Abstract
Background. Group B Streptococcus (GBS) and Streptococcus pneumoniae (SP) are leading causes of bacterial meningitis in neonates and children. Each pathogen produces a pore-forming cytolytic toxin, β-hemolysin/cytolysin (β-h/c) by GBS and pneumolysin by SP. The aim of this study was to understand the role of these pore-forming cytotoxins, in particular of the GBS β-h/c, as potential neurotoxins in experimental neonatal meningitis.
Methods. Meningitis was induced in 7- and 11-day-old rats by intracisternal injection of wild type (WT) GBS or SP and compared with isogenic β-h/c- or pneumolysin-deficient mutants, or a double mutant of SP deficient in pneumolysin and hydrogen peroxide production.
Results. GBS β-h/c and SP pneumolysin contributed to neuronal damage, worsened clinical outcome and weight loss, but had no influence on the early kinetics of leukocyte influx and bacterial growth in the cerebrospinal fluid. In vitro, β-h/c-induced neuronal apoptosis occurred independently of caspase-activation and was not preventable by the broad spectrum caspase-inhibitor z-VAD-fmk.
Conclusions. These data suggest that both cytolytic toxins, the GBS β-h/c and SP pneumolysin, contribute to neuronal damage in meningitis and extend the concept of a key role for bacterial pore-forming cytolysins in the pathogenesis and sequelae of neonatal meningitis.
Streptococcus agalactiae (group B Streptococcus, GBS) and Streptococcus pneumoniae (SP) are the leading pathogens of bacterial meningitis in newborns and infants, respectively. About one-third of all cases of meningitis in preterm infants and newborns are caused by GBS [1–3], while approximately half of meningitis in older infants is attributable to SP, in particular, in developing counties [4, 5]. Although mortality of meningitis in the very youngest age groups has dropped significantly, long-term neurological sequelae such as deafness, blindness, cerebral palsy, seizures, hydrocephalus or cognitive impairment has remained virtually unchanged in 25%–50% of survivors [6–8].
While children and adults with SP or Haemophilus influenzae meningitis benefit from adjunctive anti-inflammatory therapy with dexamethasone [9, 10], similar interventions appear to have little influence on long-term morbidity in newborn infants with GBS meningitis [11, 12]. Additionally, the mortality and morbidity of neonates suffering meningitis are exceptionally high [1].
Several experimental studies in various models involving newborn animals have shown that neuronal vulnerability is extremely high in the first week of life [13, 14]. These findings suggest a specific vulnerability of the developing brain to infection and perhaps to specific bacterial virulence factors or toxins. During this time crucial developmental processes such as migration, formation of synapses, and physiological apoptosis take place in the immature central nervous system [15].
During meningitis, antibiotic- or autolysin-induced bacterial lysis releases cell wall fragments and cytoplasmic factors. The early host response results in the activation of immune competent cells such as microglia and the influx of leukocytes into the cerebrospinal fluid (CSF). Leukocytes are essential for the elimination of replicating bacteria but also release toxic factors which add to neuronal damage [16]. In the context of SP meningitis, bacterial toxins such as the pore-forming pneumolysin and H2O2 have been identified as major neurotoxins. Pneumolysin- and hydrogen peroxide-deficient SP mutants have been shown to cause less neuronal damage in the hippocampus in experimental meningitis [17]. Meningitis in neonatal rats induced by SP and GBS has been shown to result in pronounced neuronal damage in the hippocampus as well as in the cortex [18]. However, the contribution of bacterial toxins to such damage in neonatal animals has yet to be examined.
GBS, produces a pore-foring cytolysin, the β-hemolysin/cytolysin (β-h/c). This toxin is responsible for the hallmark zone of β-hemolysis, ie, complete lysis of the erythrocytes, visible when these bacteria grow on blood agar plates. β-h/c damages other eukaryotic cells like lung epithelium and endothelium, brain endothelial cells, and macrophages [19–22]. Several animal studies suggest that β-h/c contributes to GBS virulence in the context of pneumonia, sepsis, and blood-brain barrier penetration [22–25].
In the present study, a meningitis model of 7- and 11-day-old rats was established. The aims of the study were to investigate a) the extent of neurodegeneration induced by GBS or SP in a neonatal rat model, b) the role of their bacterial pore-forming cytolysins in triggering meningeal inflammation and neuronal damage, and c) direct effects of the GBS pore-forming cytolysin on cultured neurons.
MATERIALS AND METHODS
Animal Model of Neonatal Meningitis
Wistar rats (Charles River), at ages 7 and 11 of postnatal days, were infected by intracisternal injection of 10 μL (105 colony-forming units [cfu]/mL) or 10 μL PBS as described [18]; GBS, SP, and isogenic toxin-deficient mutant strains were administered. Bacteria were dissolved in sterile pyrogen-free phosphate buffered saline. Animals were treated with a subcutaneous injection of a glucose-electrolyte-solution (Jonosteril PÄD II) every 4 hours to prevent hypoglycemia and hypotension. After 18 hours, animals were weighed and investigated clinically applying a modified score as described [26], assessing motor activity and the time to turning upright when placed on their back: 5=normal motor activity and animal turned upright in <5 seconds; 4=decreased spontaneous activity but still turned upright in <5 seconds; 3=turned upright in >5 seconds; 2=did not turn upright; 1=did not move; and 0=death. Thereafter, animals were anesthetized intraperitoneally (i. p.) with ketamine (100 mg/kg; Pharmacia und Upjohn GmbH) and xylazine (20 mg/kg; Bayer). To confirm meningitis, cisterna magna dura mater was punctured with a 27 gauge butterfly needle. Five μL CSF were serially diluted and cultured on blood agar plates (Merck) which were incubated for 24 h at 37°C with 5% CO2. Leukocytes in the CSF were quantified in a Fuchs-Rosenthal counting chamber. Subsequently, rats were perfused transcardially with .1 M PBS (pH, 7.4), followed by 4% paraformaldehyde (PFA, Sigma) in PBS, pH 7.4. Ten minutes later, heads were postfixed overnight in 4% PFA. On the next day, brains were removed from the skulls and cut into 4 coronal 5-mm slices. These slices were embedded in paraffin and cut into 5 μm sections. All animal experiments were performed in accordance to the guidelines of the Humboldt University and of the Federal Republic of Germany.
Bacterial Culture
The following GBS and SP WT bacteria and mutants were investigated for their ability to induce neuronal apoptosis in our neonatal rat meningitis model: GBS-WT COH1, an encapsulated type III strain, and its isogenic nonhemolytic mutant COH1:cylEΔcat [27]; and encapsulated type 2 SP WT D39 (Rockefeller University, New York, USA), its pneumolysin-negative mutant plnA- (D. Briles, University of Alabama, Birmingham, USA) and its double knockout (pneumolysin and hydrogen peroxide) mutant plnA-/spxB- [17]. The bacteria were kept as frozen stocks at –70°C and plated on agar plates prior to growth in liquid media. In every experiment, bacteria were plated on agar plates to confirm their quantity and viability. GBS were grown in Todd-Hewitt broth (THB; [DIFCO]) and SP were grown in casein plus yeast medium (C + Y). For the single mutant strains, 1 μg/mL of erythromycin and for the double mutant 2 μg/mL of chloramphenicol plus 1 μg/mL of erythromycin were added to the growth medium. After growing to a defined optical density (OD .5, measured at 620 nm) in a nonshaking incubator, 1 mL of bacterial suspension was backdiluted with 4 mL of the corresponding media (THB or C + Y), incubated another 1 hour at 37°C with 5% CO2 until OD reached .5 (midlogarithmic phase) again, then 2 mL of the bacterial suspension were centrifuged at 8000 rpm for 3 minutes. Bacteria were resuspended in 1 mL sterile pyrogen-free PBS (Invitrogen Life Technologies, Karlsruhe, Germany). Serial dilutions were made to reach a final concentration of 1 × 105 cfu/mL. Meningitis was induced by injection of 10 μL of this bacterial stock solution into the cisterna magna. Bacterial suspensions were prepared freshly before each experiment and were not kept on ice.
Histopathology
Hematoxylin and eosin (HE) and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining were performed on paraffin embedded sections (5 μm). To detect typical DNA-fragmentation of apoptotic cells, TUNEL was performed by using the ApopTag Peroxidase In Situ Apoptosis Detection Kit S 7100 (Intergen, Heidelberg, Germany) according to the manufacturer's instructions.
The extent of cell death was investigated in 12 different brain regions of each animal. Degenerating cells were determined in the frontal, parietal, cingulate, retrosplenial cortex, and hippocampal dentate gyrus (upper and lower blade) of both hemispheres by means of a stereological dissector, calculating mean numerical cell densities (cells/mm3). Cerebral cortex areas were measured by a counting frame (.05 × .05 mm, dissector height .01–.07 mm). To quantify the number of cells with condensed, fragmented or shrunken nuclei in the dentate gyrus, the volume of the detected gyrus dentate area was measured by using the Stereo Investigator 5.05.4 (MicroBrightField, Williston, Vermont). Dentate gyrus of both hemispheres of each brain were evaluated. The means of all 12 slices per animal were calculated.
Neuronal Cell Culture
Primary neuronal cortical cultures were obtained from E16–E18 embryos of Wistar rats (Charles River Laboratories GmbH). Cultures were prepared as previously published [28], with modifications as described [16, 17]. Cultures were kept at 36.5° C and 5% CO2 and fed with cultivating medium (starter medium without glutamate) by replacing half of the medium twice a week. Cultures were used for experiments after 7 days in vitro.
To stimulate the neurons, we used GBS-stabilized supernatants originally prepared from the highly haemolytic WT GBS strain NCTC10/84 with an activity of 25 hemolytic units per μL (HU/μL) in different dilutions (.25 HU/μL, .1 HU/μL, .05 HU/μL) [25, 29].
To block caspase-activation we used the broad-spectrum caspase inhibitor z-VAD-fmk (100 μM) and the caspase-3 inhibitor z-DEVD-fmk (100 μM) (Enzyme Systems Products, Livermore).
Fluorometric Analysis of Caspase Activities
Following incubation with GBS hemolysin or staurosporine (positive control), neurons were harvested, centrifuged at 2000 rpm, and lysed in 50 mM HEPES, 1 mM DTT, .1 mM EDTA, .1% CHAPS, pH 7.4, for 5 minutes on ice. Following centrifugation at 15,000 rpm, supernatants (20 μL) were added to 80 μL of reaction buffer (100 mM NaCl, 50 mM HEPES, 10 mM DTT, 1 mM EDTA, 10% glycerol, .1% CHAPS, pH 7.4) containing 75 μM of a specific fluorogenic caspase substrate (Ac-DEVD-AMC) (Calbiochem). After incubation at 37° C for 60 minutes, fluorescence was measured by a microplate reader (CytoFluor II, PerSeptive Biosystems). Standard AMC (7-amino-4-methyl coumarin) was used for calculating caspase activity.
Ethidium Bromide, Acridine Orange Staining and TUNEL Assay
The fluorescent DNA-binding dyes ethidium bromide (EB) (Sigma-Aldrich) and acridine orange (AO) (Sigma-Aldrich) were used to confirm apoptosis by visualization of condensed and fragmented nuclei and to distinguish necrotic from apoptotic cells as described previously [16, 17, 30]. Cells were counted and photographs were taken using a fluorescence microscope equipped with a camera (Leica).
Cells were air-dried on slides and fixed with freshly prepared fixation solution (4% PFA in PBS; pH, 7.4). TUNEL was performed using the fluorescence in situ Cell Death Detection Kit (Roche) according to the manufacturer's instructions.
Electron Microscopy
Primary rat neurons were grown in 6-well-plates and exposed to GBS hemolysin (.1 HU/μL) or staurosporine (.5 μM/μL) for 6 hours. Thereafter, neurons were rinsed with PBS and fixed overnight at 4° C with 3% glutaraldehyde and 3% paraformaldehyde in ice-cold PBS. Neurons were postfixed in a solution of osmium tetroxide (.7% in PBS) and .2 M sucrose. Neurons were dehydrated, incubated twice in pure hydroxypropylmetacrylate (HPMA), twice in a mixture of 50% HPMA and 50% epon 812 with accelerators (Fluka), twice in 100% epon with accelerators, and finally embedded in epon. Ultrathin sections were cut with a Reichert Ultracut S ultramicrotome (Leica) with a diamond knife and mounted on AGAR grids (Plano) coated with Formvar film (Fluka). These sections were stained with a Reichert UltroStainer (Leica) using uranyl acetate and lead citrate and examined in an EM900 electron microscope (Zeiss, Germany).
Statistical Analyses
Values are presented as means ± standard deviations. Comparisons were made using Mann-Whitney rank sum test or unpaired Student’s t test. A P value ≤ .05 was considered to be statistically significant.
RESULTS
GBS and SP and Their Corresponding Toxin-Deficient Mutants Induce Meningitis in Neonatal Rats Following Cisternal Injection
WT and isogenic toxin-deficient mutant bacteria (103 cfu) were injected intracisternally in neonatal rats on postnatal day 7. All bacteria consistently induced meningitis in all neonatal rats. We observed the following significant increases of leukocytes in the CSF, COH-1 (GBS WT, n = 7) 10590 ± 7498 cells/μL, COH1:cylEΔcat (GBS mutant, n = 4) 14682 ± 9925 cells/μL and D39 (SP WT, n = 5) 6443 ± 9794 cells/μL, plnA- mutant SP (n = 4) 9896 ± 11266 cells/μL and plnA-/spxB- double mutant SP (n = 7) 2589 ± 986 cells/μL. No statistically significant differences in intrathecal leukocyte numbers were detected between WT and mutant-induced meningitis in the neonatal rats. In mock infection controls (PBS, n = 7 in GBS group, and n = 5 in SP group), no bacteria were detected in the CSF and no invasion of leukocytes (13.7 ± 19 cells/μL (GBS-group), 6.7 ± 12.5 cells/μL (SP-group) was observed. (n = 12).
Bacterial titers of rats injected with WT GBS or SP bacteria or their toxin-deficient mutants were in comparable ranges of 108–109 cfu/mL. Taken together, WT bacteria and their mutants grew intrathecally to similar bacterial titers after 18 hours of experimental meningitis and induced a similar degree of leukocyte influx (without statistically significant differences).
Bacterial Meningitis Induces Neurodegeneration in the Cortex and the Hippocampus of Neonatal Rats
Meningitis induced by either GBS or SP in neonatal rats caused a significant increase of apoptotic cells in the cortex (Figure 1A and 1C, Figure 2) and a significant increase of damaged cells in the hippocampus (Figure 1B and 1D). The distribution of the damage was similar in GBS- (Figure 1A and 1B) and SP-induced meningitis (Figure 1C and 1D). In both cases, the number of degenerating cells in the hippocampus was about tenfold higher when compared with the cortex.
Figure 1.
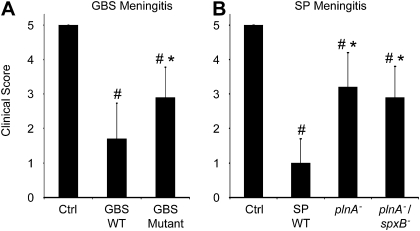
Quantification of cortical (A, C) and hippocampal (B, D) neuronal damage in neonatal rat meningitis induced by WT GBS or its isogenic mutant deficient in hemolysin/cytolysin production (COH1:cylEΔcat) (A, B) or by SP or its mutants deficient in pneumolysin production (plnA-) or in pneumolysin and hydrogen peroxide production (plnA-/spxB-) (C, D). Rats were injected into the cisterna magna 10 μL of sterile saline (control) or a bacterial suspension containing 105 cfu/mL and sacrificed 18 hours later; brains were removed and cut into 5 μm sections. Neurodegeneration was observed only in the cortex and hippocampus. It was quantified by cell shrinkage and neuronal loss in H&E-stained brain sections and by TUNEL staining. Data represent means and standard errors of the means. Cerebral cortex and dentate gyrus of both sites of twelve brain sections per animal were counted. # = P < .001 vs. control, ë = P < .05 vs. corresponding wild type bacteria, ¶ = P < .02 vs. plnA- (latter did not reach statistical significance in the hippocampus).
Figure 2.
Visualization of brain damage by applying H&E and TUNEL staining. Experimental conditions were the same as in Figure 1. GBS (first row) and SP (second row) induce apoptosis (arrows) in cortical neurons in neonatal rat meningitis. Bars = 50 μm.
Bacterial Pore-Forming Cytolysins Contribute to Neuronal Damage in Neonatal Bacterial Meningitis
The GBS mutant COH1:cylEΔcat (Figure 1A and 1B) lacking β-h/c expression as well as the SP mutant plnA- (Figure 1C and 1D) lacking pneumolysin expression induced less neuronal damage both in the cortex and the hippocampus compared with the corresponding WT parent strains. The additional loss of H2O2 in the SP double knockout plnA-/spxB- resulted in a further reduction of neuronal damage compared with pneumolysin-deficient plnA- single mutant (Figure 1C and 1D).
The spatial distribution of neuronal damage did not differ between meningitis induced by WT and cytolysin-deficient mutant bacteria. In all cases, the number of damaged cells was approximately 10 times higher in the hippocampus than in the cortex.
These data indicate that the 2 bacterial pore-forming cytolysins are essential mediators of cell death in the cortex as well as in the hippocampus.
Clinical Scores are Worse in Meningitis Induced by WT Compared with Cytolysin-Deficient Mutant Bacteria
Next, we tested whether the observed differences in neuronal cell death correlated to changes in clinical performance score [26]. Whereas control rats (PBS-challenge) had normal clinical scores (5.0 ± 0, n = 19), rats injected with WT bacteria (GBS, n = 20; SP, n = 9) had a significantly decreased clinical score (GBS 1.7 ± 1.03, P = .006; SP 1.0 ± .7, P < .001, Figure 3A and 3B). Both WT bacterial strains elicited a comparable clinical effect such that no significant difference was found between GBS and SP challenged animals. Challenge with mutants lacking the major pore-forming cytolysin (COH1:cylEΔcat n = 12, plnA- n = 10 or plnA-/spxB- n = 11) resulted in a significant improvement of the clinical score when compared with the corresponding WT bacteria (Figure 3A and 3B). Moreover, neonatal rats with meningitis induced by WT bacteria showed a significant weight loss compared with control animals at the end of the experiment (GBS 1.7 ± .65 g vs .36 ± .97 g, P < .001; SP 1.37 ± .38 g vs .06 ± .53 g, P = .001). While the GBS and SP cytolysin-deficient single mutants caused comparable weight loss to the parent strains, the absence of both SP toxins resulted in significantly less weight loss compared with rats infected with WT SP (plnA-/spxB- .36 ± .37 g vs 1.37 ± .4 g, P < .001).
Figure 3.
Clinical scores were evaluated 18 h after induction of bacterial meningitis by intracisternal injection of 10 μL of sterile saline (control) or of 10 μL of a bacterial suspension containing 105 cfu/mL either WT GBS (COH1), its isogenic β-h/c-deficient mutant (COH1:cylEΔcat) (A) or with WT SP (D39) or its isogenic pneumolysin deficient mutant (plnA-) or the pneumolysin and hydrogen peroxide deficient mutant (plnA-/spxB-) (B). Clinical scores are based on motor activity and the time of turning upright when put on their back. # = P < .001 vs. control, ë = P < .002 vs. wild type (WT), respectively.
Extent and Distribution of Neuronal Damage in Neonatal SP Meningitis is Age-Dependent
The damage previously reported in SP meningitis in adult rats is less severe than observed in the present studies [17]. Therefore, we assessed if the extent and distribution of brain damage during experimental SP meningitis are age-dependent. Histopathological changes were compared between postnatal day 7 and 11 rats after 20 hours of SP meningitis. Damage of cortical neurons was significantly more pronounced in rats on postnatal day 7 (5346 ± 814/mm3, n = 5) than in postnatal day 11 rats (999 ± 140/mm3, P < .001, n = 5). In contrast, damage of hippocampal neurons during SP meningitis did not differ significantly between rats of postnatal day 7 vs day 11 (65573 ± 8351/mm3 vs 52478 ± 28991/mm3).
GBS β-Hemolysin/Cytolysin Induces Caspase-Independent Neuronal Apoptosis
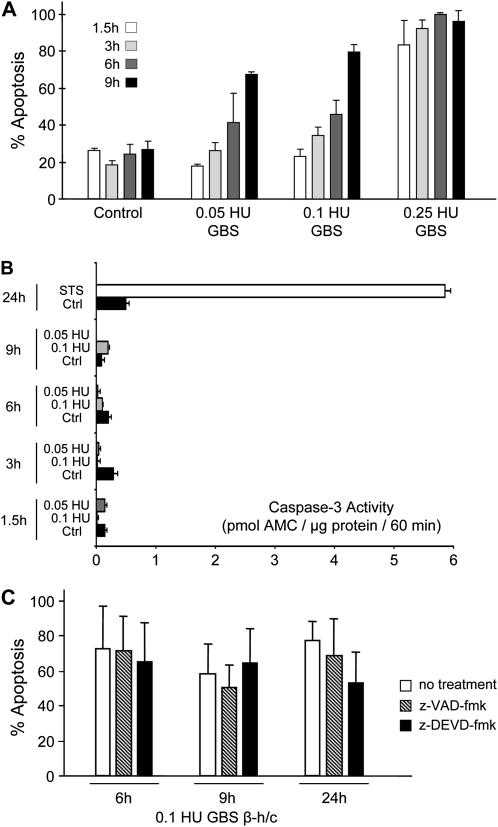
Cell-free extracts of GBS β-h/c toxin induced apoptosis in a time- and concentration-dependent fashion (Figure 4A). Electron microscopy showed condensation, shrinkage, and partial fragmentation of cells and nuclei as well as damage of mitochondria (Figure 5)
Figure 4.
(A) Primary rat cortical neurons were incubated with different concentrations of GBS β-h/c (.25, .1, and .05 hemolytic units/μL) in cell culture medium. Control neurons were untreated. Apoptosis was quantified after 1.5, 3, 6 and 9 hours applying the acridine orange/ethidium bromide assay. (B) Neurons were exposed to GBS β-h/c (.1 and .05 hemolytic units/μL) or staurosporine (STS) (.5 μM/μL) as a positive control or untreated as a negative control for various durations. Thereafter, neurons were lysed, incubated with a caspase-3 substrate and caspase-3-activity was quantified with a multiwell fluorescence reader. At no time-point did GBS β-h/c induce caspase-3 activation. (C) Neurons were incubated with either GBS β-h/c (.1 hemolytic units/μL) without or with the caspase-3-inhibitor z-DEVD-fmk (100 μM/μL) or the broad-spectrum caspase-inhibitor z-VAD-fmk (100 μM/μL). Both caspase-inhibitors failed to prevent β-h/c induced apoptosis.
Figure 5.
Electron micrographs showing nuclei (N) and mitochondria (arrows) of neurons treated with either GBS β-h/c (.1 hemolytic units/μL) or staurosporine (STS) (.5 μM/μL) as a positive control. The broad spectrum caspase-inhibitor z-VAD-fmk failed to prevent neurons from hemolysin-induced apoptosis but protected - in part - from STS-induced apoptosis. Apoptotic neurons show shrunken nuclei with marginalized and condensed chromatin and in part blebbing of the nuclear membrane as well as swollen and destroyed mitochondria (arrows). Bars = 1 μm.
Neurons were exposed to different concentrations of GBS β-h/c extracts for different durations. Using a specific assay, the positive-control staurosporine potently induced caspase-3 activation, whereas the GBS hemolysin failed to induce caspase-3 activation at all concentrations and time points tested (Figure 4B).
Next, we tested if the broad-spectrum caspase inhibitor z-VAD-fmk or the specific caspase-3 inhibitor z-DEVD-fmk protects cultured neuronal cells from GBS β-h/c-induced apoptosis. Whereas apoptosis induced by staurosporine was prevented in part (not shown) by z-VAD-fmk, apoptosis induced by the GBS β-h/c was not blocked by z-VAD-fmk nor z-DEVD-fmk (Figure 4C and Figure 5). These data indicate that, GBS β-h/c shares a caspase-independent pro-apoptotic potential with the SP cytolysin pneumolysin [17].
DISCUSSION
The present study expands the important role of bacterial toxins as a major cause of neuronal damage from adult pneumococcal meningitis to models of neonatal and group B Streptococcal meningitis. The major pore-forming cytolysin released by GBS, the leading pathogen in neonatal meningitis, and that of SP, the leading pathogen in older age groups, induced neuronal damage in neonatal meningitis. Neuronal damage correlated well with decreased clinical scores and weight loss as measures of clinical disease, and was driven by the presence of the pore-forming cytolysins in GBS and SP CNS infection. We found that the observed differences in neuronal damage and clinical deterioration could not be attributed to differences in intrathecal bacterial growth rates or leukocyte numbers when comparing WT bacterial meningitis to that produced with the cytolysin-deficient mutant bacteria. This aspect of our data is in accordance with previous studies in adult models of murine or rabbit meningitis [31, 32]. In contrast, macrophages and granulocytes play a critical role in bacterial clearance in the bloodstream and enhanced clearance of pneumolysin-deficient SP or β-h/c-deficient GBS can be appreciated in this context [23, 33]. However, following intrathecal administration, initial bacterial growth appears not to be influenced by macrophages or granulocytes, likely because these cells are virtually absent in the cerebrospinal fluid in the early stages of meningitis.
In addition to bacterial counts, the numbers of leukocytes in the CSF were comparable in meningitis induced by WT GBS or SP or the respective isogenic cytotoxin-deficient mutants. These data indicate that the observed differences in neuronal damage are substantially influenced directly by the effects of the cytolytic toxins rather than whole scale changes in inflammatory responses.
SP appears to be more potent than GBS in producing damage to cortical and hippocampal neurons. SP deficient both in pneumolysin and in H2O2 production caused a further reduction in neuronal damage compared with GBS missing only the β-h/c. However, a part of this observed difference may result from the absence [34] or much lower amounts [35] of H2O2 released by WT GBS compared with WT SP.
We found that vulnerability of cortical neurons to pore-forming cytolysin mediated damage was age-dependent, whereas damage of hippocampal neurons was similar in the 7- and 11-days-old rats, and may be a consequence of immature blood-brain barrier function or other mechanisms. Also, in other disease models, such as hypoxia and trauma, vulnerability of neurons is most pronounced in the immature rat brain within the first week of life [14, 36]. The first week of rodent brain development corresponds to the phase of brain growth spurt in humans during the last trimester of pregnancy. During that period, neurons are highly susceptible to apoptotic injury. Several mechanisms have been suggested for this observation, such as interference in the action of neurotransmitters during synaptogenesis period [37] or upregulation of p53.
Cortical and hippocampal damage has been reported previously [18] in GBS meningitis in 11-day-old Sprague Dawley rats and was ameliorated by antioxidant treatment. Differences in the degree and character of cortical damage may arise from different rat strains, age of the animals, inoculums size, and the duration of meningitis or other experimental procedures.
The GBS β-h/c had direct toxic effects on primary neurons. These data support the concept that pore-forming cytolysins, like SP pneumolysin, can be considered a major neurotoxin. GBS β-h/c-induced cell death could not be prevented by caspase inhibitors and no caspase activity was detected in neurons. These findings support previous observations in macrophages [38, 39].
Several bacteria [40–43] and bacterial toxins [44, 45] are known to induce caspase-independent apoptosis. Key mechanisms of bacterial- or bacterial toxin-induced caspase-independent apoptosis include mitochondrial damage and release of mitochondrial factors (eg, apoptosis-inducing factor or endonuclease G) [45, 46], upregulation of X-chromosome-linked inhibitor of apoptosis protein (XIAP) [45], calpain activation [46], or bacterial inhibition of phosphatidylcholine synthesis [47].
Antibodies against bacterial toxins prevent brain damage (eg, anti-suilysin antibodies) [48]. Anti-pneumolysin antibodies prevent damage of ependymal cells in experimental meningitis [49]. It remains to be shown if antibodies against pneumolysin are neuroprotective in this model of neonatal meningitis.
We conclude that the major and clinically important pathogens GBS and SP, and in particular their major pore-forming cytolysins are highly neurotoxic and contribute to neuronal damage in adult and neonatal meningitis.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 507) and the Transregio (SFB 43).
References
- 1.de Louvois J, Blackbourn J, Hurley R, Harvey D. Infantile meningitis in England Wales: A two year study. Arch Dis Child. 1991;66:603–7. doi: 10.1136/adc.66.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Synnott MB, Morse DL, Hall SM. Neonatal meningitis in England and Wales: A review of routine national data. Arch Dis Child. 1994;71:F75–F80. doi: 10.1136/fn.71.2.f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fluegge K, Supper S, Siedler A, Berner R. Serotype distribution of invasive group B streptococcal isolates in infants: Results from a nationwide active laboratory surveillance study over 2 years in Germany. Clin Infect Dis. 2005;40:760–3. doi: 10.1086/427942. [DOI] [PubMed] [Google Scholar]
- 4.Polin RA, Harris MC. Neonatal bacterial meningitis. Semin Neonatol. 2001;6:157–72. doi: 10.1053/siny.2001.0045. [DOI] [PubMed] [Google Scholar]
- 5.Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008;7:637–48. doi: 10.1016/S1474-4422(08)70139-X. [DOI] [PubMed] [Google Scholar]
- 6.de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: Sequelae at 5 years of age. Eur J Pediatr. 2005;164:730–734. doi: 10.1007/s00431-005-1747-3. [DOI] [PubMed] [Google Scholar]
- 7.Fluegge K, Siedler A, Heinrich B, et al. Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics. 2006;117:1139–45. doi: 10.1542/peds.2005-2481. [DOI] [PubMed] [Google Scholar]
- 8.Berardi A, Tzialla C, Riva M, Cerbo RM, Creti R. Group B streptococcus: Early- and late-onset infections. J Chemother. 2007;19:24–7. doi: 10.1080/1120009x.2007.11782439. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre PB, Macintyre CR, Gilmour R, Wang H. A population based study of the impact of corticosteroid therapy and delayed diagnosis on the outcome of childhood pneumococcal meningitis. Arch Dis Child. 2005;90:391–6. doi: 10.1136/adc.2003.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2007;24:CD004405. doi: 10.1002/14651858.CD004405.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Daoud AS, Batieha A, Al-Sheyyab M, Abuekteish F, Obeidat A, Mahafza T. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. Eur J Pediatr. 1999;158:230–3. doi: 10.1007/s004310051056. [DOI] [PubMed] [Google Scholar]
- 12.Coimbra RS, Loquet G, Leib SL. Limited efficacy of adjuvant therapy with dexamethasone in preventing hearing loss due to experimental pneumococcal meningitis in the infant rat. Pediatr Res. 2007;62:291–4. doi: 10.1203/PDR.0b013e318123fb7c. [DOI] [PubMed] [Google Scholar]
- 13.Bittigau P, Sifringer M, Pohl D, et al. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–35. doi: 10.1002/1531-8249(199906)45:6<724::aid-ana6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Felderhoff-Mueser U, Sifringer M, Polley O, et al. Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol. 2005;57:50–9. doi: 10.1002/ana.20322. [DOI] [PubMed] [Google Scholar]
- 15.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann O, Priller J, Prozorovski T, et al. TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest. 2007;117:2004–13. doi: 10.1172/JCI30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun JS, Sublett JE, Freyer D, et al. Pneumococcal pneumolysin und H2O2 mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leib SL, Kim YS, Chow LL, Sheldon RA, Tauber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98:2632–9. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchlewicz BA, Duncan JL. Lysis of erythrocytes by a hemolysin produced by a group B streptococcus sp. Infect Immun. 1981;34:787–94. doi: 10.1128/iai.34.3.787-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizet V, Gibson RL, Chi EY, Framson PE, Hulse M, Rubens CE. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–26. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nizet V, Kim KS, Stins M, et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–81. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu GY, Doran KS, Lawrence T, et al. Sword and shield: Linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A. 2004;101:14491–6. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puliti M, Nizet V, von Hunolstein C, et al. Severity of group B streptococcal arthritis is correlated with beta-hemolysin expression. J Infect Dis. 2000;182:824–32. doi: 10.1086/315773. [DOI] [PubMed] [Google Scholar]
- 24.Hensler ME, Liu GY, Sobczak S, Benirschke K, Nizet V, Heldt GP. Virulence role of group B streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J Infect Dis. 2005;191:1287–91. doi: 10.1086/428946. [DOI] [PubMed] [Google Scholar]
- 25.Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–44. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeffler JM, Ringer R, Hablutzel M, Tauber MG, Leib SL. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis und learning deficits in experimental pneumococcal meningitis. J Infect Dis. 2001;183:247–52. doi: 10.1086/317921. [DOI] [PubMed] [Google Scholar]
- 27.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001;39:236–47. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 28.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum and dentate gyrus. J Neurosci Res. 1995;42:674–83. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 29.Nizet V, Gibson RL, Chi EY, Framson PE, Hulse M, Rubens CE. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–26. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714–9. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedland IR, Paris MM, Hickey S, et al. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J Infect Dis. 1995;172:805–9. doi: 10.1093/infdis/172.3.805. [DOI] [PubMed] [Google Scholar]
- 32.Wellmer A, Zysk G, Gerber J, et al. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect Immun. 2002;70:6504–8. doi: 10.1128/IAI.70.11.6504-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benton KA, Everson MP, Briles DE. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–55. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolm M, Jansen WT, Schnabel R, Chhatwal GS. Hydrogen peroxide-mediated killing of Caenorhabditis elegans: A common feature of different streptococcal species. Infect Immun. 2004;72:1192–4. doi: 10.1128/IAI.72.2.1192-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pericone CD, Overweg K, Hermans PW, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000;68:3990–7. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dzietko M, Felderhoff-Mueser U, Sifringer M, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–87. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Olney JW, Wozniak DF, Farber NB, Jevtovic-Todorovic V, Bittigau P, Ikonomidou C. The enigma of fetal alcohol neurotoxicity. Ann Med. 2002;34:109–19. doi: 10.1080/07853890252953509. [DOI] [PubMed] [Google Scholar]
- 38.Fettucciari K, Rosati E, Scaringi L, et al. Group B streptococcus induces apoptosis in macrophages. J Immunol. 2000;165:3923–3933. doi: 10.4049/jimmunol.165.7.3923. [DOI] [PubMed] [Google Scholar]
- 39.Liu GY, Nizet V. Extracellular virulence factors of group B streptococci. Front Biosci. 2004;9:1794–802. doi: 10.2741/1296. [DOI] [PubMed] [Google Scholar]
- 40.Braun JS, Novak R, Murray PM, et al. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J Infect Dis. 2001;184:1300–9. doi: 10.1086/324013. [DOI] [PubMed] [Google Scholar]
- 41.Bermpohl D, Halle A, Freyer D, et al. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest. 2005;115:1607–15. doi: 10.1172/JCI23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haslinger-Löffler B, Wagner B, Brück M, et al. Staphylococcus aureus induces caspase-independent cell death in human peritoneal mesothelial cells. Kidney Int. 2006;70:1089–98. doi: 10.1038/sj.ki.5001710. [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan MP, O'Leary S, Kelly DM, Keane J. A caspase-independent pathway mediates macrophage cell death in response to Mycobacterium tuberculosis infection. Infect Immun. 2007;75:1984–93. doi: 10.1128/IAI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol. 2003;171:2354–65. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 45.Braun JS, Hoffmann O, Schickhaus M, et al. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun. 2007;75:4245–54. doi: 10.1128/IAI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fettucciari K, Fetriconi I, Mannucci R, et al. Group B streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–56. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- 47.Zweigner J, Jackowski S, Smith SH, van der Merwe M, Weber JR, Tuomanen EI. Bacterial inhibition of phosphatidylcholine synthesis triggers apoptosis in the brain. J Exp Med. 2004;200:99–106. doi: 10.1084/jem.20032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charland N, Nizet V, Rubens CE, Kim KS, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–43. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirst RA, Mohammed BJ, Mitchell TJ, Andrew PW, O'Callaghan C. Streptococcus pneumoniae-induced inhibition of rat ependymal cilia is attenuated by antipneumolysin antibody. Infect Immun. 2004;72:6694–8. doi: 10.1128/IAI.72.11.6694-6698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]