = .01) and later stage at diagnosis (70.8% vs 55.3% in stage III–IV, P = .01; Table 1). In Cox regression model, BMI greater than 25 kg/m2 was also significantly associated with younger median age of EA onset (59.9 vs 65.4 years; relative risk, 1.30; 95%confidence interval [CI], 1.03–1.64; P = .03). The other major risk factors of EA, namely sex, presence of GERD symptoms, GERD frequency/duration, and smoking habit, were not significantly different between these two groups (Table 1). Although 43 and 46 patients, respectively, who had GERD symptoms did not provide adequate information on GERD frequency and duration, their demographic data were not statistically different in the two age groups (Tables W1 and W2).
Table 1.
Characteristics of Early Onset and Later Onset EA Patients.
| Characteristics | Early Onset (≤55 Years), n = 89 | Later Onset (>55 years), n = 246 | P |
| Sex, male, n (%) | 79 (88.8) | 216 (87.8) | .81 |
| GERD,* yes, n (%) | 42 (47.2) | 131 (53.3) | .33 |
| Frequency of GERD, n (%), times/wk | |||
| ≤2 | 19 (45.2) | 48 (36.6) | .07† |
| ≥3 | 9 (21.4) | 51 (38.9) | |
| Unknown | 14 (33.4) | 32 (24.4) | |
| Duration of GERD, n (%), years | |||
| ≤10 | 10 (23.8) | 46 (35.1) | .34† |
| >10 | 19 (45.2) | 55 (42.0) | |
| Unknown | 13 (31.0) | 30 (22.9) | |
| Ever smokers, yes, n (%) | 66 (74.2) | 203 (82.6) | .09 |
| BMI at age 18 (mean ± SD), kg/m2 | 24.6 ± 4.4 | 23.3 ± 3.5 | .01 |
| BMI ≥ 25 kg/m2 at age 18, n (%) | 37 (42.6) | 67 (27.2%) | .01 |
| Stage, n (%) | |||
| I–II | 26 (29.2) | 110 (44.7) | .01 |
| III–IV | 63 (70.8) | 136 (55.3) | |
Seven (2%) missing and imputed.
Not comparing those with missing data.
Among the 14 pathways in this study, the apoptosis pathway remained significant after correcting for multiple comparisons (nominal P = .004, FDR = 0.05; Table 2). Although this pathway comprises the most number of SNPs in our GoldenGate platform, the other two pathways involving inflammation and carcinogens/procarcinogens metabolism, which also included more than 200 SNPs, were not related to the age of EA onset (Table 2).
Table 2.
Associations of Pathways with Susceptibility to Early Onset (Age ≤ 55 Years) of EA.
| Pathway | No. Genes | No. SNPs | Nominal P* | FDR |
| Apoptosis | 41 | 233 | .004 | 0.05 |
| Cell signaling | 28 | 82 | .03 | 0.22 |
| Telomere maintenance | 1 | 5 | .07 | 0.31 |
| Angiogenesis | 15 | 140 | .09 | 0.31 |
| Cell growth | 30 | 64 | .18 | 0.48 |
| DNA methylation | 4 | 14 | .23 | 0.48 |
| Immunity | 10 | 20 | .24 | 0.48 |
| Molecular transport | 8 | 16 | .28 | 0.49 |
| Metastasis | 15 | 17 | .54 | 0.84 |
| Cell cycle control | 48 | 149 | .70 | 0.93 |
| Inflammation | 37 | 219 | .80 | 0.93 |
| Carcinogen/procarcinogen metabolism | 71 | 208 | .86 | 0.93 |
| Sex hormone signaling | 11 | 65 | .86 | 0.93 |
| DNA repair | 51 | 129 | .95 | 0.95 |
Pathway-based analysis assessed by the logistic kernel machine regression.
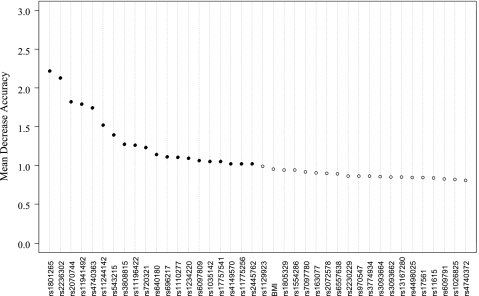
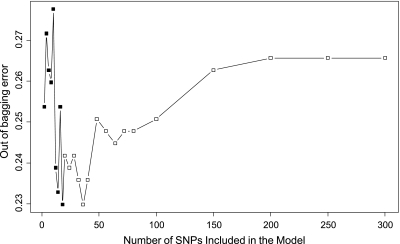
In the random forest analysis, the importance score (MDA) for each variable that quantifies the relative contribution of that variable to the prediction accuracy was obtained (Figure 1). Interestingly, the well-established EA risk factors such as smoking and GERD were not listed in the top markers to discriminate early and later onset of EA. The most important environmental factor recognized was BMI, which only ranked 22 in this algorithm (Figure 1). The SWSFS algorithm identified a set of 20 SNPs that had the lowest classification error rates (Figure 2). Therefore, the top 20 SNPs with MDA greater than 1 shown in Figure 1 were selected for further logistic regression analysis, which used subjects with later onset of EA (>55 years when diagnosed with cancer) as reference.
Figure 1.
Importance score plot of top 40 SNPs selected from 1330 SNPs using the random forest algorithm. Higher MDA means higher contribution to the prediction of early onset. MDA was estimated from 100,000 trees.
Figure 2.
Prediction error rate of 1330 SNPs using early onset (≤55 years) of EA as outcome. The x-axis denotes the number of SNPs in the model, where the SNPs are included one by one based on their MDA ranks ranging from the most (left) to the least (right) importance. The y-axis is the error rate estimated from 100, 000 trees by random forest analysis. A set of 20 SNPs that had the lowest error rates (0.23) was selected for further logistic regression analysis.
Among the top 20 SNPs, 10 remained significantly associated with age of cancer onset after adjusted for covariates and FDR (FDR < 0.05; Table 3). Of the 10 SNPs, 4 were in apoptotic genes and the 2 (rs720321, rs17757541) in BCL2 were not in linkage disequilibrium (correlation coefficient r2 = -0.04, P = .47). Compared with patients diagnosed of EA at the age older than 55 years, the presence of variant genotypes of these two SNPs in BCL2 was inversely associated with younger age of EA onset (adjusted odds ratio [AOR] = 0.40–0.45, FDR = 0.01–0.02). On the contrary, variant genotypes of NOS3 rs2070744 (AOR = 1.76, FDR = 0.01) and CYP19A1 rs2445762 (AOR = 1.63, FDR = 0.03), two SNPs categorized in carcinogen/procarcinogen metabolism pathway, were significantly more prevalent among younger EA patients. The results for the other SNPs are shown in Table 3. The allele frequencies of the 10 significant SNPs are listed in Table W3.
Table 3.
Associations between the Top 20 SNPs* and Early Onset (≤55 Years) of EA.
| SNP | Reference Genotype | Gene | Pathway | AOR (95% CI)† | P† | FDR† |
| rs2070744 | CC | NOS3 | Carcinogen/procarcinogen metabolism | 1.76 (1.25–2.48) | .001 | 0.01 |
| rs2236302 | CC | MMP14 | Metastasis | 0.27 (0.12–0.61) | .002 | 0.01 |
| rs720321 | GG | BCL2 | Apoptosis | 0.40 (0.23–0.72) | .002 | 0.01 |
| rs4740363 | AA | ABL1 | Cell cycle control | 0.16 (0.05–0.53) | .003 | 0.01 |
| rs11775256 | CC | TNFRSF10A | Apoptosis | 1.79 (1.20–2.68) | .004 | 0.02 |
| rs17757541 | CC | BCL2 | Apoptosis | 0.45 (0.26–0.78) | .005 | 0.02 |
| rs696217 | GG | GHRL | Cell growth | 0.27 (0.10–0.71) | .008 | 0.02 |
| rs1035142 | GG | CASP8 | Apoptosis | 0.61 (0.42–0.89) | .010 | 0.02 |
| rs2445762 | CC | CYP19A1 | Carcinogen/procarcinogen metabolism | 1.63 (1.11–2.41) | .014 | 0.03 |
| rs11941492 | CC | VEGFR2/KDR | Angiogenesis | 1.58 (1.07–2.32) | .020 | 0.04 |
| rs4149570 | GG | TNFRSF1A | Apoptosis | 1.46 (1.02–2.08) | .037 | 0.07 |
| rs1110277 | GG | SLC23A2 | Molecular transport | 1.48 (1.01–2.16) | .04 | 0.07 |
| rs11244142 | AA | ABL1 | Cell cycle control | 1.63 (1.01–2.63) | .04 | 0.07 |
| rs11196422 | GG | CASP7 | Apoptosis | 1.55 (0.97–2.47) | .06 | 0.09 |
| rs6097809 | TT | CYP24A1 | Carcinogen/procarcinogen metabolism | 1.87 (0.88–3.99) | .10 | 0.14 |
| rs1234220 | TT | PTEN | Apoptosis | 1.60 (0.87–2.94) | .13 | 0.16 |
| rs640180 | GG | FGFR4 | Angiogenesis | 1.34 (0.90–2.00) | .15 | 0.17 |
| rs1801265 | CC | DPYD | Carcinogen/procarcinogen metabolism | 1.33 (0.86–2.05) | .20 | 0.22 |
| rs3808815 | CC | ABL1 | Cell cycle control | 1.33 (0.74–2.38) | .34 | 0.35 |
| rs543215 | AA | PGR | Sex hormone signaling | 0.10 (0.61–1.56) | .93 | 0.93 |
Twenty SNPs that have the highest importance scores and lowest classification error rate were identified by random forest from among 1330 functional/tagging SNPs in 14 pathways.
Adjusted odds ratio used patients younger than 55 years as the reference; adjusted for sex, BMI at age 18 years (<25 vs .25), GERD (+/-), and smoking (never/ever); FDR < 0.05 was defined as significant.
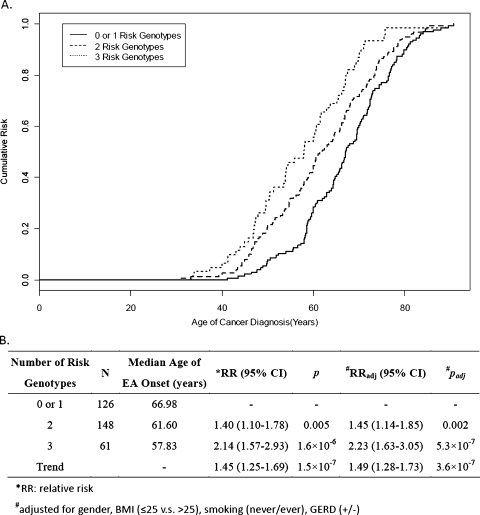
The age at EA diagnosis for the different genotypes (wide type vs variants) of the 10 significant SNPs was analyzed separately using Kaplan-Meier survival curves (Figure W1). The trend for each SNP was consistent with that found in logistic regression models shown in Table 3. NOS3 rs2070744 (FDR = 0.02), BCL2 rs720321 (FDR = 0.1), and CASP8 rs1035142 (FDR = 0.007) remained significant after adjusting for FDR in a Cox regression model (Table W4). Therefore, a combination of these three SNPs was used to evaluate the cumulative risk of EA onset (Figure 3). We found that the median age at EA diagnosis among patients with two and three risk genotypes was 5.4 and 9.2 years younger, respectively, than those with fewer than 1 risk genotype (adjusted P for trend = 3.6 x 10-7) (Figure 3B).
Figure 3.
Cumulative risk of combined genotypes of rs2070744 (NOS3), rs720321 (BCL2), and rs1035142 (CASP8) for onset age of EA. (A) Kaplan-Meier curves: cumulative risks for onset age of EA associated with the presence of fewer than one, two, or three risk genotypes. (B) Cox regression model: More risk genotypes are associated with higher risk of earlier onset of EA.
Discussion
Although GERD is the strongest risk factor for EA, nearly 50% of EA patients do not experience GERD-associated symptoms [19]. Therefore, it is unlikely that GERD is the determinant factor for early onset of EA. For instance, a previous study has showed that there was no difference in sex distribution, smoking history, or presence of GERD symptoms between early and later onset of EA patients [20]. In our study, we also observed that patients in both of early onset and later-onset groups had similar frequency and duration of GERD symptoms, suggesting that GERD was not a confounding factor for the effect of genetic polymorphisms on the age of EA onset. Consistent with previous studies [3,21], we found a significantly higher proportion of stage III, IV disease among younger-aged EA patients. This finding implies that younger-aged patients are prone to be underdiagnosed or EA is more aggressive if it develops at a younger age.
We used a case-only design, which had a greater power than case-control study, to investigate the determinant SNPs discriminating early and later onset of EA. To avoid false-positive results arising from the correlation between significant candidate genotypes and age, we also examined the independent and interactive effects of the top 10 SNPs in a case-control manner (Table W5). The control subjects were described in our previous study [11]. We found that the trend of the interaction ORs in the case-control study was consistent with the ORs in this case-only study, although not all of the P values in the case-control model were significant (P < .05).
This is the first pathway-based genetic association study of patients with early versus later onset EA. Our pathway analysis indicated that the apoptosis pathway was important in distinguishing the two groups. In addition, results from both logistic regression (Table 3) and survival analysis (Figure W1) revealed that SNPs in the apoptotic pathway had stronger association with early onset of EA than SNPs in other pathways. Apoptosis is essential for normal tissues to regulate cell number and to eliminate DNA-damaged or aging cells as an organism develops. Evading apoptosis, a characteristic of transformed cells, has been well documented in the carcinogenesis of EA [22,23]. Previous studies have linked polymorphisms in apoptotic genes to different risks of esophageal cancer [11,14,24]. In the current analysis, four SNPs in three apoptotic genes (BCL2, CASP8, and TNFRSF10A) were significantly associated with younger-aged EA. BCL2 protein is one of the products of BCL2 family, which has both proapoptotic and antiapoptotic functions. BCL2 protein has been found aberrantly expressed in EA carcinogenesis [25,26]. TNFRSF10A, or TNF-related apoptosis-inducing ligand (TRAIL) receptor 1, is one of the cognate receptors that binds to TNF superfamily ligands to activate caspase 8 and 10, thus initiating the caspase cascade of extrinsic apoptotic pathway [27]. Genetic polymorphisms in the apoptotic genes such as BCL2 have been found to be implicated in certain aging-related diseases, such as Alzheimer disease, in which progressive cellular apoptosis leads to brain dysfunction [28]. In animal models of myocardial infarction, cardiomyocyte apoptosis activities among older rats were higher than that of younger rats [29].
Two SNPs (rs2070744 of NOS3 and rs2445762 of CYP19A1) in the carcinogen/procarcinogen metabolism pathway were also associated with younger-aged EA. The product of endothelial constitutive nitric oxide synthase 3 (NOS3) is one of the isoforms of NO synthases, which catalyze the formation of NO. NOS3 participates in many immunologic processes including apoptosis [30]. Therefore, NOS3 is an apoptosis-related gene, although it is categorized in the carcinogen/procarcinogen metabolism pathway in this study. Overexpression of NOS3 has been detected in esophageal adenocarcinoma, especially those with advanced diseases (T3/4, positive nodal metastasis, and stage III/IV) [31]. Cytochrome P450 aromatase, the product of CYP19, is an enzyme that catalyzes the synthesis of estrogens from androgens. Genetic variants in CYP19A1 have been linked to hormone-related cancers, especially prostate and breast cancers [32,33].
We also found significant associations between polymorphisms in VEGFR2/KDR, ABL1, GHRL, and MMP14 and early onset of EA. Gockel et al. [34] reported positive immunostaining of VEGFR2/KDR, a receptor tyrosine kinase related to angiogenesis, in 94% (47/50) of human EA tissues. However, the protein expressions of the other three genes in EA or Barrett metaplasia are not clear. Polymorphisms in VEGFR2/KDR and a cell cycle gene, ABL1, are related to many human cancers but rarely studied in esophageal cancer. GHRL is an obesity-related gene. rs696217, a missense SNP in GHRL, has been reported to have modest association with EA risk (AOR, 1.69; 95% CI, 1.14–2.50; P = .2 after Bonferroni correction) [35]. Matrixmetalloproteinases (MMPs) are proteases that digest the extracellular matrix and contribute to cancer invasion and metastasis. rs2236302 of MMP14 is a synonymous SNP that is located in exon 5. However, its association with cancer has not been reported.
Moreover, a combination of NOS3 rs2070744, BCL2 rs720321, and CASP8 rs1035142 significantly discriminate the early and later onset of EA. The cumulative risk for developing EA at a younger age increases when more risk genotypes in these three apoptosis-related genes are present. This result further supports the importance of genetic variants in apoptosis pathway in the early onset of EA. Although many significant SNPs identified in this study were tag SNPs located in introns with unclear functions, it was interesting that most of those genes are involved in the earlier stage of cancer formation, such as pathways involving carcinogen metabolism, cell cycle control, cell growth, and apoptosis (Table 3).
We acknowledge that our study has several limitations. First, the case number in this study is relatively small because EA is an uncommon disease even among whites. However, we have more than 80% power to identify a significant effect of the SNPs if the minor allele frequency approaches 10% and an association OR of 1.45 [36]. Second, we were not able to replicate in another population because no other data set is available at this stage. Third, there is lack of information about the presence of Barrett esophagus in our patients. Last, the generalization of our results to other ethnic population or histologic types (e.g., SCC) of esophageal cancer should be used with caution because we only included white EA patients.
In conclusion, this study suggests that polymorphisms in cancer-related genes, especially SNPs in apoptotic pathway, may play an important role in the early onset of EA. In addition to the typical alarm symptoms in current clinical guidelines, this set of three SNPs has the potential to become a clinical tool to screen for those at high risk of EA at a relatively young age. Previous studies have reported overexpression of NOS3, BCL2, and VEGFR2/KDR in EA tissues, supporting their role in Barrett carcinogenesis. Replication in other populations and further studies are required to validate our results and to elucidate the functions of important SNPs, individually or jointly, in relation to early onset of EA.
Supplementary Material
Acknowledgments
The authors thank the physicians and patients from the Massachusetts General Hospital Thoracic Oncology Center and the Dana-Farber Cancer Institute GI Oncology Center for their cooperation and participation and the assistance of researchers from Excellence for Cancer Research Center (DOH100-TD-C-111-002; Department of Health, Executive Yuan, Taiwan, ROC).
Abbreviations
- EA
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- MDA
mean decrease of accuracy
- FDR
false discovery rate; 95% CI, 95% confidence interval
- Padj
adjusted P value
Footnotes
This study was funded by National Institutes of Health grants R01CA109193, R03CA110822, R01CA074386, and ES00002; CIHR operating grant; the Kevin Jackson Memorial Fund; Alan B. Brown Chair in Molecular Genomics; Posluns Family Fund PMH Foundation; and the Flight Attendant Medical Research Institute Award No. 062459. The Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278 from the National Center for Research Resources.
All authors disclosed no conflict of interest; the institutes that provided the grant supports did not participate in the study design, the collection, analysis, or interpretation of data.
This article refers to supplementary materials, which are designated by Tables W1 to W5 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamouda A, Forshaw M, Rohatgi A, Mirnezami R, Botha A, Mason R. Presentation and survival of operable esophageal cancer in patients 55 years of age and below. World J Surg. 2010;34:744–749. doi: 10.1007/s00268-010-0407-6. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–2337. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–1780. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Park YH, Sohn SK, Kim JG, Lee MH, Song HS, Kim MK, Jung JS, Lee JJ, Kim HJ, Kim DH. Interaction between BCL2 and interleukin-10 gene polymorphisms alter outcomes of diffuse large B-cell lymphoma following rituximab plus CHOP chemotherapy. Clin Cancer Res. 2009;15:2107–2115. doi: 10.1158/1078-0432.CCR-08-1588. [DOI] [PubMed] [Google Scholar]
- 7.Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, Bailey J, Lee JH, Bresalier R, Rashid A, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24:259–267. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Cescon DW, Zhai R, Zhou W, Kulke MH, Ma C, Xu W, Su L, Asomaning K, Heist RS, et al. p53 Arg72Pro, MDM2 T309G and CCND1 G870A polymorphisms are not associated with susceptibility to esophageal adenocarcinoma. Dis Esophagus. 2010;23:36–39. doi: 10.1111/j.1442-2050.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung WY, Liu G. Genetic variations in esophageal cancer risk and prognosis. Gastroenterol Clin North Am. 2009;38:75–91. doi: 10.1016/j.gtc.2009.01.009. viii. [DOI] [PubMed] [Google Scholar]
- 10.Zhai R, Liu G, Asomaning K, Su L, Kulke MH, Heist RS, Nishioka NS, Lynch TJ, Wain JC, Lin X, et al. Genetic polymorphisms of VEGF, interactions with cigarette smoking exposure and esophageal adenocarcinoma risk. Carcinogenesis. 2008;29:2330–2334. doi: 10.1093/carcin/bgn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CY, Wu MC, Chen F, Ter-Minassian M, Asomaning K, Zhai R, Wang Z, Su L, Heist RS, Kulke MH, et al. A large-scale genetic association study of esophageal adenocarcinoma risk. Carcinogenesis. 2010;31:1259–1263. doi: 10.1093/carcin/bgq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorbin LH, Wittkind CL. TNM Classification of Malignant Tumors. 6th ed. New York, NY: Wiley & Sons; 2002. Union Internationale Contre le Cancer. [Google Scholar]
- 13.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 14.Zhai R, Chen F, Liu G, Su L, Kulke MH, Asomaning K, Lin X, Heist RS, Nishioka NS, Sheu CC, et al. Interactions among genetic variants in apoptosis pathway genes, reflux symptoms, body mass index, and smoking indicate two distinct etiologic patterns of esophageal adenocarcinoma. J Clin Oncol. 2010;28:2445–2451. doi: 10.1200/JCO.2009.26.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman D, Fennerty MB, Morris CD, Holub J, Eisen G, Sonnenberg A. Endoscopic evaluation of patients with dyspepsia: results from the national endoscopic data repository. Gastroenterology. 2004;127:1067–1075. doi: 10.1053/j.gastro.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Moore JH, Asselbergs FW, Williams SM. Bioinformatics challenges for genome-wide association studies. Bioinformatics. 2010;26:445–455. doi: 10.1093/bioinformatics/btp713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang R, Tang W, Wu X, Fu W. A random forest approach to the detection of epistatic interactions in case-control studies. BMC Bioinformatics. 2009;10(suppl 1):S65. doi: 10.1186/1471-2105-10-S1-S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Yekutieli D. Quantitative trait Loci analysis using the false discovery rate. Genetics. 2005;171:783–790. doi: 10.1534/genetics.104.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemi N, Loren D, DiMarino AJ, Cohen S. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci. 2009;54:1708–1712. doi: 10.1007/s10620-008-0565-7. [DOI] [PubMed] [Google Scholar]
- 21.Portale G, Peters JH, Hsieh CC, Tamhankar AP, Almogy G, Hagen JA, Demeester SR, Bremner CG, Demeester TR. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg. 2004;70:954–958. [PubMed] [Google Scholar]
- 22.Dvorakova K, Payne CM, Ramsey L, Bernstein H, Holubec H, Chavarria M, Bernstein C, Sampliner RE, Riley C, Prasad A, et al. Apoptosis resistance in Barrett's esophagus: ex vivo bioassay of live stressed tissues. Am J Gastroenterol. 2005;100:424–431. doi: 10.1111/j.1572-0241.2005.40932.x. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald RC. Molecular basis of Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2006;55:1810–1820. doi: 10.1136/gut.2005.089144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain M, Kumar S, Lal P, Tiwari A, Ghoshal UC, Mittal B. Role of BCL2 (Ala43Thr), CCND1 (G870A) and FAS (A-670G) polymorphisms in modulating the risk of developing esophageal cancer. Cancer Detect Prev. 2007;31:225–232. doi: 10.1016/j.cdp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Katada N, Hinder RA, Smyrk TC, Hirabayashi N, Perdikis G, Lund RJ, Woodward T, Klingler PJ. Apoptosis is inhibited early in the dysplasia-carcinoma sequence of Barrett esophagus. Arch Surg. 1997;132:728–733. doi: 10.1001/archsurg.1997.01430310042007. [DOI] [PubMed] [Google Scholar]
- 26.Mobius C, Stein HJ, Spiess C, Becker I, Feith M, Theisen J, Gais P, Jutting U, Siewert JR. COX2 expression, angiogenesis, proliferation and survival in Barrett's cancer. Eur J Surg Oncol. 2005;31:755–759. doi: 10.1016/j.ejso.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Carlo-Stella C, Lavazza C, Locatelli A, Vigano L, Gianni AM, Gianni L. Targeting TRAIL agonistic receptors for cancer therapy. Clin Cancer Res. 2007;13:2313–2317. doi: 10.1158/1078-0432.CCR-06-2774. [DOI] [PubMed] [Google Scholar]
- 28.Lukiw WJ, Bazan NG. Inflammatory, apoptotic, and survival gene signaling in Alzheimer's disease. A review on the bioactivity of neuroprotectin D1 and apoptosis. Mol Neurobiol. 2010;42:10–16. doi: 10.1007/s12035-010-8126-4. [DOI] [PubMed] [Google Scholar]
- 29.Gallogly MM, Shelton MD, Qanungo S, Pai HV, Starke DW, Hoppel CL, Lesnefsky EJ, Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFκB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signal. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 31.Chandra R, Haines GK, III, Bentz BG, Shah P, Robinson AM, Radosevich JA. Expression of nitric oxide synthase type 3 in reflux-induced esophageal lesions. Otolaryngol Head Neck Surg. 2001;124:442–447. doi: 10.1067/mhn.2001.114254. [DOI] [PubMed] [Google Scholar]
- 32.Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, Berglund G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, et al. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:2734–2744. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidokoro K, Ino K, Hirose K, Kajiyama H, Hosono S, Suzuki T, Kawase T, Hiraki A, Hamajima N, Tanaka H, et al. Association between CYP19A1 polymorphisms and sex hormones in postmenopausal Japanese women. J Hum Genet. 2009;54:78–85. doi: 10.1038/jhg.2008.11. [DOI] [PubMed] [Google Scholar]
- 34.Gockel I, Moehler M, Frerichs K, Drescher D, Trinh TT, Duenschede F, Borschitz T, Schimanski K, Biesterfeld S, Herzer K, et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep. 2008;20:845–850. [PubMed] [Google Scholar]
- 35.Doecke J, Zhao ZZ, Stark MS, Green AC, Hayward NK, Montgomery GW, Webb PM, Whiteman DC. Single nucleotide polymorphisms in obesity-related genes and the risk of esophageal cancers. Cancer Epidemiol Biomarkers Prev. 2008;17:1007–1012. doi: 10.1158/1055-9965.EPI-08-0023. [DOI] [PubMed] [Google Scholar]
- 36.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.