Abstract
Hypothalamic–pituitary–adrenal underactivity has been reported in rheumatoid arthritis (RA). This phenomenon has implications with regard to the pathogenesis and treatment of the disease. The present study was designed to evaluate the secretion of the adrenal androgen dehydroepiandrosterone sulfate (DHEAS) and its relation to clinical variables in RA, spondyloarthropathy (Spa), and undifferentiated inflammatory arthritis (UIA). Eighty-seven patients (38 with RA, 29 with Spa, and 20 with UIA) were studied, of whom 54 were women. Only 12 patients (14%) had taken glucocorticoids previously. Age-matched, healthy women (134) and men (149) served as controls. Fasting blood samples were taken for determination of the erythrocyte sedimentation rate (ESR), serum DHEAS and insulin, and plasma glucose. Insulin resistance was estimated by the homeostasis-model assessment (HOMAIR). DHEAS concentrations were significantly decreased in both women and men with inflammatory arthritis (IA) (P < 0.001). In 24 patients (28%), DHEAS levels were below the lower extreme ranges found for controls. Multiple intergroup comparisons revealed similarly decreased concentrations in each disease subset in both women and men. After the ESR, previous glucocorticoid usage, current treatment with nonsteroidal anti-inflammatory drugs, duration of disease and HOMAIR were controlled for, the differences in DHEAS levels between patients and controls were markedly attenuated in women (P = 0.050) and were no longer present in men (P = 0.133). We concluded that low DHEAS concentrations are commonly encountered in IA and, in women, this may not be fully explainable by disease-related parameters. The role of hypoadrenalism in the pathophysiology of IA deserves further elucidation. DHEA replacement may be indicated in many patients with IA, even in those not taking glucocorticoids.
Keywords: Dehydroepiandrosterone sulfate, inflammatory arthritis
Introduction
Decreased hypothalamic–pituitary–adrenal activity, particularly a blunted response to activation of the immune system, is strongly implicated in the onset and persistence of inflammatory arthritis (IA) [1]. Dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) are the steroid hormones most abundantly produced by the human adrenal cortex, and therefore are probably of biological importance [2,3]. Low serum concentrations of these weak androgens more sensitively confirm hypothalamic–pituitary–adrenal hypofunction than does glucocorticoid secretion [4]. In young women with rheumatoid arthritis (RA), decreased DHEA and DHEAS levels are significantly correlated with low early-morning cortisol concentrations and high basal levels of interleukin-6 [5]. Both glucocorticoids and testosterone, of which DHEA is a precursor, attenuate cytokine production by synovial inflammatory cells [1].
DHEAS concentrations before the onset of RA in pre-menopausal women were reported to be reduced in two studies by Masi et al [6,7] and normal in a study by Heikilla et al [8], respectively. However, the findings in the latter report may have been related to a variation in the use of laboratory methods to assay DHEA levels or a genetically different type of RA in Finnish patients [6]. The concentration of DHEAS in both serum and synovial tissue is decreased in established RA [9]. The decrease is more pronounced in patients taking glucocorticoids [10]. DHEA replacement in the latter circumstance has been recommended as a means of attenuating glucocorticoid-induced side effects [10]. Recently, the acute phase response and the severity of disease were reported to correlate with decreased basal DHEA levels in RA [5]. Treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) similarly attenuates hypothalamic–pituitary–adrenal axis function in RA [11]. Also, insulin resistance, which may be a common disturbance in RA [12], is associated with loss of the diurnal rhythm and hyporesponsiveness of the hypothalamic–pituitary–adrenal axis to stress [13].
In the present study, we compared serum DHEAS concentrations in 87 patients who had IA (RA, spondyloarthropathy [Spa], or undifferentiated inflammatory arthritis [UIA]) with the concentrations in controls matched for age and sex, and investigated whether decreased serum DHEAS concentrations in IA could be accounted for by the acute-phase response, previous glucocorticoid usage, current NSAID treatment, duration of disease, and insulin resistance.
Materials and methods
Patients, controls, and investigations
Eighty-seven consecutive nondiabetic patients were enrolled. Their age (mean ± SD) was 49 ± 14 years (range 18–81). Eighty were Caucasian and 7 were Asian. Fifty-four were women, of whom 26 had RA (FRA), 14 Spa (FSpa), and 14 UIA (FUIA). Of the 33 men, 12 had RA (MRA), 15 Spa (MSpa), and 6 UIA (MUIA). The duration of disease (mean ± SD) was 4.8 ± 7.8 years. Patients with RA met the 1987 criteria of the American Rheumatism Association for the classification of RA [14] and patients with Spa met the criteria of the European Spondyloarthropathy Study Group (ESSG) for the classification of spondyloarthropathy [15]. Patients with UIA had swelling of at least two joint areas for a minimum of 6 weeks without meeting the criteria for either RA or Spa. Patients who had used glucocorticoids within the previous 2 months or who were taking agents known to affect insulin sensitivity or lipid metabolism were excluded. Twenty-six patients were on no medication. Agents taken were NSAIDs (n = 51), opioid agonists of low efficacy (n = 7), paracetamol (n = 6), methotrexate (n = 5), sulfasalazine (n = 4), misoprostol (n = 3), lansoprazole (n = 2), clonazepam (n = 2), mianserin (n = 2), alendronate (n = 1), and amitryptiline (n = 1). Glucocorticoids had been taken by 12 patients (6 with FRA, 2 with FUIA, 1 with MRA, 2 with MSpa, and 1 with MUIA).
The duration of disease was recorded and the body mass index (kg/m2) determined in each patient. Fasting blood samples were taken (between 0800 and 1000) for determination of the erythrocyte sedimentation rate (ESR), serum DHEAS (chemiluminescent enzyme immunoassay; Diagnostic Products Co, Los Angeles, CA, USA), lipids and insulin (microparticle enzyme immunoassay on the Axsym system; Abbott, IL, USA), and plasma glucose. The intra-assay and interassay coefficients of variance were, respectively, 1.9% and 1.2% for insulin, and 8.2% and 12.0% for DHEAS. Insulin resistance was estimated by the homeostasis-model assessment (HOMAIR) [16] using the formula fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5. Normal values for DHEAS were obtained from 134 age-matched healthy women (mean age 48 ± 17 years) and 149 age-matched healthy men (mean age 52 ± 16 years).
Statistical analyses
Statistical analyses were made using the Statistical Package for the Social Sciences (SPSS) program. DHEAS concentrations were compared between patients and controls using the one-way analysis of variance (ANOVA). Multiple intergroup (FRA, FSpa, FUIA, MRA, MSpa, MUIA) comparisons were done by the one-way ANOVA and the Bonferroni post hoc test. The dependencies of DHEAS levels on the ESR, previous glucocorticoid usage, current NSAID treatment, duration of disease, and were evaluated using simple linear regression HOMAIR analyses and analyses of covariance. Results were expressed as mean ± SD except in boxplots representing DHEAS concentrations in the controls.
Results
DHEAS concentrations in patients and controls
The DHEAS concentrations in patients and controls are reported in Table 1 and Figs 1 to 4. DHEAS concentrations were significantly lower in both women and men with IA than in their respective controls, and in 14 women (26%) and 10 men (30%) with IA, these concentrations fell below the extreme ranges (Figs 1 and 2) found in age-matched controls. The values found for the various subsets of disease are given in Table 2. Multiple intergroup comparisons of DHEAS concentrations revealed no significant differences apart from higher levels in MSpa than in MUIA (P = 0.04). Even in patients with MSpa, DHEAS concentrations were lower than in male controls (4.61 ± 1.87 vs 6.19 ± 4.12 μmol/l, P < 0.001). This difference remained equally significant after age was controlled for.
Table 1.
Serum DHEAS concentrations (μmol/l) in patients with IA and in controls
| Serum DHEAS (μmol/l) | |||
| Gender | Controls | Patients | P |
| Women | 3.65 ± 2.84 | 2.21 ± 2.06 | <0.001 |
| Men | 6.19 ± 4.12 | 3.66 ± 2.02 | <0.001 |
Values are shown as mean ± SD.
Figure 1.
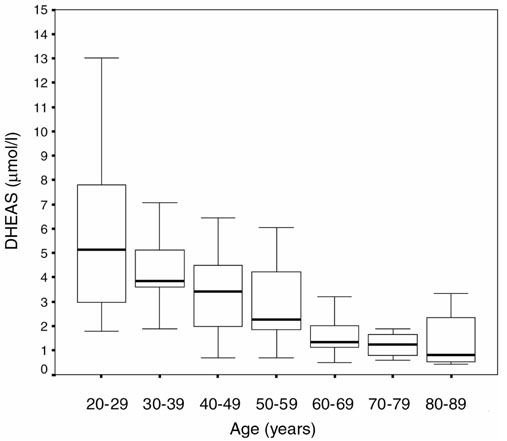
Serum DHEAS concentrations in female controls according to their age.
Figure 4.
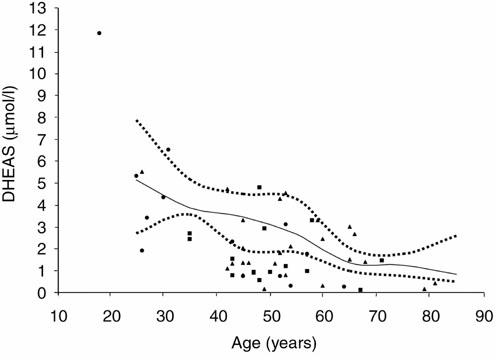
Serum DHEAS concentrations in male controls. Symbols as in Fig. 3.
Figure 2.
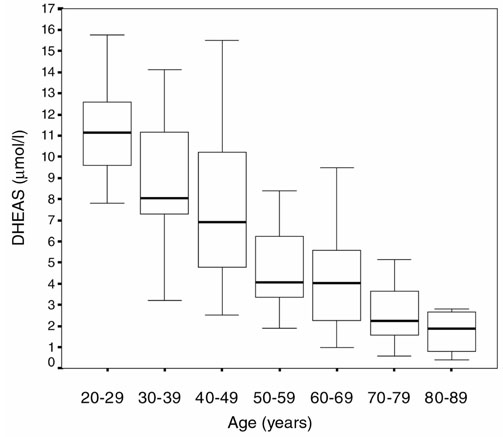
Serum DHEAS concentrations in male controls according to their age.
Table 2.
Serum DHEAS concentrations (μmol/l) in subsets of IA
| IA subset | DHEAS |
| FRA | 2.01 ± 1.50 |
| Fspa | 3.04 ± 3.20 |
| FUIA | 1.74 ± 1.30 |
| MRA | 3.18 ± 2.07 |
| Mspa | 4.61 ± 1.87* |
| MUIA | 2.23 ± 1.09 |
*vs MUIA: P = 0.04.
The dependencies of DHEAS on clinical variables
The dependencies of DHEAS on clinical variables in patients with IA are represented in Tables 3 and 4. None of the evaluated variables contributed significantly to the variance of DHEAS concentrations, except for NSAID treatment in men with IA. After we had controlled for the ESR, previous glucocorticoid usage, duration of disease, current NSAID treatment, and HOMAIR, the differences in DHEAS concentrations between patients and controls were markedly attenuated in women (P = 0.050) and were no longer present in men (P = 0.133).
Table 3.
Dependencies of DHEAS on disease-related variables in IA in females
| Variable | aR2 | P |
| ESR (mm/hour) | 0.016 | 0.519 |
| Duration of disease (months) | 0.015 | 0.512 |
| Glucocorticoid therapy | 0.003 | 0.339 |
| HOMAIR (μU.mmol/ml.l) | 0.014 | 0.529 |
| NSAID therapy | 0.004 | 0.383 |
aR2, adjusted R2.
Table 4.
Dependencies of DHEAS on disease-related variables in IA in males
| Variable | aR2 | P |
| ESR (mm/hour) | 0.017 | 0.497 |
| Duration of disease (months) | 0.004 | 0.361 |
| Glucocorticoid therapy | 0.006 | 0.371 |
| HOMAIR (μU.mmol/ml.l) | 0.013 | 0.449 |
| NSAID therapy | 0.042 | 0.003 |
aR2, adjusted R2.
Since the acute-phase response may not correlate with disease activity in inflammatory arthritides other than RA [17], a subgroup analysis was made in the 26 patients with FRA (Table 5). In these, previous glucocorticoid usage predicted low DHEAS concentrations. However, even when this variable was controlled for, DHEAS concentrations remained lower in FRA than in female controls (P = 0.037).
Table 5.
Dependencies of DHEAS on disease-related variables in RA in females
| Variable | aR2 | P |
| ESR (mm/hour) | 0.040 | 0.109 |
| Duration of disease (months) | 0.044 | 0.092 |
| Glucocorticoid therapy | 0.049 | 0.037 |
| HOMAIR (μU.mmol/ml.l) | 0.023 | 0.305 |
| NSAID therapy | 0.015 | 0.433 |
aR2, adjusted R2.
Similarly, since DHEAS concentrations were higher in MSpa than in MUIA, a subgroup analysis was made (Table 6). In the MSpa patients, the HOMAIR and NSAID treatment accounted for 15.2% and 16.7%, respectively, of the variance in DHEAS concentrations. After the respective variables were controlled for, DHEAS concentrations were no longer different between patients and controls (P = 0.766). The body mass index was higher (28.8 ± kg/m2) in the MSpa subgroup than in the other groups of patients. The detailed results on the body mass indices, lipids, and insulin resistance are the subject of a separate report (submitted for publication).
Table 6.
Dependencies of DHEAS on disease-related variables in Spa in males
| Variable | aR2 | P |
| ESR (mm/hour) | 0.025 | 0.172 |
| Duration of disease (months) | 0.002 | 0.259 |
| Glucocorticoid therapy | 0.023 | 0.160 |
| HOMAIR (μU.mmol/ml.l) | 0.152 | 0.083 |
| NSAID therapy | 0.167 | 0.073 |
aR2, adjusted R2.
Discussion
In the present study, we found decreased DHEAS concentrations in both women and men with RA, Spa, or UIA. This finding was previously documented in RA [9]. With regard to Spa, Hedman et al [18] reported normal DHEAS concentrations in 25 patients who had psoriatic arthritis, reactive arthritis, ankylosing spondylitis, or enteropathic arthritis. Both elevated and decreased DHEAS concentrations have been reported in ankylosing spondylitis [19,20]. In one of the latter reports, a significant correlation between low DHEAS concentrations and a high acute-phase response was found [20], as was recently reported for RA [5]. The role of sex steroids in the pathogenesis of Spa remains incompletely elucidated [19]. Of our 29 patients with Spa, 9 had ankylosing spondylitis, 7 psoriatic arthritis, and 13 reactive or undifferentiated spondyloarthropathy. Although the DHEAS concentrations were highest in our patients with MSpa, the respective concentrations were still significantly lower than in age- and gender-matched controls.
The number of patients enrolled in our study allowed us to control for disease-related factors, i.e. the acute-phase response, previous glucocorticoid usage, NSAID treatment, duration of disease, and insulin resistance. The overall disease activity was low in our patients (mean ESR=26 mm/hour), which may explain why the investigated disease-related parameters did not significantly predict low DHEAS concentrations except for previous glucocorticoid usage in RA women and NSAID treatments in IA men. Even after disease-related factors were controlled for, DHEAS concentrations tended to remain lower in IA women than in their control subjects. This finding indirectly supports the intriguing paradigm in which IA may select for patients with subtle adrenal insufficiency, as proposed by Masi et al [7]. Our finding of similarly decreased concentrations in the various subsets of the disease supports the observation that risk factors for RA and inflammatory arthritides other than RA are alike [21]. In contrast, DHEAS concentrations were no longer different in men with IA from those in their controls after disease-related parameters had been controlled for. Underactivity of the hypothalamic–pituitary–gonadal axis, with decreased testosterone production, was documented in men with RA [22], and this underactivity may participate in the pathogenesis in these patients [1]. The roles of hypoadrenalism (decreased glucocorticoid as well as adrenal androgen production) and hypogonadism in the onset and persistence of disease in various IA subgroups and in both genders deserves further investigation.
In 44 (51%) of our patients, serum DHEAS concentrations were below the 25th percentile obtained in controls and in 24 patients (28%), these levels were below normal. Robinzon and Cutolo [10] recently attributed several side effects of glucocorticoid therapy, including catabolism, muscular atrophy, osteopenia, osteoporosis, and avascular necrosis, to the suppressant effect of these agents on the hypothalamic–pituitary–adrenal axis, resulting in decreased DHEA secretion. Also, low DHEAS concentrations constitute an independent risk factor for cardiovascular disease [3] and recently DHEA was shown to form an intergral part of low-density lipoprotein and to protect the latter potently from oxidation [23]. This is of interest in the present context, since a 70% excess cardiovascular mortality was reported in RA [24,25,26]. Indeed, IA and cardiovascular disease may share a common predisposition [27].
DHEA replacement is associated with improvement of physical and general wellbeing in elderly subjects [28] and with lessening of disease activity in lupus [29] and of osteoporosis in postmenopausal women [30]. It constitutes part of the optimal treatment of frank adrenal insufficiency [31]. Robinzon and Cutolo suggested the use of DHEA replacement, guided by monitoring of its concentration in the blood, in RA patients taking glucocorticoids [10]. Although short-term DHEA replacement therapy was not associated with an improvement in disease activity in 11 patients with RA, the findings mentioned above together with our results suggest that the indications for DHEA replacement in IA should not be restricted to patients on glucocorticoids. The role of DHEA supplementation in the treatment of IA requires further study.
Conclusion
In a controlled study on patients with IA, we found that secretion of DHEAS is similarly reduced in RA, Spa, and UIA. After we had controlled for the acute-phase response, previous glucocorticoid usage, current NSAID therapy, duration of disease, and insulin resistance, the differences in DHEAS concentrations between patients and controls matched for age and sex were attenuated in women and were no longer present in men. The contribution of low DHEAS concentrations to the pathogenesis of IA deserves further study. Also, in view of the impaired health status associated with low DHEAS concentrations, the role of DHEA replacement therapy in IA needs to be investigated.
Abbreviations
DHEA = dehydroepiandrosterone; DHEAS = dehydroepiandrosterone sulfate; ESR = erythrocyte sedimentation rate; FRA = rheumatoid arthritis in females; FSpa = spondyloarthropathy in females; FUIA = undifferentiated inflammatory arthritis in females; HOMAIR = homeostasis-model assessment of insulin resistance; IA = inflammatory arthritis; MRA = rheumatoid arthritis in males; MSpa = spondyloarthropathy in males; MUIA = undifferentiated inflammatory arthritis in males; NSAID = nonsteroidal anti-inflammatory drugs; RA = rheumatoid arthritis; Spa = spondyloarthropathy; UIA = undifferentiated inflammatory arthritis.
Figure 3.
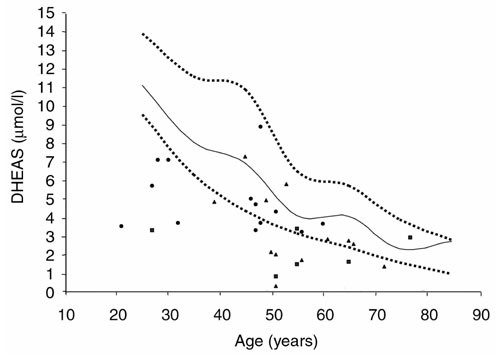
Serum DHEAS concentrations in female controls (unbroken line = median; lower dotted line = 25th percentile; upper dotted line = 75th percentile) and patients (dots = RA; triangles = Spa; squares = UIA).
Acknowledgments
Acknowledgements
This study was supported by the Pain Research and Relief Unit of the University of the Witwatersrand and Lancet Laboratories, Johannesburg, South Africa.
References
- Cutolo M, Straub RH. Recent aspects of gonadal hormone and neurotransmitter interactions with synovial and immune cells: implications in rheumatoid arthritis. Ann Rheum Dis. 2000;59:657–661. doi: 10.1136/ard.59.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi AT, Chrousos GP, Bornstein ST. Enigmas of adrenal androgen and glucocorticoid dissociation in premenopausal onset rheumatoid arthritis. J Rheumatol. 1999;26:247–250. [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SSC. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Masi AT, Chrousos GP. hypothalamic–pituitary–adrenal-glucocorticoid axis function in rheumatoid arthritis. J Rheumatol. 1996;23:577–581. [PubMed] [Google Scholar]
- Cutolo M, Foppiani L, Prete C, Ballarino P, Sulli A, Villaggio B, Seriolo B, Giusti M, Accardo S. Hypothalamic-pituitary-adreno-cortical axis function in premenopausal women with rheumatoid arthritis not treated with glucocorticoids. J Rheumatol. 1999;26:282–288. [PubMed] [Google Scholar]
- Masi AT, Chatterton RT, Aldag JC, Comstock GW, Helzlsouer KJ, Hoffman SC, Malmet RL, Agopian MS. Decreased serum dehydroepiandrosterone sulfate levels in menstruating women prior to onset of RA before age 50: independent reference laboratory assays validate previous significant results [abstract]. Arthritis Rheum. 1998;41:S126. [Google Scholar]
- Masi AT, Chatterton RT, Aldag JC. Perturbations of hypothalamic–pituitary–gonadal axis and adrenal androgen functions in rheumatoid arthritis: an odyssey of hormonal relationships to the disease. Ann N Y Acad Sci. 1999;876:53–62. doi: 10.1111/j.1749-6632.1999.tb07622.x. [DOI] [PubMed] [Google Scholar]
- Heikkila R, Aho K, Heliovaara M, Knekt P, Reunanen A, Aromaa A, Leino A, Palosuo T. Serum androgen-anabolic hormones and the risk of rheumatoid arthritis. Ann Rheum Dis. 1998;57:281–285. doi: 10.1136/ard.57.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre B, Hedman M, Nilsson E, Oleson O, Throner A. Relationship between blood and joint tissue DHEAS levels in rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 1993;11:597–601. [PubMed] [Google Scholar]
- Robinzon B, Cutolo M. Should dehydroepiandrosterone replacement therapy be provided with glucocorticoids? Rheumatology. 1999;38:488–495. doi: 10.1093/rheumatology/38.6.488. [DOI] [PubMed] [Google Scholar]
- Hall J, Morand EF, Medbak S, Zaman M, Perry L, Goulding NJ, Maddison PJ, O'Hare JP. Abnormal hypothalamic–pituitary–adrenal axis function in rheumatoid arthritis. Effects of non-steroidal antiinflammatory drugs and water immersion. Arthritis Rheum. 1994;37:1132–1137. doi: 10.1002/art.1780370804. [DOI] [PubMed] [Google Scholar]
- Svenson KLG, Pollare T, Lithell H, Hallgren R. Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism. 1988;37:125–130. doi: 10.1016/s0026-0495(98)90005-1. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS. The American Rheumatology Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Kahn MA, van der Linden SM. A wider spectrum of spondylo-arthropathies. Semin Arthritis Rheum. 1990;20:107–112. doi: 10.1016/0049-0172(90)90023-9. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- Koh WH, Pand I, Samuels A, Jones S, Calin A. Low dose amytriptyline in ankylosing spondylitis. A short term, double-blind placebo controlled study. J Rheumatol. 1997;24:2158–2161. [PubMed] [Google Scholar]
- Hedman M, Milsson E, de la Torre B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin Exp Rheumatol. 1992;10:25–30. [PubMed] [Google Scholar]
- Giltay EJ, van Schaardenburg D, Gooren LJ, Popp-Snijders C, Dijkmans BA. Androgens and ankylosing spondylitis: a role in the pathogenesis? Ann N Y Acad Sci. 1999;876:340–364. doi: 10.1111/j.1749-6632.1999.tb07658.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Urena S, Ponce-Tellez A, Tello-Jimenez M, Zaragoza-Gonzalez R, Ramos-Remus C. Serum levels of dehydroepiandrosterone (DHEA-S) in ankylosing spondylitis [abstract]. Arthritis Rheum. 1999;41:S114. [Google Scholar]
- Symmons D, Harrison B. Early inflammatory polyarthritis: results from the Norfolk Arthritis register with a review of the literature. I. Risk factors for the development of inflammatory polyarthritis and rheumatoid arthritis. Rheumatology. 2000;39:835–843. doi: 10.1093/rheumatology/39.8.835. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Balleari E, Giusti M, Monachesi M, Accardo S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonatotropin stimulation. Arthritis Rheum. 1988;31:1314–1317. doi: 10.1002/art.1780311015. [DOI] [PubMed] [Google Scholar]
- Khalil A, Fortin JP, LeHoux JG, Fulop T. Age-related decrease of dehydroepiandrosterone concentrations in low density lipoproteins and its role in the susceptibility of low density lipoproteins to lipid peroxidation. J Lipid Res. 2000;41:1552–1561. [PubMed] [Google Scholar]
- Koota K, Isomaki H, Mutru O. Death rate and causes of death in RA patients during a period of five years. Scand J Rheumatol. 1977;6:241–244. doi: 10.3109/03009747709095458. [DOI] [PubMed] [Google Scholar]
- Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. Br Med J (Clin Res Ed) 1985;290:1797–1799. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson LT, Knowler WC, Pillemer S, Hanson RL, Pettitt DJ, Nelson RG, del Puente A, McCance DR, Charles MA, Bennett PH. Rheumatoid arthritis and mortality. A longitudinal study in Pima Indian. Arthritis Rheum. 1993;36:1045–1053. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- Dessein PH, Stanwix AE, Moomal Z. Rheumatoid arthritis and cardiovascular disease may share similar risk factors. Rheumatology. 2001. [DOI] [PubMed]
- Morales AJ, Molan JJ, Nelson JE, Yen SSC. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven RF, Morabilo LM, Engleman HG, McGuire JL. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. J Rheumatol. 1998;25:285–289. [PubMed] [Google Scholar]
- Labrie R, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3498–3505. doi: 10.1210/jcem.82.10.4306. [DOI] [PubMed] [Google Scholar]
- Wiebke A, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341:1013–1020. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]


