Abstract
Background. Maternal human immunodeficiency virus (HIV) RNA load, CD4 cell count, breast-feeding, antiretroviral use, and malaria are well-established factors associated with mother-to-child transmission (MTCT) of HIV; the role of maternal tuberculosis (TB), however, has not been well established.
Methods. The study population was 783 HIV-infected Indian mother-infant pair participants in randomized and ancillary HIV-infected cohorts of the Six Week Extended-Dose Nevirapine (SWEN) Study, a study comparing extended nevirapine versus single-dose nevirapine, to reduce MTCT of HIV among breast-fed infants. Using multivariable logistic regression, we assessed the impact of maternal TB occurring during pregnancy and through 12 months after delivery on risk of MTCT.
Results. Of 783 mothers, 3 had prevalent TB and 30 had incident TB at 12 months after delivery. Of 33 mothers with TB, 10 (30%) transmitted HIV to their infants in comparison with 87 of 750 mothers without TB (12%; odds ratio [OR], 3.31; 95% confidence interval [CI], 1.53–7.29; P = .02). In multivariable analysis, maternal TB was associated with 2.51-fold (95% CI, 1.05–6.02; P = .04) increased odds of HIV transmission adjusting for maternal factors (viral load, CD4 cell count, and antiretroviral therapy) and infant factors (breast-feeding duration, infant nevirapine administration, gestational age, and birth weight) associated with MTCT of HIV.
Conclusions. Maternal TB is associated with increased MTCT of HIV. Prevention of TB among HIV-infected mothers should be a high priority for communities with significant HIV/TB burden.
An estimated 2 billion people are latently infected with Mycobacterium tuberculosis, of whom more than 9 million develop active disease every year, with women accounting for 3.6 million cases [1]. Tuberculosis (TB) is the leading infectious cause of death in women worldwide and is the most important cause of morbidity and mortality in human immunodeficiency virus (HIV)–infected women residing in low-income settings such as sub-Saharan Africa and India. Women now account for 50% of adults infected with HIV globally and up to 70% of HIV-infected adults in sub-Saharan Africa [2]. The greatest burden of HIV infection and TB among women occurs during childbearing years (ie, ages 15–49 years). When TB occurs during the pregnancy or postpartum period, it is associated with adverse maternal outcomes and significant infant morbidity, TB, and death [3–5].
Maternal HIV RNA load, CD4 cell count, duration of breast-feeding, type of antiretroviral therapy (ART), and malaria coinfection are well-established independent maternal factors associated with increased mother-to-child transmission (MTCT) of HIV [6]. Given the high risk of maternal TB and its impact on the survival of both HIV-infected mothers and their infants, it is important to also determine whether maternal TB increases the risk of MTCT of HIV. Thus, the aim of our study was to assess whether maternal TB is associated with an increased risk of MTCT of HIV and if an association was found, to determine whether it is independent of other known MTCT risk factors. Identifying maternal TB as a risk factor for MTCT would suggest a new important and modifiable factor to be addressed by prevention of MTCT programs in communities at high risk for HIV and TB coinfection.
METHODS
Study Population
Our study population included 742 HIV-infected mother-infant pairs enrolled in the India Six Week Extended-Dose Nevirapine (SWEN) Study [7], a National Institutes of Health (NIH)–funded phase 3 randomized trial for prevention of MTCT of HIV, which is assessing the efficacy of extended nevirapine prophylaxis given to breast-fed infants for the first 6 weeks of life. We included an additional 41 mother-infant pairs who were enrolled in an ancillary HIV-infected cohort; these additional participants were either ineligible for or did not want to enroll in the SWEN trial but were willing to be followed up with the same procedures and at the same time points as the SWEN trial participants. The primary outcome of the SWEN trial and ancillary cohort was to assess HIV transmission at 6 months of life, and key secondary objectives were to assess HIV transmission at 12 months as well as maternal and infant morbidity, including that caused by TB.
The methods of the SWEN trial and eligibility criteria are described in detail elsewhere [7]. The participants were enrolled and followed up between August 2002 and September 2007. The study site was a large, urban, public teaching hospital in Pune, Maharashtra, India (Sassoon Hospital, affiliated with Byramji Jeejeebhoy Medical College [BJMC]), which serves a primarily peri-urban population. The study was approved by the institutional review boards of both Johns Hopkins Medicine and Pune and the Byramji Jeejeebhoy Medical College Ethics Committee.
HIV-infected women were enrolled during their third trimesters, at delivery, or within 1 week postpartum. Mother-infant pairs were followed up prospectively until 12 months postpartum. Women who were enrolled antepartum were seen every 4 weeks for scheduled visits until week 36, then every 2 weeks until delivery. After delivery, at each scheduled visit (at weeks 1, 2, 3, 4, 5, 6, 10, and 14, and at months 6, 9, and 12), women and their infants underwent TB-symptom screening, clinical examination, and select laboratory investigations. Infants underwent HIV-1 DNA polymerase chain reaction (PCR) testing within 48 h of birth, and at all scheduled visits except at weeks 3 and 5; positive HIV-infection status was confirmed by quantitative HIV-1 RNA load (viral load of >5000 copies/mL). HIV-infection status was externally quality-assured as described elsewhere [7].
Ascertainment of Tuberculosis and Case Definitions
A woman was classified as having prevalent TB if she entered the study with a prior diagnosis of TB and was undergoing treatment for TB. Incident TB was defined as a new diagnosis of maternal TB during study follow-up. At each study visit, women were screened for active TB using symptom assessment (reported cough, fever, weight loss, and night sweats) and physical examination. Sputum and culture for acid-fast bacilli (AFB) were performed if TB was suspected either by symptom screen or by physician assessment. We performed TB culture using the manual Lowenstein-Jensen (LJ) method. We used World Health Organization (WHO) definitions of confirmed, probable, or suspected TB [8]. TB was confirmed when M. tuberculosis was cultured from the mother, and was probable when (1) AFB were detected on maternal sputum smear microscopy, or (2) histological features suggested TB. TB was suspected when the mother had only clinical and radiological evidence suggesting TB and had a response to anti-TB therapy.
Statistical Analysis
We analyzed data using STATA statistical software (version 9.0). The t test for means was used when continuous variables were normally distributed. We used a nonparametric Mann-Whitney U test when continuous variables were not normally distributed, χ2 tests for discrete variables, and Fisher exact tests when cell size was ≤5 observations. We used univariable and multivariable logistic regression with HIV transmission by 12 months as the outcome variable. We categorized continuous variables into clinically meaningful groups (ie, CD4 cell count of <350, 350–500, and >500 cells/μL). Mixed feeding was defined as ingestion of any solid food or liquid other than breast milk combined with breast milk. We assessed maternal TB (prevalent or incident) as the explanatory variable, adjusting for the following factors associated with HIV transmission: maternal factors (older age, lower educational status, mode of delivery, lower CD4 cell count, higher HIV-1 RNA load, use of antepartum zidovudine or single-dose nevirapine, and maternal highly active antiretroviral therapy [HAART]) and infant factors (premature birth, infant sex, low birth weight, duration of breast-feeding, and extended use of nevirapine). We assessed colinearity by calculating the variance inflation factors, which were all <2.5. We used multivariate logistic regression to analyze variables that were significantly associated in the univariate analyses. We explored interactions between age and other covariates. We assessed model fit by the likelihood ratio test to determine the best model for the relationship between any maternal TB and MTCT of HIV.
RESULTS
A total of 783 women who gave birth to live-born infants were included in the study; the median age of the mothers was 23 years (interquartile range [IQR], 21–25 years) and median parity was 1 (IQR, 0–2) (Table 1). Of the 783 mothers, 157 (20%) underwent cesarean delivery. The median CD4 cell count and HIV RNA load closest to delivery were 472 cells/μL (IQR, 316–667 cells/μL) and 6294 copies/mL (IQR, 898—34,200 copies/mL), respectively. For maternal ART, 271 of 783 mothers (35%) received antepartum zidovudine, 510 (65%) received single-dose nevirapine, and 54 (7%) started HAART during study follow-up. Notable infant characteristics included 86 of 783 infants (11%) born prematurely, 301 (38%) with low birth weight (<2500 g), 323 (42%) breast-fed beyond 4 months of age, and 367 (47%) having received extended nevirapine up to 6 weeks of age.
Table 1.
Characteristics, unadjusted and adjusted odds ration of risk factors associated with mother-to-child HIV transmission by 12 month of life in a cohort of HIV-infected women, Pune India 2002-2007
| Total N (%) | HIV transmitted N = 97 | No HIV transmitted N = 686 | Unadjusted Odds Ration (95% CI) | Adjusted Odds ration (95% CI) | |
| MATERNAL CHARACTERISTICS | |||||
| Median Age, years (IQR) | 23 (21–25) | 24 (22–62) | 23 (21–25) | 1.05 (0.98, 1.11) | |
| Mode of delivery, n (%) | |||||
| C-section | 157 (20) | 18 (19) | 139 (20) | Ref | |
| Vaginal delivery | 626 (80) | 79 (81) | 547 (80) | 0.69 (0.65, 1.92) | |
| Median CD4 cell/μL (IQR) | 472 (316–667) | 356 (206–540) | 485 (333–682) | ||
| > 500 | 350 (45) | 29 (30) | 321 (47) | Ref | Ref |
| 350-500 | 179 (23) | 19 (20) | 160 (23) | 1.31(0.71, 2.42) | 1.18 (0.63, 2.22) |
| < 350 | 250 (32) | 2.70 (1.65, 4.21)*** | 2.20 (1.19, 3.48) | ||
| Median HIV RNA copies/ml (IQR) | 6294 (898–34200) | 48187 (11500–126101) | 4480 (687–24258) | ||
| ≤ 3 log10 HIV RNA | 207 (26) | 7 (7) | 200 (29) | Ref | Ref |
| 3−5 log10 HIV RNA | 491 (63) | 62 (64) | 429 (63) | 4.13 (1.86, 9.18) | 3.67(1.61, 8.32)** |
| > 5 log10 HIV RNA | 82 (11) | 14.8(6.14, 35.70) | 10.8 (4.25, 27.70)** | ||
| Antepartum zidovudine | |||||
| Yes | 510 (65) | 56 (58) | 454 (66) | Ref | Ref |
| No | 271 (35) | 41 (42) | 230 (34) | 1.45 (0.94, 2.23) | 1.25(0.76, 2.05) |
| Single-does nevirapine | |||||
| Yes | 510 (65) | 56 (58) | 454 (66) | Ref | Ref |
| No | 271 (35) | 41 (42) | 230 (34) | 1.45 (0.94, 2.23) | 1.25 (0.76, 2.70) |
| Maternal HAART use | |||||
| Yes | 54 (7) | 5 (5) | 49 (7) | Ref | Ref |
| No | 727 (9.) | 92 (95) | 635 (93) | 1.42 (0.55, 3.66) | 1.40 (0.50, 3.87) |
| Maternal TB (Prevalent or incident) | |||||
| No | 750 (96) | 87 (90) | 663 (97) | Ref | Ref |
| Yes | 33 (4) | 10 (10) | 23 (3) | 3.31 (1.53, 6.20)* | 2.51 (1.05, 6.02)* |
| INFANT CHARACTERISTICS | |||||
| Premature birth | |||||
| No | 697 (89) | 81 (84) | 616 (90) | Ref | |
| Yes | 86 (11) | 16 (16) | 70 (10) | 1.74 (0.96, 3.14) | |
| Infant sex | |||||
| Male | 413 (53) | 48 (49) | 365 (63) | Ref | |
| Female | 369 (47) | 49 (51) | 320 (47) | 1.16 (0.76, 1.78) | |
| Birth weight, gms | |||||
| >2500 | 430 (62) | 51 (53) | 430 (63) | Ref | |
| <2500 | 301 (38) | 46 (47) | 255 (37) | 1.52 (0.99, 2.330 | |
| Breastfeeding | |||||
| < 4 month | 438 (58) | 46 (47) | 392 (59) | Ref | Ref |
| >4 month | 323 (42) | 51 (53) | 272 (41) | 1.60 (1.04, 2.45)* | 1.72 (1.70, 2.65) |
| Extended nevirapine | |||||
| Yes | 367 (47) | 41 (42) | 326 (48) | Ref | Ref |
| No | 433 (53) | 56 (58) | 357 (52) | 1.23 (0.81, 1.92) | 1.24 (0.79, 1.97) |
Table Legend: Characteristics that were assessed at time of delivery were CD4 cell count and HIV-1 RNA Logistic regression was used to calculate unadjusted and adjusted odd ration. Maternal religion, parity, hemoglobin at delivery, education status and mode of delivery were also assessed but were not fount to be significantly associated (data not shown). *p ≤ 0.05, **p<0.01,***p<0.001
HAART = highly active antiretroviral therapy
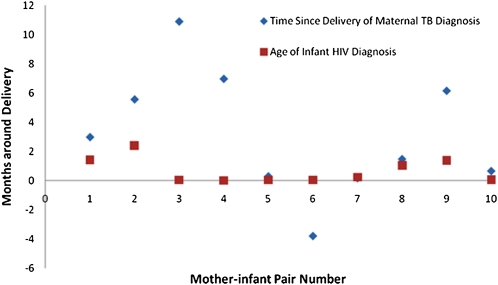
Of the 783 mothers, 97 (12%) had transmitted HIV to their infants by 12 months of life; of these transmissions, 29 (30%) occurred in utero (ie, infant HIV DNA PCR was positive within 48 h of birth). There were 33 maternal TB cases in total; 3 were prevalent and 30 were incident cases at 12 months postpartum, of which 79% were diagnosed within 6 months postpartum. Thirteen (39.4%) were culture-confirmed TB, 9 (27.3%) were probable (ie, AFB test was positive), and 11 (33.3%) were suspected (ie, cases where there was clinical and radiological evidence and TB treatment was given, but AFB test or culture was not positive or was not performed). The temporal distribution of maternal TB cases in relation to infant HIV transmission is shown in Figure 1. In univariate analysis, MTCT of HIV was associated with high maternal viral load, low CD4 cell count, maternal TB disease, and longer duration of breast-feeding and was marginally associated with infant low birth weight (Table). In this study, 10 of 33 mothers with TB (30%) transmitted HIV to their infants compared with 87 of 750 mothers without TB (12%; odds ratio [OR], 3.31; 95% confidence interval [CI], 1.53–7.19; P < .01). (An 11th mother-infant pair was excluded from analysis because the mother had prevalent TB [diagnosed in pregnancy], but her infant was stillborn and HIV infection status was not ascertained.) We found no clear relationship between the type of maternal TB diagnosis (ie, culture-confirmed, probable, or suspected TB) and the proportion of HIV transmissions (data not shown). Because mixed feeding can increase the risk of MTCT of HIV, we also specifically compared mixed-feeding practices of mothers with TB who transmitted HIV with those of mothers who transmitted HIV but did not have TB. Of 10 mothers with TB who transmitted HIV, 0 (0%) practiced mixed feeding prior to MTCT of HIV compared with 25 of 87 mothers without TB who transmitted HIV (29%). In a multivariate analysis that included all cases of maternal TB (prevalent and incident), maternal TB was associated with 2.51-fold (95% CI, 1.05–6.02; P = .04) increased odds ratio of MTCT of HIV adjusting for maternal factors (eg, CD4 cell count, viral load, and antiretroviral use) as well as infant factors (eg, nevirapine administration and breast-feeding duration). When we restricted analysis to only maternal-incident cases, we found similar results (adjusted OR, 2.55; 95% CI, 1.01–6.45; P = .049). In 5 mother-infant pairs, the maternal TB diagnosis arrived close to the infant HIV diagnosis (median, 13 d; range, 2–48 d), and in a sixth mother-infant pair, the mother received a diagnosis with TB 116 d prior to delivery and her infant was HIV-infected at birth. We reviewed the clinical descriptions and symptom duration for the 5 cases in which MTCT of HIV occurred prior to maternal TB diagnosis. For cases 1, 2, and 9, there was a delayed diagnosis of TB. For case 1, the mother, a 19-year-old woman with a CD4 cell count of 330 cells/μL and an HIV RNA load of 48,676 copies/mL, had been diagnosed with a right upper lobe consolidation within a week of delivery. She was given antimicrobials and referred for sputum for AFB, but she did not follow through. She had persistence of cough and pulmonary infiltrate, and was started on antitubercular therapy 2 months later by her outside provider. Her infant was found to be HIV-infected at day 43 of life. In case 2, a 25-year-old woman with a CD4 cell count of 342 cells/μLand an HIV RNA load of 248,722 copies/mL had malaise for >2 months. She was noted to have chest pain and cervical adenopathy, and radiologic imaging showed bilateral chest infiltrates and hepatosplenomegaly with splenic microabscesses 2 months later, at which time treatment for disseminated TB began. The infant was found to be HIV-infected at day 73 of life, whereas the mother was diagnosed with TB 3 months after her infant’s HIV infection diagnosis. For case 9, the mother, a 30-year-old woman with a CD4 cell count of 390 cells/μL and an HIV RNA load of 30,900 copies/mL, presented with severe disseminated TB 4 months after her infant was found to be infected with HIV. She died within 10 d of diagnosis, and her autopsy showed disseminated TB. The duration of her symptoms was unknown. For cases 3 and 4, the duration of symptoms consistent with TB was recorded as ≤3 months.
Figure 1.
Temporal relationship between diagnosis of maternal tuberculosis (TB) and infant human immunodeficiency virus (HIV) infection among 10 mother-infant pairs, Pune, India.
DISCUSSION
We identified maternal TB clinical disease as an important independent risk factor associated with MTCT of HIV. Maternal TB was associated with 2.5-fold increased odds of MTCT of HIV, adjusting for maternal factors (eg, CD4 cell count, viral load, and antiretroviral use) as well as infant factors (eg, nevirapine administration and breast-feeding duration). Although maternal TB was associated with a relatively small fraction of HIV transmissions (ie, small attributable risk), maternal TB is potentially preventable with improved infection control, improved community-based intensive case-finding, and implementation of isoniazid prevention therapy—the “3 I's” TB-control strategy recommended by WHO—as well as by scale-up of HAART [9].
Data assessing TB as a risk factor for MTCT of HIV are very limited. Whereas maternal coinfections such as malaria and some sexually transmitted infections are established risk factors for MTCT of HIV, TB has not been adequately assessed or identified as an independent risk factor to date [4, 6]. A study of 42 HIV-infected women with TB during pregnancy found a high proportion (19%) of these women transmitted HIV in utero (infant HIV PCR positive within 3 d of life) [10]. This study, however, was small and did not adjust for several factors associated with MTCT of HIV, including maternal HIV RNA load. Our study of women who breast-fed their infants and whose TB was predominantly diagnosed <6 months postpartum provides for the first evidence that maternal TB is independently associated with an increased risk of MTCT to infants.
Why might maternal TB be associated with MTCT of HIV in a breast-feeding population? There are at least 3 possible explanations. First, maternal TB may increase maternal HIV infectiousness. Increased maternal infectiousness could be mediated by HIV-1 RNA load, which has been shown to increase in the setting of TB both in vitro and in vivo in most TB cases [11–14]. Although our analysis controlled for maternal plasma viral load at the time of delivery, maternal TB could be associated with a transient increase in viral load that was not measured in our study. In addition, compartmentalization of increased viral load in breast milk or cell-associated virus, in the setting of maternal TB, was not measured in our study. Another potential pathway of increased maternal infectiousness may be mediated by immune activation and inflammation due to maternal TB, which has been suggested as a mechanism to explain the association of sexually transmitted infections and placental malaria with an increased risk for HIV MTCT [15, 16]. Multiple studies demonstrate that TB is associated with increased immune activation and HIV replication [11–13, 17]. Immune activation has been associated with HIV disease progression [18] and may be associated with increased HIV transmission, even independent of the HIV RNA load pathway. Furthermore, it is plausible that maternal immune activation may allow more HIV virus to enter into the breast milk compartment, thereby making the mother more infectious. Another possibility is that maternal TB may increase the infant’s risk of HIV acquisition. This increased infant “susceptibility” may be mediated by maternal TB-induced immune activation, which may lead to a process whereby the infant's CD4-expressing immune cells become more susceptible to infection because of maternal immune activation factors (eg, cytokines or activated T cells) [16]. A third possibility is that maternal TB is associated with an MTCT risk factor that our analysis did not sufficiently control for or measure. We did not measure placental malaria, which has been associated with MTCT of HIV, but this condition is of very low prevalence in our setting (prevalence of malaria, <.7%; data not shown). We also did not adjust for maternal sexually transmitted infections, but we found a low prevalence of syndromic genital ulcer disease or syphilis (<6%; unpublished data). Women with TB could be more likely to mix-feed their infants because of their TB illness and therefore could be more likely to transmit, but we assessed this risk and did not identify this in our cohort. We did not measure factors that are uncommonly evaluated but have been shown in limited studies to be associated with MTCT, such as maternal-infant HLA status or presence of CCR5Δ32 mutation or maternal vitamin-D status [19]. However, we adjusted for important known maternal and infant factors associated with MTCT of HIV.
Our study has some potential limitations. First, this analysis was a secondary endpoint of the clinical trial combined with an observational cohort, which was not originally designed or powered to address our hypothesis that maternal TB was a risk factor for MTCT of HIV. In addition, not all TB disease diagnoses were culture-confirmed, so some misclassification bias is possible. Reverse causality is also possible, because some of the HIV transmission events preceded the maternal TB diagnosis. However, for 3 of the 5 cases in which this occurred, we observed a delay in diagnosing the maternal TB; therefore, the mothers likely had TB disease at the time they transmitted HIV to her their infants. Delays in TB diagnoses are a well-recognized problem and an important contributor to morbidity and mortality in HIV-infected persons [20]. Furthermore, several of the maternal TB cases were discovered near to the infant HIV diagnoses. Therefore, despite these acknowledged limitations, we feel that our observation of maternal TB as an independent risk factor is likely to be a true and important association.
TB in HIV-infected mothers has been previously shown to be associated with higher maternal and infant mortality [3, 4]. In this study, we have shown that maternal TB is also independently associated with an increased risk of HIV transmission to exposed infants. Prevention of maternal TB among HIV-infected mothers should be a high priority for programs to prevent MTCT of HIV in communities with significant HIV-TB coinfection rates.
Funding
This work was supported by the US National Institutes of Health (NIH), US National Institute of Allergy and Infectious Diseases (R01AI45462); the Fogarty International Center NIH Program of International Training Grants in Epidemiology Related to AIDS (D43-TW000010-22); and the NIH Byramji Jeejeebhoy Medical College HIV Clinical Trials Unit (U01AI069497 to A.G., N.G., V.K., J.S., V.M., S.P., and R.C.B.).
Acknowledgments
We thank the study participants and study staff for their immense contributions.
References
- 1.World Health Organization. Global tuberculosis control: A short update to the 2009. Available at http://www.who.int/tb/publications/global_report/2009/update/en/index.html. Accessed 5 September 2010. [Google Scholar]
- 2.United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS epidemic update: November 2009. Available at http://data.unaids.org/pub/Report/2009/JC1700_Epi_update_2009_en.pdf. Accessed 5 September 2010. [Google Scholar]
- 3.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–9. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 4.Pillay T, Khan M, Moodley J, Adhikari M, Coovadia H. Perinatal tuberculosis and HIV-1: Considerations for resource-limited settings. Lancet Infect Dis. 2004;4:155–65. doi: 10.1016/S1473-3099(04)00939-9. [DOI] [PubMed] [Google Scholar]
- 5.Adhikari M. Tuberculosis and tuberculosis/HIV co-infection in pregnancy. Semin Fetal Neonatal Med. 2009;14:234–40. doi: 10.1016/j.siny.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Paintsil E, Andiman WA. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr. 2009;21:94–101. doi: 10.1097/MOP.0b013e32831ec353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedri A, Gudetta B, Isehak A, et al. for the Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: An analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. TB/HIV: A clinical manual. 2nd ed. Geneva, Switzerland: World Health Organization;; 2004. [Google Scholar]
- 9.World Health Organization. WHO three I's meeting: Intensified case finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for people living with HIV. Available at http://www.who.int/tb/publications/2009/who_3is_meeting_report.pdf. Accessed 5 September 2010. [Google Scholar]
- 10.Pillay T, Adhikari M, Coovadia HM, Moodley J, Khan M, Sullivan JL. In utero HIV infection in pregnancies complicated by tuberculosis in Durban, South Africa. Arch Dis Child Fetal Neonatal Ed. 2004;89:F468–9. doi: 10.1136/adc.2003.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day JH, Grant AD, Fielding KL, et al. Does tuberculosis increase HIV load? J Infect Dis. 2004;190:1677–84. doi: 10.1086/424851. [DOI] [PubMed] [Google Scholar]
- 12.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication: Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 13.Zhang Y, Nakata K, Weiden M, Rom WN. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;95:2324–31. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toossi Z, Mayanja-Kizza H, Hirsch CS, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–8. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayisi JG, van Eijk AM, Newman RD, et al. Maternal malaria and perinatal HIV transmission, western Kenya. Emerg Infect Dis. 2004;10:643–52. doi: 10.3201/eid1004.030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001;14:753–77. doi: 10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederman MM, Georges DL, Kusner DJ, Mudido P, Giam CZ, Toossi Z. Mycobacterium tuberculosis and its purified protein derivative activate expression of the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994;7:727–33. [PubMed] [Google Scholar]
- 18.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and the treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]