Abstract
Background
The main symptom of postoperative ileus (POI) is an intestinal motility disorder in which monocytes/macrophages and neutrophils play crucial roles. Prokinetic 5-hydroxytryptamine 4 receptor (5-HT4R) agonists and dopamine receptor antagonists are potential therapeutic agents for directly ameliorating the motility disorder associated with POI.
Aim
To determine the effects of the 5-HT4R agonists mosapride citrate (MOS) and CJ-033466 on intestinal smooth muscle contractility relative to immune reactions after POI.
Methods
Intestinal manipulation (IM) was applied to the rat distal ileum. Both MOS (0.3 and 1 mg/kg, s.c.) and CJ-033466 (1 mg/kg, s.c.) were administered to the animals before and after IM. At 24 h after IM, isolated intestinal smooth muscle contractile activity in vitro, gastrointestinal transit in vivo, inflammatory mediator expression and leucocyte infiltration were measured.
Results
After IM, ileal circular muscle contractility in vitro and gastrointestinal transit in vivo were reduced and the number of macrophages and neutrophils increased in the inflamed muscle layer, resulting in the induction of inflammatory mediators such as interleukin 1 β (IL-1β), IL-6, tumour necrosis factor α (TNFα), monocyte chemoattractant protein 1 (MCP-1) and inducible nitric oxide synthase (iNOS). Both MOS and CJ-033466 significantly attenuated not only the intestinal motility dysfunction but also the leucocyte infiltration and inflammatory mediator expression after IM. The autonomic ganglionic blocker hexamethonium (1 mg/kg, i.p.) and the α7-nicotinic acetylcholine receptor (α7nAChR) antagonist methyl lycaconitine citrate (0.087 mg/kg, i.p.) blocked MOS-mediated ameliorative actions. Immunohistochemically, α7nAChR is expressed by monocytes/macrophages but not by neutrophils in the inflamed intestine.
Conclusion
Stimulating the 5-HT4R accelerates acetyl choline (ACh) release from cholinergic myenteric neurons, which subsequently activates α7nAChR on activated monocytes/macrophages to inhibit their inflammatory reactions in the muscle layer. In addition to their gastroprokinetic action, 5-HT4R agonists might serve as novel therapeutic agents for POI characterised by anti-inflammatory potency.
Keywords: Prokinetic agents, motility disorder, abdominal surgery, macrophages, inflammation, macrophages, motility disorders, prokinetic agent
Significance of this study.
What is already known about this subject?
The 5-HT4R agonist mosapride is actually used in clinical practice as a gastrointestinal prokinetic drug.
Recent clinical trials showed that mosapride, that is benzamide analogues of cisapride, reduce postoperative ileus although pharmacological mechanisms are not well understood.
Stimulation of the vagus nerve prevents postoperative ileus through α7nAChR in macrophages.
What are the new findings?
The 5-HT4R agonist mosapride dramatically inhibits postoperative ileus through anti-inflammatory reaction in addition to its gastrointestinal prokinetic action.
The anti-inflammatory action is mediated by acetylcholine (ACh) release from cholinergic myenteric neurons, which subsequently activates α7nAChR on activated monocytes/macrophages to inhibit inflammation.
For the first time, we found a new subset of activated macrophages expressed α7nAChR during inflammation in small intestine.
How might it impact on clinical practice in the foreseeable future?
5-HT4R agonists, such as mosapride, could be clinically used not only as a gastrointestinal prokinetic drug but also as anti-inflammatory drug to prevent postoperative ileus.
Intestinal macrophages are unique immunoreactive cells inducing α7nAChR during inflammation, which give a significant impact on medical sciences.
5-HT4R could be a new therapeutic molecular target for anti-inflammatory gastrointestinal diseases.
Introduction
Various 5-HT4R agonists, such as tegaserod, cisapride and mosapride, have been clinically validated as treatment for gastrointestinal disorders characterised by dysmotility. Originally, 5-HT4R agonists were believed to induce prokinetic potency only in the upper part of the gastrointestinal tract. Therefore, 5-HT4R agonists are still clinically used to treat gastroparesis and functional dyspepsia.1–3 However, 5-HT4R agonists can also induce prokinetic ability in the lower part of the gastrointestinal tract in experimental animals and humans,4 5 and they are clinically applied to treat chronic constipation and constipation-predominant irritable bowel syndrome.6 7 Indeed, immunohistochemical studies have identified 5-HT4R in the myenteric and submucosal ganglia of gastrointestinal tissues.8–10
Postoperative ileus (POI) is a common complication after intra-abdominal surgery that is accompanied by increased morbidity and prolonged hospitalisation, increasing hospital costs.11 Neurogenic, inflammatory and inflammatory–neuronal interactive mechanisms are generally considered to induce POI.12 Sympathetic reflexes, the activation of non-adrenergic, non-cholinergic nerves, inhibitory humoural agents and the influence of anaesthetics play crucial roles in the pathogenesis of POI at the acute stage during and shortly after (up to 3 h) laparotomy and abdominal surgery.13 14 Local inflammatory responses in the manipulated intestine additionally participate in disordered motility during the later stage of POI (24 h after surgery).15–19 Many resident macrophages are distributed in the subserosal, myenteric plexus regions of the intestinal muscle layer and inside the circular smooth muscle layer under healthy conditions.20 21 Although these ramified forms of resident macrophages normally remain inactive,22 23 inflammatory stimuli and/or mechanical stress induced by IM activates them to produce prostaglandins, nitric oxides, inflammatory cytokines and chemokines that cause muscularis inflammation and intestinal motility disorders.15–19 24
The pharmacological management of POI is important to inhibit morbidity rates and reduce hospital costs and length of hospital stay. Gastrointestinal prokinetic agents might be useful for managing or preventing POI according to some clinical trials.25–28 The effects of various gastrointestinal prokinetic agents on postoperative ileus have also been investigated in experimental animals.29 Peripherally acting μ-opioid receptor antagonists, such as alvimopan and methylnaltrexone, offer promising results for preventing prolonged POI 28 and 5-HT4R agonists that are benzamide analogues of cisapride and MOS also reduce POI, whereas the partial 5-HT4R agonistic and dopamine D2 antagonistic agent metoclopramide does not have preventive action.28 30 These results suggest that the ameliorative actions of cisapride and MOS are not simply mediated by gastrointestinal prokinetic actions through 5-HT4R, and that other mechanisms are involved. In the present study, therefore, we investigated how 5-HT4R agonists prevent POI in a rat model.
Materials and methods
Animal model of POI
Male Sprague–Dawley rats (250–400 g; Charles River Japan, Yokohama, Japan) were manipulated and cared for in strict compliance with the Guide to Animal Use and Care published by the University of Tokyo. The Institutional Review Board of the Graduate School of Agriculture and Life Sciences of the University of Tokyo approved the study protocol.
All animals were anaesthetised with pentobarbital sodium (Schering–Plough Corp., Kenilworth, New Jersey, USA) and the animal model of POI was made by intestinal manipulation (IM) previously reported.15–19
Experimental design
The rats were randomly assigned to the following groups: Control, no treatment with fasting; IM + Vehicle, sterilised physiological saline was subcutaneously injected at 2 h before and at 2 and 6 h after IM; IM + MOS and IM+CJ, 5-HT4R agonist MOS citrate (0.3, 1 mg/kg, donated by Dainippon Sumitomo Pharma) or CJ-033466 (CJ; 1 mg/kg, donated by Pfizer) were similarly administered three times, respectively; IM + MOS + GR, the 5-HT4R antagonist GR113808 (GR; 1 or 3 mg/kg, Sigma Aldrich, St. Louis, Missouri, USA) was similarly administered by three intraperitoneal (i.p.) injections with MOS; IM + MOS + HEX, the non-specific autonomic ganglionic blocker hexamethonium chloride (1 mg/kg; Nacalai Tesque, Kyoto, Japan) was applied at 1 h before IM and MOS was applied at 2 h before and at 2 and 6 h after IM; IM + MOS + MLA, the α7-nicotinic acetylcholine receptor (α7nAChR) antagonist methyl lycaconitine (MLA) citrate (0.087 mg/kg; TOCRIS, Bristol, UK) was injected i.p. at 30 min before each MOS application. Both MOS and GR113808 were dissolved in 1% of lactic acid with physiological saline and other substances were dissolved in distilled water.
Contraction studies
The manipulated ileal portion was isolated from POI model rats at 24 h after IM. The ileum was cut open along the mesenteric attachment, and the mucosal and submucosal layers were gently removed. Circular strips were suspended along the circular axis in a tissue bath filled with a normal physiological salt solution comprising (in mM) 136.9 NaCl, 5.4 KCl, 1 CaCl2, 1.5 MgCl2, 23.8 NaHCO3, 5.5 glucose, and 0.01 EDTA (pH 7.4). Muscle strips were maintained at 37°C and aerated with 95% O2–5% CO2. Responses of the strips were measured isometrically under a resting tension of 10 mN. The magnitude of absolute force was normalised to the wet weight of each strip.
Intestinal transit determination
After 18 h of fasting, the rats were randomly assigned to four groups (Control, IM + Vehicle, IM + MOS (MOS 1 mg/kg), IM + MOS + MLA (MOS 1 mg/kg, MLA 0.087 mg/kg) to measure gastrointestinal transit. Twenty-two hours after IM, 200 μl of the non-absorbable marker 0.25% (w/v) phenol red in 5% (w/v) glucose was orally administered to the rats via a gastric tube. After 1 h later, the gastrointestinal part was isolated and stomach and intestine were separated as a single segment (Sto) and divided into ten segments (Sl1–Sl10), respectively. The measurement of volume of each segment and calculation of the geometric center of distribution of phenol red were performed as previously reported.17 18
Whole mount immunohistochemistry
Whole mount muscularis preparations were basically performed in an orderly manner previously reported.23 24 Each first and second antibody are listed in table 1. Preparations were immunohistochemically analysed using an LSM510 confocal microscope (Carl Zeiss Japan, Tokyo, Japan) and a Digital Eclipse C1 (Nikon, Tokyo, Japan). Immunopositive cells in the myenteric plexus and the subserosal layers of three randomly selected areas in each preparation were counted and then the averaged value was calculated. The same experiment was performed at least four times to calculate means ± SE.M.
Table 1.
Antibodies used for immunohistochemistry
| Antibody | Dilution |
| Anti-ED1mouse monoAb (BMA) | 1:500 |
| Alexa Fluor 488 conjugated anti-mouse IgG donkey polyAb (Invitrogen) | 1:500 |
| Alexa Fluor 568 conjugated anti-mouse IgG donkey polyAb (Invitrogen) | 1:500 |
| Anti-ED2 mouse monoAb (BMA) | 1:500 |
| Alexa Fluor 488 conjugated anti-mouse IgG donkey polyAb (Invitrogen) | 1:500 |
| Anti-PGP9.5 rabbit polyAb (UltraClone) | 1:3000 |
| Alexa Fluor 568 conjugated anti-rabbit IgG goat polyAb (Invitrogen) | 1:500 |
| Anti-α7nAChR(C-20) goat polyAb (Santa Cruz Biotechnology) | 1:200 |
| Alexa Fluor 488 conjugated anti-goat IgG rabbit polyAb (Invitrogen) | 1:500 |
| FITC-conjugated α-bungarotoxin (Biotium, Hayward) | 5 μg/ml |
Myeloperoxidase staining
Whole mount preparations of ileal muscularis region were cut into 0.5×1 cm pieces and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at 4°C. The preparations were washed with Tris-buffered saline (TBS) for 1 h, incubated in physiological salt solution containing 0.1% (w/v) Hanker–Yates reagent (Polysciences, Warrington, Pennsylvania, USA) and 0.03% (v/v) hydrogen peroxidase (Mitsubishi Gas Chemical Company, Tokyo, Japan) for 10 min and then rinsed in PBS for 10 min. Cells that were obviously myeloperoxidase (MPO)-positive (neutrophils) in the muscularis and subserosal layers were counted under a microscope (Nikon ACT-1C for DXM1200; Nikon, Tokyo, Japan) in five randomly selected areas of each preparation. Some preparations were also analysed immunohistochemically using ED1 or ED2 after MPO staining.
Quantitative RT-PCR
Quantitative RT-PCR proceeded as described.24 The sequences of the primer and expected product size are listed in table 2. Amplification proceeded in a PCR Thermal Cycler MP (TaKaRa Biomedicals, Shiga, Japan) using 30–34 cycles (two-cycle intervals), each consisting of 98°C for 10 s, 52–58°C for 30 s, and 72°C for 1 min. The products of each cycle were resolved on 2% agarose gels containing 0.1% ethidium bromide, and the optimal number of PCR cycles for quantification was selected. The density of detectable fluorescent bands was measured using NIH Image software (Image J, Ver. 1.38x).
Table 2.
Sequences of PCR primers and their Tm values and product sizes
| Gene | Sequences (S: sense, As: antisense) | Tm (°C) | Product size (bp) |
| GAPDH |
|
58 | 308 |
| IL-1β |
|
52 | 246 |
| IL-6 |
|
48 | 614 |
| TNFα |
|
52 | 248 |
| MCP-1 |
|
54.5 | 600 |
| iNOS |
|
52 | 196 |
| 5-HT4R |
|
58 | 354 |
| α7-nAChR |
|
58 | 204 |
Statistics
Results are expressed as means ± SEM. Data were statistically evaluated using unpaired Student t tests for comparisons between two groups and by one-way analysis of variance (ANOVA) followed by Dunnett's test for comparisons among three or more groups. Values of p<0.05 were considered statistically significant.
Results
5-HT4R stimulation ameliorates motility dysfunction induced by intestinal manipulation in a post-operative ileus model rat intestine
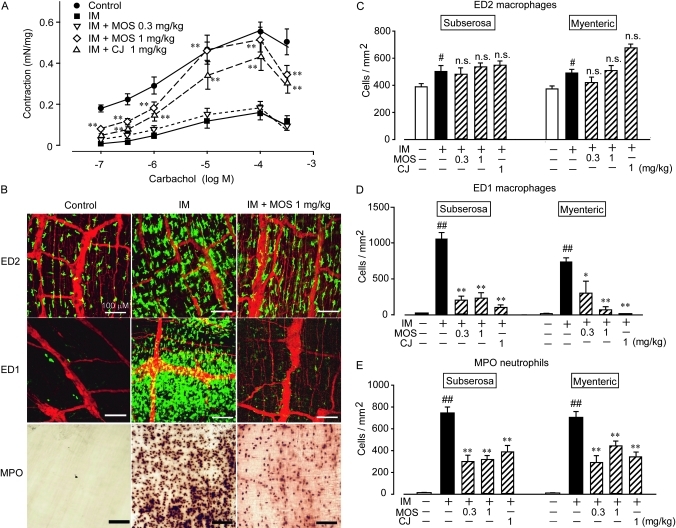
Carbachol (CCh) concentration-dependently induced and significantly diminished contractions in ileal circular smooth muscle tissues isolated from the small intestines of normal and POI model rats, respectively. The nitric oxide synthase inhibitor, NG-nitro-l-arginine methyl ester (l-NAME, 300 μM; n=4; IM (CCh 1 μM), 0.0455±0.0090 mN/mg; IM+l-NAME (CCh; μM), 0.2714±0.0662 mN/mg; p<0.01) almost completely recovered the decreased contractility, suggesting that the motility dysfunction induced by IM is mediated by NO generated from inducible nitric oxide synthase. Figure 1A shows that the decreased intestinal motility was almost completely recovered in the ileal tissue isolated from POI model rats treated with 1 mg/kg s.c. of either of the specific 5-HT4R agonists MOS or CJ-033466.
Figure 1.
Effects of 5-HT4R agonists on motility dysfunction and leucocyte infiltration induced by intestinal manipulation (IM). (A) Effects of IM on carbachol-induced contractions in intestinal circular smooth muscles and effect of mosapride citrate (MOS) and CJ-033466 (CJ) on contractility. **Significantly different from IM (n=4 each). (B) Typical results of whole mount immunohistochemical staining of myenteric plexus region to detect ED2-positive resident macrophages, ED1-positive monocyte-derived macrophages and MPO-positive neutrophils. Red signals indicate PGP9.5-positive myenteric nerves. Green signals indicate ED2- or ED1-positive macrophages. (C–E) Quantification of leucocytes in subserosal and myenteric plexus regions; # and ##, significantly different from control at p<0.05 or p<0.01, respectively; * and **, significantly different from number of leucocytes in IM intestine at p<0.05, and p<0.01, respectively. MOS (0.3 or 1 mg/ml, s.c.) and CJ-033466 (1 mg/kg, s.c.) were administered as described in Materials and methods. Bars show means ± SEM from four independent experiments.
5-HT4 receptor stimulation inhibited inflammation induced by intestinal manipulation
We immunohistochemically monitored changes in ED2-positive resident macrophages, ED1-positive monocyte-derived macrophages, and MPO-stained neutrophils (figure 1B–E). Many ED2-positive resident macrophages were detected in the myenteric plexus and subserosal regions of the control rat ileal muscle layer (figure 1B, C). On the other hand, ED1-positive monocyte-derived infiltrating macrophages and MPO-positive infiltrating neutrophils were undetectable in control animals (figure 1B, D). The ED2-positive resident macrophage population was increased by 25% in the inflamed muscle layer of the intestine of the POI model rat compared with that of control rats. On the other hand, many ED1-positive monocyte-derived macrophages and MPO-stained neutrophils infiltrated into the myenteric plexus region and subserosal region 24 h after the intestinal inflammation. Interestingly, monocyte-derived macrophages and neutrophil populations were significantly inhibited in both regions of ileal tissues isolated from the POI model rat intestine treated with MOS and CJ-033466 (figure 1D, E). The increased number of ED2-positive muscularis resident macrophages after IM was not changed by 5-HT4R stimulation with MOS and CJ-033466 (figure 1C). Neither MOS nor CJ-033466 at 1 mg/kg, s.c. affected the macrophage and neutrophil populations in the muscle layer of control rats (n=4 each; data not shown).
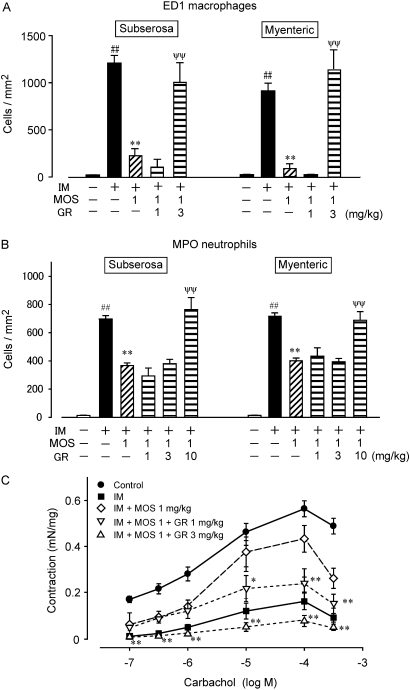
We further investigated the effects of GR113808, a 5HT4 receptor selective antagonist, on the MOS-induced anti-inflammatory reactions and its ameliorative effect on intestinal motility dysfunction (figure 2). GR113808 (1, 3, 10 mg/kg, i.p.) abolished the inhibitory effect of MOS on the infiltration of monocyte-derived macrophages and neutrophils into the inflamed muscle layer (figure 2A, B). In addition, GR113808 suppressed the ameliorative effect of MOS on the IM-mediated motility dysfunction (figure 2C). GR113808 (1 mg/kg, i.p.) did not affect the populations of ED1-positive macrophages and neutrophils in the muscle layer of control rat intestine (n=4, data not shown).
Figure 2.
Effect of the 5-HT4R antagonist GR113808 on MOS-induced leucocyte infiltration and motility dysfunction. Numbers of ED1-positive monocyte-derived macrophages (A) and MPO-stained neutrophils (B) in myenteric plexus region; ##, significantly different from control at p<0.01; **, significantly different from IM at p<0.01; ψψ, significantly different from IM with MOS (IM+MOS). Each bar shows mean±SEM from three independent experiments. (C) Changes in intestinal muscle contraction induced by CCh. MOS (1 mg/kg) and GR113808 (1, 3 and 10 mg/kg) were administered as described in Materials and methods. * and **, significantly different from IM+MOS (n=4 each). CCh, carbachol chloride; IM, intestinal manipulation; MOS, mosapride citrate; MPO, myeloperoxidase.
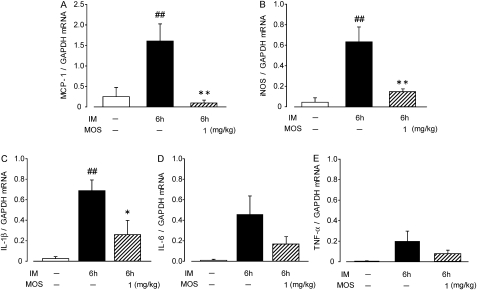
To evaluate the inflammation in the muscle layer of ileum after IM, we investigated changes in interleukin-1 β (IL-1β), IL-6, tumour necrosis factor α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) and inducible nitric oxide synthase (iNOS) levels at 6 h after IM by quantitative RT-PCR. The mRNA expression of IL-1β, MCP-1 and iNOS was significantly elevated by IM, which was remarkably attenuated by MOS. The mRNA expression of TNFα and IL-6 tended to increase and MOS also inhibited the tendency (figure 3).
Figure 3.
Effects of 5-HT4R activation on expression of inflammatory mediators in inflamed muscle layer of a post-operative ileus (POI) model rat small intestine. Detailed procedures and predicted sizes of PCR products are described in Materials and methods. ##, significantly different from control at p<0.01; ##, significantly different from control at p<0.01; **, significantly different from intestinal manipulation (IM). Each bar shows mean ± SEM from four independent experiments.
Anti-inflammatory reaction induced by 5-HT4R stimulation is caused by a neurogenic reaction
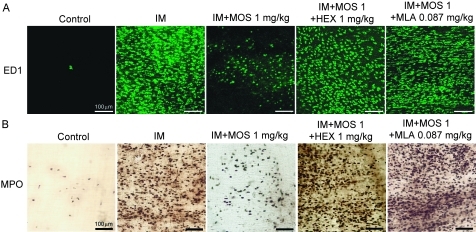
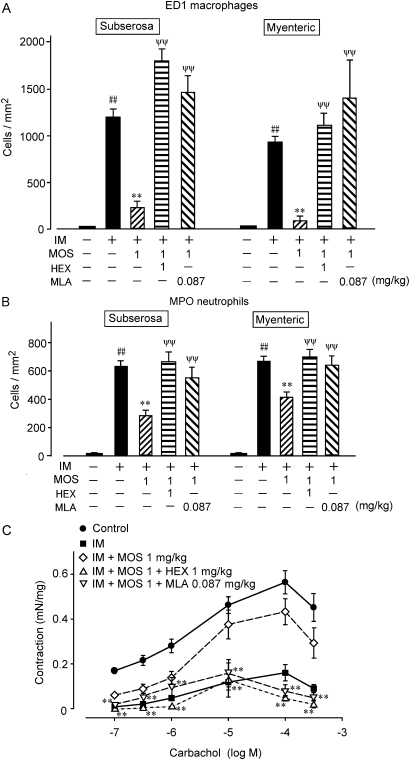
Stimulating the 5-HT4R activates myenteric plexus cholinergic neurons to release acetyl choline (ACh), which in turn induces gastroprokinetic action in the gastro intestine.4 5 Therefore, we used the autonomic ganglionic blocker HEX to investigate whether the MOS-mediated anti-inflammatory actions are neurogenically mediated (figures 4 and 5). HEX (1 mg/kg, i.p.) did not affect the populations of ED1-positive macrophages and MPO-positive neutrophils in the myenteric plexus or in the subserosal region of the control rat intestine (n=4; values are cells/mm2; ED1: myenteric, 6.76±6.43; subserosa, 5.46±1.22; MPO: myenteric 23.32±6.98; subserosa, 5.39±1.42). HEX also did not affect the increase in infiltration by ED1-positive macrophages and neutrophils induced by IM (n=4; values are cells/mm2; ED1: myenteric, 1245.92±186.57; subserosa, 1150.93±115.43; MPO: myenteric, 911.05±60.49; subserosa, 1011.19±83.68). However, HEX significantly reduced the ability of MOS to inhibit infiltration by ED1-positive macrophages and MPO-stained neutrophils (figures 4 and 5A,B).
Figure 4.
Effects of the autonomic ganglionic blocker hexamethonium and α7nAChR antagonist methyl lycaconitine (MLA) on infiltration of ED1-positive monocyte-derived macrophages and myeloperoxidase (MPO)-stained neutrophils into subserosal region after intestinal manipulation (IM). Data are typical findings from four independent experiments. Mosapride (MOS; 1 mg/kg, s.c.), hexamethonium (1 mg/kg, i.p.) and MLA (0.087 mg/kg, i.p.) were administered as described in Materials and methods.
Figure 5.
Quantification of antagonistic effects of hexamethonium and MLA on MOS-induced anti-inflammatory activities determined as leucocyte infiltration and intestinal motility dysfunction in POI model rats. Numbers of ED1-positive monocyte-derived macrophages (A) and MPO-stained neutrophils (B). #, ** and ψψ, significantly different from control, IM (IM), and IM plus MOS (IM + MOS), respectively. Bars indicate means ± SEM from four independent experiments. Changes in intestinal muscle contraction by CCh (C). MOS citrate (1 mg/kg, s.c.), hexamethonium (1 mg/kg, i.p.) and MLA citrate (0.087 mg/kg, i.p.) were administered as described in Materials and methods; **, significantly different from IM+MOS (n=4 each). CCh, carbachol chloride; IM, intestinal manipulation; MLA, methyl lycaconitine; MOS, mosapride citrate; MPO, myeloperoxidase; POI, post-operative ileus.
We then investigated the effect of HEX on the MOS-induced amelioration of motility dysfunction by IM (figure 5C). HEX (1 mg/kg, i.p.) did not affect the CCh (1 μm)-induced contractility of ileal tissue isolated from normal and POI model rats (n=4 each; values are mN/mg; normal rat, 0.2815±0.0648; normal rat with HEX, 0.2363±0.0575; POI model rat, 0.0440±0.0090; POI model rat with HEX, 0.0750±0.0156). However, HEX abolished the ability of MOS to improve the motility dysfunction in the POI model rat intestine.
Methyl lycaconitine citrate, an α7nAChR antagonist, abolished the anti-inflammatory action induced by 5-HT4R stimulation
Cholinergic neuronal stimulation induces immuno-suppressive actions through α7nAChR on immunoreactive cells such as macrophages and T cells,31 suggesting that 5-HT4R stimulation activates cholinergic neurons to release ACh, which may secondarily result in α7nAChR activation in immunoreactive cells. We therefore investigated the effect of the α7nAChR antagonist methyl lycaconitine citrate (MLA) on MOS-induced anti-inflammatory reactions in the POI model rat intestine.
We confirmed that MLA (0.087 mg/kg, i.p.) did not affect infiltration by ED1-positive macrophages and MPO-stained neutrophils in control and POI model rat intestines (n=4; data not shown). However, MLA completely suppressed the MOS-induced anti-inflammatory activity determined as macrophage and neutrophil infiltration into the muscle layer (figures 4 and 5A, B).
We also examined effect of MLA on the MOS-induced ameliorative effect on IM-mediated motility dysfunction (figure 5C). MLA (0.087 mg/kg i.p.) did not affect the CCh (1 μM)-induced contraction of ileal tissue in control and POI model rats (n=4 each; values are mN/mg; normal rat, 0.2815±0.0648; normal rat with MLA, 0.2512±0.0291; postoperative ileus model rat, 0.0440±0.0090; postoperative ileus model rat with MLA, 0.0565±0.0101). Figure 5C shows that MLA (0.087 mg/kg i.p.) inhibited the ameliorative action of MOS on intestinal dysmotility caused by IM.
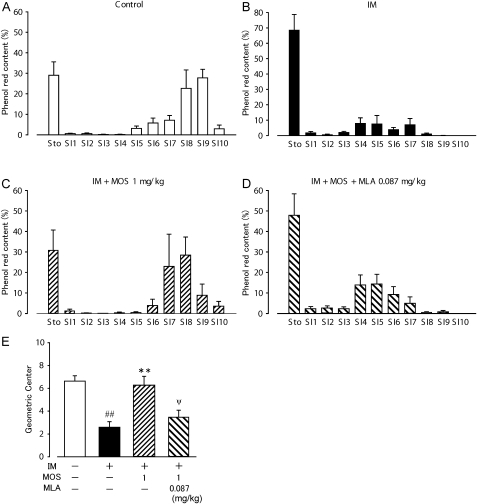
To confirm the effect of MLA on MOS-induced ameliorative action for IM-mediated gastrointestinal motility disorder in vivo, gastrointestinal transit was measured at 22–23 h after IM. About 30% of the orally administered phenol red labelled content remained inside the stomach and 70% of it was transported down the intestine to the distal end of ileum with a peak (SI9) in control healthy rats (figure 6A). The averaged calculated geometric centre for the control animals was 6.63±0.41 for 11 segments of the gastrointestinal tract (figure 6E). In contrast, IM caused a significant delay in gastrointestinal transit after a 1-h transit period, and 70% of the orally administered content remained in the stomach, whereas 30% of the transported content was moved around the jejunum and proximal ileum. The geometric centre was 2.60±0.49 (n=4, figure 6B, E). The administration of MOS significantly recovered the delayed gastrointestinal transit and reduced the value of the geometric centre after IM (figure 6C, E). Furthermore, MLA (0.087 mg/kg s.c.) significantly inhibited the ameliorative action of MOS on the delayed gastrointestinal transit caused by IM (figure 6D, E) in which 50% of the orally administered content remained in the stomach, and 50% of the transported content was moved around the jejunum and proximal ileum (geometric centre value: 3.47±0.61, n=4). MOS did not affect gastrointestinal transit of control healthy rat (6.73±0.92, n=4), suggesting that MOS does not induce gastrointestinal prokinetic action in the current experimental condition.
Figure 6.
Ameliorative action of 5-HT4R activation and negative effect of MLA on gastrointestinal transit in POI model rats. (A–D) Detailed procedures are described in Materials and methods. Gastrointestinal transit 1 h after food intake was measured. Each bar indicates means ± SEM from four independent experiments. (E) Values of geometric centre of four groups (control, IM + vehicle, IM + MOS, IM + MOS + MLA) were shown. ##, ** and ψψ, significantly different from control, IM (IM), IM plus MOS (IM + MOS), respectively. IM, intestinal manipulation; MLA, methyl lycaconitine; MOS, mosapride citrate; POI, post-operative ileus.
ED1- and ED2-positive macrophages express α7nAChR whereas neutrophils do not
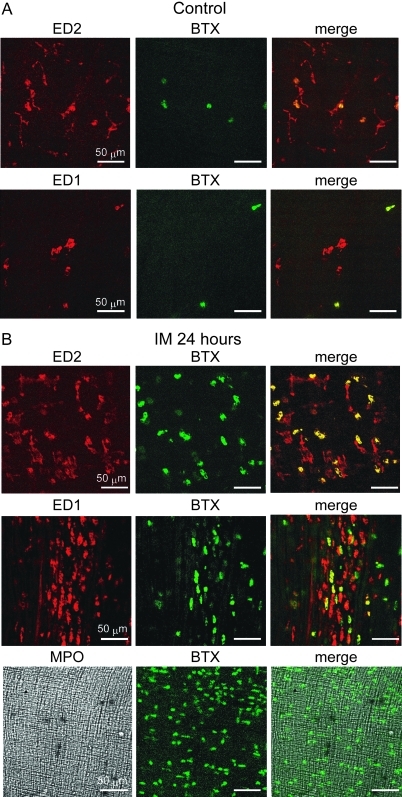
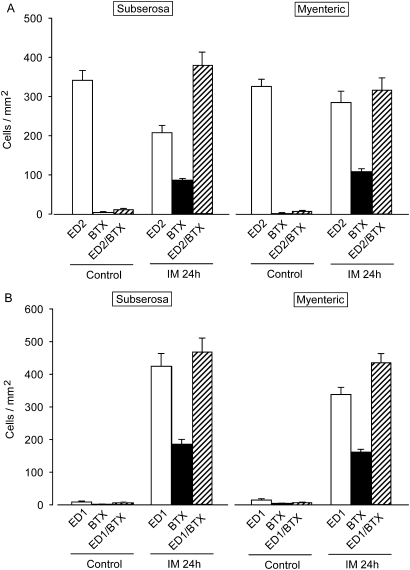
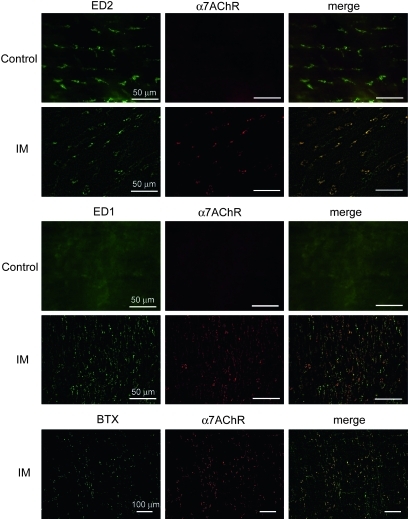
We investigated which cells in the intestinal wall express α7nAChR cells after IM using α-bungarotoxin (α-BTX) conjugated with fluorescein isothiocyanate (FITC) (figures 7 and 8). We rarely detected α-BTX-bound cells in the myenteric plexus and serosal regions of control ileal tissues (myenteric plexus and subserosal regions, 10.4±1.86 and 5.66±1.72 cells/mm2, respectively; n=4). In contrast, many α-BTX-positive cells were detected in both regions of inflamed ileal tissues (myenteric plexus and subserosal regions, 760.5±40.67 and 750.49±59.53 cells/mm2; n=4 each). We double-stained specimens of the inflamed muscle layer with ED1- and ED2-antibody or MPO and FITC-labelled α-BTX. Over 50% of the population of round ED2-positive activated resident macrophages bound to α-BTX and the ratios of ED1-positive infiltrating macrophages that bound to α-BTX were similar in both regions of the inflamed muscle layer (figures 8). In contrast, MPO-positive neutrophils did not react with α-BTX (figure 7).
Figure 7.
Double staining with α-bungarotoxin and ED1- or ED2-positive macrophages or myeloperoxidase (MPO)-stained neutrophils in myenteric plexus region isolated from normal and a post-operative ileus (POI) model rat ileum. Data are typical findings from four independent experiments. Green signals, α-bungarotoxin-bound cells possibly indicating α7nAChR expression; red signals, anti-ED1 or anti-ED2 antibody stained macrophages.
Figure 8.
Summary of cell populations stained with α-bungarotoxin and ED1- or ED2-positive macrophages in myenteric plexus and subserosal region isolated from normal and POI model rat ileum. Each column shows mean ± SEM from four independent experiments. The quantitative method is shown in the Materials and methods.
We further performed immunohistochemical double staining using anti-α7nAChR and anti-ED1 or anti-ED2 antibodies. Many cells were double stained with anti-α7nAChR and anti-ED1 or anti-ED2 antibodies in the inflamed myenteric plexus region at 24 h after IM (figure 9A, B: ED2 and anti-α7nAChR, 136.61±27.98 cells per mm2; ED1 and anti-α7nAChR, 256.44±48.21 cells per mm2. n=4 each). We also performed double staining using anti-α7nAChR antibody and α-BTX. The results indicated that almost all anti-α7nAChR antibody-positive and α-BTX-positive cells merged (figure 9C, n=4).
Figure 9.
Double staining with anti-α7nAChR antibody and ED1- or ED2-positive macrophages in the myenteric plexus region isolated from normal and post-operative ileus (POI) model rat ileum. Data are typical findings from five independent experiments. (A and B) Green signals, anti-ED2 or anti-ED1 antibody-positive macrophages; red signals, anti-α7nAChR antibody-positive cells. (C) Immunohistochemistry stained with anti-α7nAChR antibody and α-bungarotoxin (BTX). Green signals, FITC conjugated α-bungarotoxin-positive cells; red signals, anti-α7nAChR antibody-positive cells.
Discussion
Muscularis inflammation induces a motility disorder at the prolonged phase of POI.12 We demonstrated here that the gastroprokinetic agent MOS ameliorates the motility dysfunction in the POI. Furthermore, we showed that MOS significantly suppressed macrophage and neutrophil infiltration into the inflamed region, suggesting that an anti-inflammatory reaction is involved. It is possible that the ameliorative action of MOS on the motility dysfunction might be induced by gastrointestinal prokinetic ability due to MOS remaining in the isolated ileal tissue under assay conditions. However, this is unlikely because rats rapidly eliminate MOS with a t½ of 1.9 h,32 and we isolated intestinal tissues at 18 h after the final administration of MOS (at the time of measuring contractile activity), when the MOS concentration in the muscle tissue would be insufficient to induce a prokinetic reaction. We then questioned whether the ameliorative actions of MOS both on motility dysfunction and inflammation are mediated by selective action through the 5-HT4R, because MOS metabolites have antagonistic effects on 5-HT3 receptors.33 The potent and selective 5-HT4R agonist CJ-03346634 35 ameliorated the motility dysfunction and the infiltration of leucocytes induced by IM. In addition, the selective 5-HT4R antagonist GR113808 36 abolished the effects of MOS. We thus concluded that 5-HT4R stimulation restores the motility dysfunction in POI via an anti-inflammatory mechanism that is independent of prokinetic ability.
Immune reactive cells such as dendritic cells also express 5-HT4R.37 Activation of the 5-HT4R inhibits TNFα but increases the production of IL-1β and IFNγ. Thus, 5-HT4R agonists might directly act on inflammatory cells such as macrophages and neutrophils. Therefore, we next examined whether the effect of 5-HT4R agonists is mediated through direct actions on these immune cells or through the neurogenic mechanisms. We found that the non-specific autonomic ganglionic blocker HEX completely suppressed the 5-HT4R stimulation-mediated anti-inflammatory reaction, suggesting that 5-HT4R stimulation in POI exerts neuronal anti-inflammatory actions. Unlike the observation in human dendritic cells,37 5-HT4R mRNA expression was undetectable in rat peritoneal macrophages (supplementary figure 1). Regarding the neurogenic mechanism of anti-inflammatory actions induced by 5-HT4R agonists, recent understanding of the control mechanisms of intestinal inflammation exerted by autonomic nervous systems should be considered. For instance, Tanila and Kauppila reported that a selective α2-adrenergic antagonist reversed laparotomy-induced ileus, even at the prolonged phase of POI.38 Kreiss et al. 39 demonstrated that macrophages infiltrating the muscularis after IM express α2-adrenergic receptors. Furthermore, RAW264.7 macrophages are capable of synthesising catecholamines,40 suggesting that released catecholamines can react with α2-adrenergic receptors on macrophages in an autocrine and a paracrine manner to complicate POI. As an alternative autonomic regulation of inflammation, several investigators have suggested that vagus nerve stimulation attenuates gastrointestinal inflammations.41–45 Wang et al recently found that nicotine inhibits the production of pro-inflammatory cytokines from macrophages more efficiently than ACh.42 On the other hand, many type of immune cells such as lymphocytes, dendritic cells and monocyte/macrophages are now recognised to express α7nAChR.31 The activation of α7nAChR triggers a spectrum of anti-inflammatory signalling mechanisms that directly or indirectly inhibit NF-κB activation and/or Jak/STAT3 pathway in inflammatory cells.31 We found here that the α7nAChR antagonist MLA almost completely suppressed the anti-inflammatory action mediated by 5-HT4R stimulation. The amelioration of intestinal dysmotility by 5-HT4R agonists was also abolished by MLA. These results suggest that α7nAChR is involved in the ameliorative action of 5-HT4R stimulation on POI.
The present study focused on the role of monocyte/macrophage lineage cells because evidence suggests that these cells express abundant α7nAChR.31 Our immunohistochemical study of inflamed intestinal tissues showed that some ED1- and ED2-positive macrophages, but not MPO-stained neutrophils, had binding affinity for α-BTX and were stained with anti-α7nAChR antibody. Although the variety of nAChR subunits allows for a diversity of combinations, the MLA-sensitive receptors with high affinity for α-BTX could be α7nAChR, because α-BTX or MLA has binding affinity for the α1, α7 and α9, or α6 and α7 isoforms of nAChR.46 We further analysed the distribution of α7nAChR in more detail in control and inflamed small intestine musculature. We detected only a small population of α-BTX or anti-α7nAChR antibody-positive leucocytes in the myenteric plexus and subserosal regions of the normal rat small intestine. In contrast, 24 h after the inflammation induced by IM, some ED2-positive activated resident macrophages with a round form and ED1-positive monocyte-derived infiltrating macrophages expressed α7nAChR in POI model rats. ED2-positive activated resident macrophages are derived from self-multiplication at the early stage of intestinal inflammation.47 ED1-positive infiltrating macrophages might also transform to ED2-positive resident macrophages, which become stainable for both ED1 and ED2.47–49 These reports also indicate the possibility that ED1 and ED2 double positive macrophages may also express α7nAChR.
Although many reports indicate that α7nAChR is expressed in macrophages and neutrophils, several others suggest otherwise. Therefore, Van Der Zanden and colleagues posited that α7nAChR expression on leucocytes is questionable.50 We did not detect α7nAChR mRNA expression in rat resident peritoneal macrophages (supplementary figure 1). In contrast, α7nAChR mRNA was detectable in peritoneal macrophages stimulated with LPS (1 μg/ml) for 20 h, although not at very high levels. Recent studies have also indicated that α7nAChR expression is upregulated in macrophage-like U937 cells stimulated with LPS51 and in alveolar macrophages by acid-induced acute lung injury.52 Taken together, we support the idea that α7nAChR expression is modulated by the maturation and transformation of either resident or monocyte-derived intestinal muscularis macrophages by inflammatory mediators.
Neutrophils apparently express nicotinic receptors, although whether one of these receptor types is α7nAChR remains unclear.31 53 One recent study found that neutrophils in the injured lung express α7nAChR.52 However, neutrophils expressing α7nAChR are unlikely in the inflamed intestine, because α-BTX did not bind to infiltrated neutrophils stained with MPO in the present study. On the other hand, Saeed et al reported that microvascular endothelial cells express α7nAChR.54 They clarified that vagus nerve stimulation and cholinergic agonists significantly block leucocyte migration through α7nAChR in the carrageenan air pouch model. However, we did not detect microvascular tubes that were both α-BTX-positive and stained with anti-α7nAChR antibody in the myenteric plexus region of the small intestine either before or after inducing inflammation by IM.
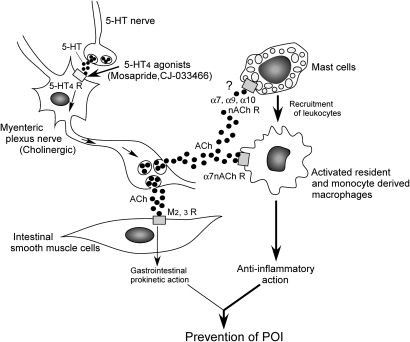
We found in this study that 5-HT4R stimulation of myenteric neurons ameliorates intestinal inflammation induced by IM, which results in recovered motility function. The 5-HT4R might mediate anti-inflammatory actions by stimulating cholinergic neurons of the myenteric plexus to release ACh, which in turn activates α7nAChR on muscularis resident macrophages and monocyte-derived infiltrating macrophages to suppress inflammation due to IM (figure 10). On the other hand, it has been suggested that intestinal muscularis mast cells play a pivotal role in the inflammation induced by IM55; that is, mast cell activation induces leucocyte infiltration to accelerate muscularis inflammation in POI. A recent report has shown that the RBL2H3 rat mast cell line expresses α7, α9 and α10 isoforms of nAChR.56 The authors suggested that these three isoforms functionally interact, indicating the possibility of a hybrid nAChR. Therefore, the released ACh might also act on the nAChRs of mast cells to inhibit leucocyte infiltration. Further investigation is necessary to clarify this point.
Figure 10.
Anti-inflammatory mechanisms of 5-HT4R stimulation in post-operative ileus (POI). Stimulation of 5-HT4R in myenteric plexus nerve results in release of acetyl choline (ACh), which in turn activates α7nAChR on activated resident macrophages and infiltrating monocyte-derived macrophages (but not on neutrophils), which prevents leucocyte infiltration and subsequently inhibits inflammation and motility disorders. ACh released from myenteric plexus nerve might also act on α7, α9 and α10 isoforms of nAChRs of mast cells to prevent inflammation (see de Jonge et al55 and Mishra et al56).
In conclusion, although 5-HT4R agonists such as MOS are clinically validated as therapies for gastrointestinal disorders characterised by dysmotility, the present findings provide new insights indicating that 5-HT4R agonists can also serve as anti-inflammatory agents to treat diseases associated with gastrointestinal motility.
Acknowledgments
We thank Dainippon Sumitomo Pharma Co. Ltd. and Pfizer Inc. for supplying MOS and CJ-033466, respectively.
Footnotes
Funding: This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education (to MH, no.18380173 and no. 21380178; to HO, no. 20228005; and to TM, no. 19688014).
Competing interests: HO received grant support from Dainippon Sumitomo Pharma Co. Ltd. The remaining authors have declared no financial interests.
Patient consent: Not needed.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Abell TL, Camilleri M, DiMagno EP, et al. Long-term efficacy of oral cisapride in symptomatic upper gut dysmotility. Dig Dis Sci 1991;36:616–20 [DOI] [PubMed] [Google Scholar]
- 2.Appel-Dingemanse S. Clinical pharmacokinetics of tegaserod, a serotonin 5-HT4 receptor partial agonist with promotile activity. Clin Pharmacokinet 2002;41:1021–42 [DOI] [PubMed] [Google Scholar]
- 3.Deruyttere M, Lepoutre L, Heylen H, et al. Cisapride in the management of chronic functional dyspepsia: a multicenter, double-blind, placebo-controlled study. Clin Ther 1987;10:44–51 [PubMed] [Google Scholar]
- 4.Kim HS, Choi EJ, Park H. The effect of mosapride citrate on proximal and distal colonic motor function in the guinea-pig in vitro. Neurogastroenterol Motil 2008;20:169–76 [DOI] [PubMed] [Google Scholar]
- 5.Cellek S, John AK, Thangiah R, et al. 5-HT4 receptor agonists enhance both cholinergic and nitrergic activities in human isolated colon circular muscle. Neurogastroenterol Motil 2006;18:853–61 [DOI] [PubMed] [Google Scholar]
- 6.Baker DE. Rationale for using serotonergic agents to treat irritable bowel syndrome. Am J Health Syst Pharm 2005;62:700–11; quiz 12–13. [DOI] [PubMed] [Google Scholar]
- 7.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397–414 [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Geddis MS, Wen Y, et al. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol 2005;289:G1148–63 [DOI] [PubMed] [Google Scholar]
- 9.Poole DP, Xu B, Koh SL, et al. Identification of neurons that express 5-hydroxytryptamine 4 receptors in intestine. Cell Tissue Res 2006;325:413–22 [DOI] [PubMed] [Google Scholar]
- 10.Prins NH, Akkermans LM, Lefebvre RA, et al. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol 2000;131:927–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston EH, Passaro EP., Jr Postoperative ileus. Dig Dis Sci 1990;35:121–32 [DOI] [PubMed] [Google Scholar]
- 12.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil 2004;16(Suppl 2):54–60 [DOI] [PubMed] [Google Scholar]
- 13.Boeckxstaens GE, Pelckmans PA, Rampart M, et al. Pharmacological characterization of 5-hydroxytryptamine receptors in the canine terminal ileum and ileocolonic junction. J Pharmacol Exp Ther 1990;254:652–8 [PubMed] [Google Scholar]
- 14.De Winter BY, Boeckxstaens GE, De Man JG, et al. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br J Pharmacol 1997;120:464–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 1999;117:378–87 [DOI] [PubMed] [Google Scholar]
- 16.Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 2000;118:316–27 [DOI] [PubMed] [Google Scholar]
- 17.Schwarz NT, Kalff JC, Turler A, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 2001;121:1354–71 [DOI] [PubMed] [Google Scholar]
- 18.Wehner S, Behrendt FF, Lyutenski BN, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 2007;56:176–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt J, Stoffels B, Moore BA, et al. Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterology 2008;135:926–36 e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikkelsen HB, Thuneberg L, Rumessen JJ, et al. Macrophage-like cells in the muscularis externa of mouse small intestine. Anat Rec 1985;213:77–86 [DOI] [PubMed] [Google Scholar]
- 21.Ozaki H, Kawai T, Shuttleworth CW, et al. Isolation and characterization of resident macrophages from the smooth muscle layers of murine small intestine. Neurogastroenterol Motil 2004;16:39–51 [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res 1992;270:273–9 [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita K, Horiguchi K, Fujisawa M, et al. Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem Cell Biol 2007;127:41–53 [DOI] [PubMed] [Google Scholar]
- 24.Hori M, Kita M, Torihashi S, et al. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol 2001;280:G930–8 [DOI] [PubMed] [Google Scholar]
- 25.Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000;87:1480–93 [DOI] [PubMed] [Google Scholar]
- 26.Seta ML, Kale-Pradhan PB. Efficacy of metoclopramide in postoperative ileus after exploratory laparotomy. Pharmacotherapy 2001;21:1181–6 [DOI] [PubMed] [Google Scholar]
- 27.Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: where next? Trends Pharmacol Sci 2008;29:465–71 [DOI] [PubMed] [Google Scholar]
- 28.Zeinali F, Stulberg JJ, Delaney CP. Pharmacological management of postoperative ileus. Can J Surg 2009;52:153–7 [PMC free article] [PubMed] [Google Scholar]
- 29.De Winter BY, Boeckxstaens GE, De Man JG, et al. Effect of different prokinetic agents and a novel enterokinetic agent on postoperative ileus in rats. Gut 1999;45:713–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narita K, Tsunoda A, Takenaka K, et al. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis Colon Rectum 2008;51:1692–5 [DOI] [PubMed] [Google Scholar]
- 31.de Jonge WJ, Ulloa L. The α7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 2007;151:915–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakashita M, Mizuki Y, Hashizume T, et al. Pharmacokinetics of the gastrokinetic agent mosapride citrate after intravenous and oral administrations in rats. Arzneimittelforschung 1993;43:859–63 [PubMed] [Google Scholar]
- 33.Yoshida N, Omoya H, Kato S, et al. Pharmacological effects of the new gastroprokinetic agent mosapride citrate and its metabolites in experimental animals. Arzneimittelforschung 1993;43:1078–83 [PubMed] [Google Scholar]
- 34.Mikami T, Ochi Y, Suzuki K, et al. 5-Amino-6-chloro-N-[(1-isobutylpiperidin-4-yl)methyl]-2-methylimidazo[1,2-α]pyridine-8-carboxamide (CJ-033,466), a novel and selective 5-hydroxytryptamine 4 receptor partial agonist: pharmacological profile in vitro and gastroprokinetic effect in conscious dogs. J Pharmacol Exp Ther 2008;325:190–9 [DOI] [PubMed] [Google Scholar]
- 35.Toga T, Kohmura Y, Kawatsu R. The 5-HT4 agonists cisapride, mosapride, and CJ-033466, a novel potent compound, exhibit different human ether-a-go-go-related gene (hERG)-blocking activities. J Pharmacol Sci 2007;105:207–10 [DOI] [PubMed] [Google Scholar]
- 36.Grossman CJ, Kilpatrick GJ, Bunce KT. Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br J Pharmacol 1993;109:618–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 2004;172:6011–19 [DOI] [PubMed] [Google Scholar]
- 38.Tanila H, Kauppila T, Taira T. Inhibition of intestinal motility and reversal of postlaparotomy ileus by selective α2-adrenergic drugs in the rat. Gastroenterology 1993;104:819–24 [DOI] [PubMed] [Google Scholar]
- 39.Kreiss C, Toegel S, Bauer AJ. α2-Adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents. Am J Physiol Gastrointest Liver Physiol 2004;287:G658–66 [DOI] [PubMed] [Google Scholar]
- 40.Brown SW, Meyers RT, Brennan KM, et al. Catecholamines in a macrophage cell line. J Neuroimmunol 2003;135:47–55 [DOI] [PubMed] [Google Scholar]
- 41.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62 [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003;421:384–8 [DOI] [PubMed] [Google Scholar]
- 43.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, et al. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 2006;131:1122–30 [DOI] [PubMed] [Google Scholar]
- 44.The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 2007;133:1219–28 [DOI] [PubMed] [Google Scholar]
- 45.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 2005;6:844–51 [DOI] [PubMed] [Google Scholar]
- 46.Whiteaker P, Marks MJ, Christensen S, et al. Synthesis and characterization of 125I-α-conotoxin ArIB[V11L;V16A], a selective α7 nicotinic acetylcholine receptor antagonist. J Pharmacol Exp Ther 2008;325:911–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hori M, Nobe H, Horiguchi K, et al. MCP-1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am J Physiol Cell Physiol 2008;294:C391–401 [DOI] [PubMed] [Google Scholar]
- 48.Schwarz NT, Beer-Stolz D, Simmons RL, et al. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg 2002;235:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–64 [DOI] [PubMed] [Google Scholar]
- 50.Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil 2009;21:6–17 [DOI] [PubMed] [Google Scholar]
- 51.Chernyavsky AI, Arredondo J, Skok M, et al. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int Immunopharmacol 2010;10:308–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su X, Lee JW, Matthay ZA, et al. Activation of the α7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol 2007;37:186–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies BD, Hoss W, Lin JP, et al. Evidence for a noncholinergic nicotine receptor on human phagocytic leukocytes. Mol Cell Biochem 1982;44:23–31 [DOI] [PubMed] [Google Scholar]
- 54.Saeed RW, Varma S, Peng-Nemeroff T, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 2005;201:1113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Jonge WJ, The FO, van der Coelen D, et al. Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice. Gastroenterology 2004;127:535–45 [DOI] [PubMed] [Google Scholar]
- 56.Mishra NC, Rir-sima-ah J, Boyd RT, et al. Nicotine inhibits FcvarepsilonRI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through α7/α9/α10-nicotinic receptors. J Immunol 2010;185:588–96 [DOI] [PMC free article] [PubMed] [Google Scholar]