Abstract
Zoster of the sciatic nerve, the longest nerve in the human body, is an uncommon event. We cared for a child with sciatic nerve zoster who had severe pain over the lower back 6 days before appearance of vesicular rash on the foot in the L4 dermatome. On the basis of the clinical data, we calculated an anterograde velocity for the varicella zoster virion of 5.55 mm/h or .0015 mm/s. Because there is no good animal model of varicella zoster virus reactivation from latency, this experiment of nature fills a notable gap in our knowledge about varicella zoster virus neuronal transportation.
The classic epidemiological study of herpes zoster was performed by Hope-Simpson [1], whose practice included 3500 persons in Cirencester, England. During 1947–1962, he examined 192 persons with zoster. He carefully recorded the dermatomal distribution and observed that the single most frequently involved ganglion was the trigeminal, although the majority of cases occurred between thoracic dermatomes 5–10. The rarest sites of zoster were the lower lumbar and sacral dermatomes. In particular, he did not observe a single case of shingles of the L4 dermatome.
The velocity at which varicella zoster virus (VZV) travels in a human neuron has never been determined. The severe combined immunodeficient mouse with explants of human tissue is the best available animal model for VZV infection [2]. However, there is no ideal animal model that fully replicates all the features of VZV reactivation from latency and subsequent anterograde spread of virus. Therefore, VZV velocity studies have not been performed in a neuron in an animal model under these conditions. Recently, we observed a child who had developed shingles of the foot. A careful history allowed us to estimate the time required for the virus to transit from the lumbosacral ganglion to the foot. This velocity correlates very closely with the neuronal transit rate of another evolutionarily related herpesvirus of pigs, pseudorabies virus (PRV), which has been investigated in an animal model. Thus, this clinical case provides a valuable supplement to the natural history of herpes zoster provided in the past by Hope-Simpson [1] and the Nobel laureate Weller [3].
MATERIALS AND METHODS
The kit for detection of VZV antigens in vesicle fluid samples was purchased from Meridian Bioscience. The immunofluorescent probe in the kit is a mouse monoclonal antibody against the most abundant VZV antigen in infected human tissues, namely the gE glycoprotein (previously called VZV gpI) [4]. The rapid antigen detection kit for herpes simplex virus (HSV) was purchased from Chemicon. Standards for children's height and weight were based on data collected in a reference atlas [5].
Case Report
A 9-year-old boy was admitted to the hospital on 4 December with acute severe back pain of 4 days’ duration. His very first symptom on 1 December was pain referred to his left hip when he stepped into the family car. His weight was 28.3 kg (50th percentile), and his height was 1.3 meters (40th percentile) [5]. He was afebrile. Of note, he had received a diagnosis of acute lymphocytic leukemia at age 4 years. He had responded well to both induction and maintenance chemotherapy and was now in remission. At the time of admission, he was not receiving any chemotherapy. Nevertheless, the history of leukemia prompted the current admission. During examination, the child appeared to be well nourished and well developed. No rashes or bruises were evident anywhere on the body. During an initial neurological examination, his cranial nerves were intact and deep tendon reflexes were demonstrable. Sensation to light touch was equivalent in dermatomes over both legs and on the dorsal surfaces of both feet. He was able to move his quadriceps, hamstrings, gastrocnemius, soleus, tibialis anterior, extensor hallucis longus, and flexor hallucis longus muscles in both legs. However, he was unable to stand because of exquisite pain in his left lower back and left leg. He stated that any movement of the left leg led to substantial pain in the left flank. Pressure over the left iliac crest also was painful.
CT of the lower spine, pelvis, and legs detected no boney abnormalities or soft tissue swelling. Abdominal plain films were read as normal. No evidence for relapse of leukemia was discovered after an extensive hematologic evaluation.
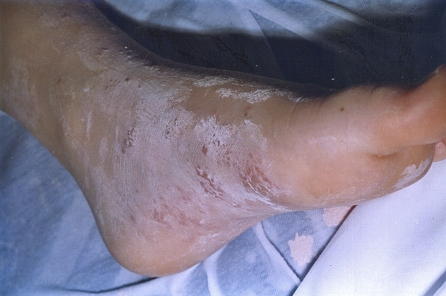
On the evening of the patient’s third day in the hospital, 6 December, a vesicular rash erupted over the medial side of his left foot (Figure 1). A scraping was obtained for examination by an immunofluorescent rapid VZV diagnostic test. The cells stained positively for VZV antigens. The diagnosis of zoster of the left foot was made. The child immediately began receiving treatment with intravenous acyclovir (30 mg/kg/day), followed by treatment with oral acyclovir after discharge from the hospital. He had a complete recovery without postherpetic neuralgia. He was well at a 1-year follow-up examination, without any relapse of leukemia.
Figure 1.
Exanthem on medial side of left foot. Cells were obtained from the fluid in the vesicular exanthem and examined for varicella zoster virus (VZV) and herpes simplex virus (HSV) antigens. The VZV antigen test result was positive, and the HSV antigen test result was negative.
Of note, the boy had never received the live attenuated varicella vaccine at age 1 year. However, the patient had a positive VZV antibody titer when screened after the diagnosis of leukemia. The mother recalled that the patient had been exposed to siblings with chickenpox prior to the cancer diagnosis.
DISCUSSION
Because of the history of acute leukemia, the mother had become an excellent historian. She was clearly aware of the timing of the first complaints of pain in the lower back by her son. In fact, on the second day of pain, she had taken her son to an emergency department of a local hospital. Therefore, we consider the painful episode to be an accurate indicator of VZV replication in the lumbosacral ganglion. Because the VZV replication cycle is ∼16 h [6], we cannot exclude the possibility that the onset of zoster was actually a day earlier than the onset of pain. We consider the zoster episode to be linked to prior chemotherapy. Before the approval of varicella vaccination in 1995, we conducted a prospective 9-year survey of zoster in all our children receiving treatment for acute lymphocytic leukemia [7]. During the 9 years, a total of 14 children manifested 17 episodes of zoster. Almost 75% of the episodes occurred within the first 2 years after diagnosis of leukemia. However, a few cases extended to 65 months after diagnosis of leukemia, a period similar to the current case.
The sciatic nerve is the longest nerve in the human body [8]. Movement and sensation in the legs and feet are largely dependent on normal functioning of the sciatic nerve. After supervising the care of this child, we surmised that we had a singular opportunity to calculate the velocity of the VZ virion in a human nerve. The duration from onset of acute left lower back pain (ganglionitis) to appearance of the zoster exanthem on the medial left foot was 6 days in our patient. This rash was in the L4 dermatome [9]. If the sciatic nerve is assumed to be ∼80 cm in this child [5], then the VZ virions would have traveled 800 mm in 6 days, or 133 mm in 1 day. This corresponds to a rate of 5.55 mm/h, or .092 mm/min or .0015 mm/s (1.5 μm/s). If we allow 1 extra day for viral replication in and initial transit from the lumbosacral ganglion before onset of symptomatic ganglionitis, the VZ virions would have traveled 800 mm in 7 days, or 114 mm in 1 day. This velocity corresponds to .0013 mm/s (1.3 μm/s).
In summary, this uncommon case of sciatic zoster is an experiment of nature that provides considerable insight into the anterograde transit time of the varicella zoster virus in a sensory human neuron during clinical zoster. This transit time overlaps with that of the closely related PRV, when examined in an animal model. In the PRV laboratory, the investigators calculated an anterograde velocity of 1.97 μm/s and a retrograde velocity of 1.28 μm/s for PRV when traveling in sensory axons removed from embryonic chick embryos [10]. In an earlier study, investigators studying HSV-1 in the giant axon of the squid estimated a retrograde velocity of 2.2 μm/s [11]. In one sense, therefore, the human zoster case validates that the fast-axonal transport mechanism proposed for herpesviruses in 2 widely divergent animal models can be applied to VZV transportation in human neurons [12].
Funding
This work was supported by the National Shingles Foundation, and National Instituted of Health.
References
- 1.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Arvin AM. Investigations of the pathogenesis of varicella zoster virus infection in the SCIDhu mouse model. Herpes. 2006;13:75–80. [PubMed] [Google Scholar]
- 3.Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1434–40. doi: 10.1056/NEJM198312083092306. [DOI] [PubMed] [Google Scholar]
- 4.Weigle KA, Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro in chickenpox and zoster vesicles. J Infect Dis. 1983;148:630–8. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- 5.Saul RA, Geer JS, Seaver LH, Phelan MC, Sweet KM, Mills CM. Growth references: third trimester to adulthood. Greenwood, SC: Greenwood Genetic Center; 1998. [Google Scholar]
- 6.Rapp F, Vanderslice D. Spread of zoster virus in human embryonic lung cells and the inhibitory effect of iododeoxyuridine. Virology. 1964;22:321–30. doi: 10.1016/0042-6822(64)90023-6. [DOI] [PubMed] [Google Scholar]
- 7.Grose C, Giller RH. Varicella-zoster virus infection and immunization in the healthy and the immunocompromised host. Crit Rev Oncol Hematol. 1988;8:27–64. doi: 10.1016/s1040-8428(88)80004-0. [DOI] [PubMed] [Google Scholar]
- 8.Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Walton JGR, Hutchinson M, McArdle MJF, O'Brien MD, Thomas PK, Willison RG. Aids to the examination of the peripheral nervous system. London, UK: Bailliere Tindall; 1986. [Google Scholar]
- 10.Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A. 2001;98:3466–70. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci U S A. 2000;97:8146–50. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–14. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]