A dramatic intersection between the infectious diseases and oncology fields has emerged from the appreciation that many cancers arise in the setting of chronic infection. For certain viral-induced cancers, such as human papilloma virus (HPV)–associated cervical cancer, the integrated virus encodes oncogenes that directly drive the transformation of infected cells. However, the vast majority of infection-associated cancers arise from chronic immune responses that, although ineffective in eliminating the inciting microbe, collaborate with microbial products to drive carcinogenesis. As many as one-third of cancers worldwide, particularly epithelial cancers, are associated with identified single microbial infections, leading to the conceptual paradigm that chronic infection with specific microbes causes these cancers, independent of other components of the ambient microbial community. In addition to cervical cancer and HPV, other well-established examples include gastric cancer and Helicobacter pylori, liver cancer and hepatitis B and C viruses, urinary bladder cancer and Schistosoma hematobium, and biliary tree cancer, Clonorchis sinensis, and Opisthorchis viverrini.
A contrasting view of microbial carcinogenesis has been applied to colon cancer, the second leading cause of death due to cancer among adults in the United States. On the basis of increasingly high through put methods, we now understand that the colon is home to one of the most dense and diverse communities of bacteria in the body and that, although clustering of the colon microbiome sequences may occur in families or under similar close-contact conditions, we are all remarkably unique in our bacterial make up [1–2]. Indeed, beginning in the 1960s, investigators postulated and pursued links between the complex colon commensal flora and colon cancer [3].
Our recent work demonstrating the capacity of a specific human colonic bacterium, enterotoxigenic Bacteroides fragilis (ETBF), to induce colon tumors in multiple intestinal neoplasia (Min) mice [4] leads us to propose the “Alpha-bug hypothesis,” which integrates the single microbe and microbiome community views of microbial carcinogenesis. While we focus on colon carcinogenesis as an example, we propose that the Alpha-bug hypothesis might apply to other cancer types as well as other chronic immune-based pathology such as inflammatory bowel disease (IBD).
POTENTIAL HYPOTHESES
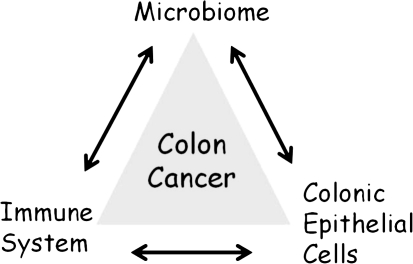
Understanding how our colon bacterial communities contribute to the pathogenesis of colon cancer will require understanding the tripartite relationship depicted in Figure 1. This tripartite relationship positions members of the colon microbiome at its apex to suggest that bacteria are the key drivers of the mucosal immune response and subsequent changes in epithelial cell function and genetics that underpin oncogenic transformation. Colonic epithelial cells (CEC) possessing genetic or epigenetic mutations that disorder cell signaling and function are the cells of origin for colon cancer. It is estimated that clinical colon cancer emerges after a 20- to 40-year span in which sufficient CEC mutations accumulate to permit oncogenic transformation. Mucosal inflammation is integral to understanding colon cancer pathogenesis based on the strong clinical link of intestinal neoplasia and IBD such as Crohn’s disease and ulcerative colitis together with the recent demonstration of key molecular links between innate and adaptive immune responses and epithelial cancers. Further microbiome studies in IBD patients serve to well illustrate that microbiome dysbiosis is usual with chronic intestinal inflammation [5].
Figure 1.
Tripartite relationship contributing to colon cancer pathogenesis. The microbiome, colonic mucosal immune balance, and colonic epithelial cell (CEC) responses (to the microbiome and mucosal immune responses) and CEC genetics are proposed to collectively contribute to the pathogenesis of colon cancer.
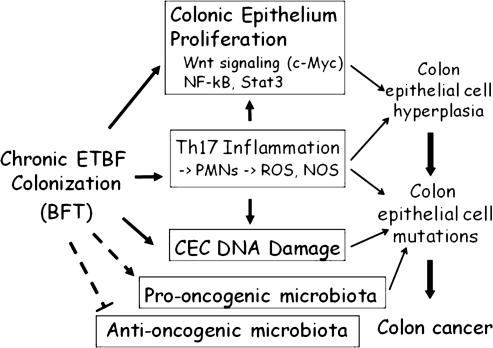
Colon cancer has not yet been epidemiologically linked to a single microbe, raising the alternative idea that it is driven by multiple members of the complex colonic microbial community [6–8 ]. So how might the microbiota play a role in colon oncogenesis? One hypothesis might be that the colonic bacterial species act collectively to induce colon cancer in specific hosts (with possibly host gene polymorphisms leading to subtle pro-tumor mucosal immune responses), particularly if the host is ingesting a cancer-prone diet. This builds on the often-cited epidemiologic data indicating that the colon cancer risk of a migrating population will assume that of their resident country [7]. The more conventional hypothesis is that select microbiome members are sufficiently dominant that they alone define the oncogenic risk. This is, in essence, our clinical construct for diarrheal disease pathogenesis. If an individual develops diarrhea and a Shigella spp. is isolated from his or her feces, the person is designated as a case of shigellosis. While numerous organisms have been suggested as potential inducers of colon oncogenesis over time [6–8], the data are conflicting and meager, with no longitudinal studies and only a few studies seeking to assess the colon bacterial environment concurrent with the diagnosis of colonic neoplasia [9]. We propose here an integrated hypothesis. Namely, certain microbiome members possessing unique virulence traits—bacteria that we term Alpha-bugs—not only are directly pro-oncogenic but are capable of remodeling the colonic bacterial community to one that enhances and further promotes Alpha-bug induction of mucosal immune responses and CEC changes resulting in colon cancer. As such, the Alpha-bug does not act alone but, rather, co-opts the microbial community to aid in its nefarious undertaking. Additionally, Alpha-bugs may enhance carcinogenesis by selectively “crowding out” cancer-protective microbial species (Figure 2). As discussed below, our concept arose from our studies of one candidate Alpha-bug, enterotoxigenic Bacteroides fragilis (ETBF).
Figure 2.
Model of colon cancer induction by Alpha-bugs. The model uses data on enterotoxigenic Bacteroidesfragilis (ETBF) as an example of a putative Alpha-bug. Detailed description of the model is contained in the text. Abbreviations: ROS = reactive oxygen species; NOS = nitric oxide synthase.
ETBF AS AN ALPHA-BUG: A CANDIDATE BACTERIAL INDUCER OF COLON ONCOGENESIS
B. fragilis commonly colonize the human colon, with estimates ranging from 30 to 70% of populations based primarily on older studies using culture-based approaches in relatively small populations. Although commonly present in the human microbiota, B. fragilis constitute a minority of the bacterial community (estimated to be ∼1–2% or less) but emerge as the leading anaerobe in invasive disease, such as abscess formation or bacteremia. ETBF are a molecular class of B. fragilis distinguished by their secretion of a 20kDa zinc-dependent metalloprotease toxin termed the B. fragilis toxin (BFT) [10]. Metalloproteases are common enzymes produced by bacteria, and specifically, BFT is structurally related to other bacterial toxins such as diphtheria toxin or tetanus toxin among others that are important in human disease. There are, to date, 3 described isotypes of BFT (BFT-1, BFT-2, BFT-3) that are at least 92% identical in amino acid sequence, and in in vitro studies BFT toxin isotypes appear to affect CECs similarly. Both BFT-1- and BFT-2-secreting ETBF appear to be globally distributed, with BFT-1-secreting ETBF being most common. BFT-3-secreting ETBF, so far, appear concentrated in Southeast Asia.
ETBF represents a class of colonic bacterium that appears to behave in humans as either pathogen or commensal. ETBF are associated with inflammatory diarrheal illnesses, resembling Shigella sonnei infections, in children and adults [11]. However, asymptomatic colonization with ETBF also occurs in a sizable number of individuals (∼4–30% in different studies) [10]. An important caveat is that no studies have yet examined if asymptomatic ETBF colonization is associated with colonic pathology.
A number of observations led to the idea that ETBF might induce colon tumors. In vitro studies of the mechanism of action of BFT (studies predominantly conducted with BFT-2) indicated that the toxin rapidly, but indirectly, stimulated cleavage of E-cadherin, the structural protein comprising the zonula adherens of CECs and a protein that acts to suppress colon tumorigenesis [12–13]. Cleavage of E-cadherin by BFT causes increased colonic permeability, exposing the colon submucosa to luminal bacterial antigens [14–15]. This pathophysiologic change is thought to be one factor contributing to the induction of colon inflammation in IBD, a known precursor of intestinal cancer. Cleavage of E-cadherin by BFT also precipitates β-catenin nuclear signaling [16]. β-catenin is a known activator of Wnt signaling, a cellular pathway critical to cell development and activated by one or more mechanisms in nearly all colon neoplasia[17]. Activation of β-catenin signaling by BFT further triggers augmented proliferation of already cancerous CECs [16]. In addition, BFT stimulates CECs to synthesize and secrete pro-inflammatory cytokines (such as IL-8 and TNF-α, among others) through activation of nuclear factor-κB (NF-κB) signaling [10].
These results meshed with our work identifying that ETBF induces human inflammatory diarrhea and that in C57Bl/6 mice, a mouse strain usually relatively resistant to colitis, ETBF colonization results in an initial symptomatic inflammatory colitis followed by lifelong colonization associated with chronic low-level asymptomatic colonic inflammation and hyperplasia [14]. Concomitantly, the work of a wide array of investigators continued to strengthen the association between chronic inflammation and carcinogenesis. Hence, specific details of the pathogenesis of ETBF infections and the mechanism of action of BFT combined with a broader scientific context converged into an unanticipated hypothesis deserving of investigation—namely, ETBF promotes colon inflammation and possibly colon oncogenesis.
This hypothesis was pursued in the multiple intestinal neoplasia (Min) mouse strain that is considered the classic murine model of colon tumorigenesis and is characterized by being heterozygous for the adenomatosis polyposis coli (APC) allele (Apc+/-). Mutations in APC occur in nearly all human colon cancers and are the defining molecular defect in the familial adenomatosis polyposis syndrome that inexorably leads to colon cancer in affected individuals [17]. One poorly understood feature of the Min mouse tumor model is that adenomas arise over months predominantly in the small bowel with typically few (<5) colon adenomas. Death ultimately occurs in Min mice due to intestinal obstruction and anemia by about 6 months of age.
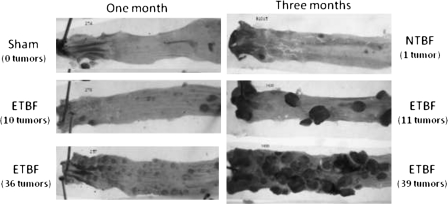
The classic picture of Min mouse tumorigenesis was dramatically altered by colonization of Min mice with ETBF (Figure 3) [4]. ETBF colonization rapidly led to the induction of excess colon tumors in Min mice without altering small-bowel tumor formation, consistent with the fact that the colon is the home for ETBF colonization. ETBF colonization led to significantly increased and visible colon tumors after only 4 weeks compared to controls, with large colon tumors present in ETBF-colonized Min mice by 3 months resulting in premature murine death. ETBF-induced tumors cluster in the distal colon, which is also the predominant site of human colon tumors, further enhancing the potential relevance of this new colon tumor model. ETBF-induced oncogenesis is exceedingly swift, with microscopic adenomas detectable in the Min mouse colons after only one week of colonization, a time point at which adenomas are never identified in control mice, and the extent of colon inflammation induced by ETBF correlates significantly with the numbers of ETBF-induced colon tumors. This latter observation led us to hypothesis that the type of inflammation induced by ETBF was critical to ETBF-induced colon tumorigenesis.
Figure 3.
Enterotoxigenic Bacteroides fragilis (ETBF)–induced colon tumors in multiple intestinal neoplasia (Min) mice. Colons from Min mice colonized with ETBF or nontoxigenic B. fragilis (NTBF) or sham-inoculated mice for one or 3 months are shown. Colons are stained with methylene blue to facilitate enumeration of colon tumors by dissecting microscope examination. The number of colon tumors identified in each depicted mouse colon is shown in parentheses.
To test this hypothesis, we analyzed the T cell–dependent mucosal immune responses in the mouse colons. While prior data had largely focused on innate immune responses in carcinogenesis [18], we reasoned that T cells are critical regulators of the majority of inflammatory responses and are typically critical in host responses to infections. Our results identified that ETBF rapidly induce nearly exclusive activation of Stat3 (signal transducer and activator of transcription), a member of a family of transcription factors that mediate, in part, T cell lineage development and which also serve as key regulators of oncogenesis [19]. Stat3 activation (usually by tyrosine kinase phosphorylation with nuclear translocation) is required for the induction of Th17 immune responses whose signature effector cytokine is IL-17. In contrast, Stat1, Stat2, and Stat4 contribute to Th1 cell development and Stat6 directs Th2 cell development with the secretion of the key Th1 and Th2 cytokine effectors, γ-interferon and IL-4, respectively. Consistent with the detection of predominant Stat3 activation, 2 populations of T cells, CD3+CD4+ (TCRαβ+) and CD3+CD4– (TCRγδ+), were identified to be producing IL-17 in the colon mucosa of ETBF-colonized mice but not controls. Blockade of IL-17 alone or in combination with the IL-23 receptor (IL-23 is required for amplification of Th17 cell populations) or depletion of CD4+ T cells significantly inhibited gross or microscopic ETBF-induced colon tumorigenesis, whereas blockade of γ-interferon or depletion of γδ-T cells did not inhibit ETBF-induced tumorigenesis. Preliminary data suggest that IL-17 induction is long-lived in asymptomatic ETBF-colonized mice consistent with the long lag time between the initiation and clinical presentation of colon tumors. Together these data identified Stat3/Th17 inflammation as mediating, at least in part, colon tumorigenesis induced by ETBF.
The ETBF Min mouse model offers new opportunities to understand colon oncogenesis and the contributions of the microbiota. Strengths of the model include a disease course (acute illness to persistent colonization) that mimics what likely occurs in some individuals who become infected with ETBF. Tumor induction by ETBF localizes to the distal colon as in humans. Our data suggest that at least one host cell mutation, ie, APC mutation,which is nearly universal in human colon cancer, facilitates the induction of colon tumors by ETBF.
Our results, as in all new observations, also lead to abundant new questions. Relevant to the Alpha-bug hypothesis, does ETBF act alone to induce colon tumors, or do microbiota collaborators contribute? Do ETBF and/or other microbiota collaborators promote mutation of the second Apc allele or other CEC gene alterations that initiate colon tumors? As noted earlier, we favor the hypothesis that the synergistic actions of ETBF and other microbiota members are key to colon oncogenesis. This is based on our preliminary observations that treatment of ETBF-colonized Min mice with antibiotics that modify the colonic microbial community without altering the level of ETBF colonization can change the carcinogenesis rate. We are currently using colon microbiome–profiling techniques to assess how, in the absence of antibiotics, ETBF might alter the colonic microbiota. Furthermore, at least some ETBF strains are fatal for germfree mice, suggesting critical interactions between other microbiota members and ETBF that influence colon disease development [14].
Do all ETBF induce colon tumors experimentally? Does BFT isotype influence disease pathogenesis? Our studies to date have focused on a single BFT-2-secreting ETBF strain, and, thus, it is imperative that additional ETBF strains be examined for their pathogenetic potential. Our preliminary data suggest that colonizing ETBF strains do induce colon inflammation and tumors in C57Bl/6 and/or Min mice, but not unexpectedly, ETBF may vary in their colonization potential. Additional experiments with ETBF recombinant strains and/or strains in which bft isotype alleles are deleted as well as studies of the interaction of ETBF with the colonic mucosa are necessary to understand what virulence factors of ETBF regulate tumor induction. Colon inflammation induced by ETBF requires expression of BFT [14]; thus, it is likely that BFT expression is one essential virulence determinant of tumor-inducing potential.
Does chronic colonization with ETBF induce asymptomatic colon hyperplasia and inflammation in humans as we observed in mice? Have patients with colon adenomas or cancer been exposed to ETBF, and/or are they colonized with ETBF at the time of disease diagnosis? These critical translational questions require the development of sensitive and specific, potentially BFT-isotype-specific, diagnostic tools to identify ETBF exposure and/or colonization. With the development of these tools, the opportunity to better define ETBF as well as B. fragilis epidemiology will emerge. Because it is possible that ETBF induction of colon oncogenesis could occur by a “hit and run” mechanism, it will be equally important to determine if ETBF can imprint specific colon immune responses, ie, Th17 responses, that may drive human colon tumor formation even after ETBF has been cleared by the host. Little is known about the colon immune responses or mucosal immune balance present in human colon cancer, but our preliminary data and those of others suggest that Stat3 activation characterizes at least a subset of these tumors [19–20]. Such data lead us to speculate that studies to define how Stat3/Th17 immune responses promote colon tumors in ETBF-colonized mice as well as studies to further define the epidemiology of ETBF infection in humans with and without colon tumors may lead to insights into the pathogenesis of human colon cancer with the potential to change current approaches to the prevention, diagnosis, and treatment of these very common malignancies.
A MODEL OF COLON CANCER PATHOGENESIS
Colon cancer is, by definition, a genetic disease of CECs in which accumulated epigenetic and/or genetic mutations disorder CEC signal transduction, promoting CEC hyperplasia and dysplasia, ultimately resulting in cancer [17]. This model of colon cancer pathogenesis, first proposed in 1990 by Fearon and Vogelstein, is termed, by some, the “Vogelgram”[21]. In our discussions with Dr Bert Vogelstein, however, he has pointed out that the triggers leading to the genetic progression culminating in colon cancer are not yet defined. Thus, our model suggests that ETBF, a putative Alpha-bug, is one potential trigger for a cascade that could result in colon neoplasia. In this model, ETBF, through the secretion of at least BFT, alters CEC and mucosal immune function to promote oncogenic mucosal events as well as the intraluminal environment to create partners in the oncogenic process. Critical CEC events include changes in signal transduction that result in the activation of pathways solidly linked to oncogenesis, including Wnt, NF-κB, and Stat3 signaling. Concomitantly, ETBF leads to colon mucosal immune changes favoring a predominant and oncogenic Th17 immune response that may be additive or even synergistic with the CEC events associated with ETBF colonization of the colon, particularly in the distal colon. DNA damage, likely critical to the induction of the genetic and/or epigenetic mutations pivotal to the induction of colon neoplasia, may occur through direct (eg, BFT action on CECs) and/or indirect (eg, reactive oxygen or nitrogen species released by inflammatory cells) mechanisms. We suggest that ETBF further remolds the colon microbiota to create collaborators that contribute to colon oncogenesis. Our thinking incorporates other concepts suggested to contribute to colon oncogenesis such as diet, which can also remodel the microbiota and/or promote the production of intraluminal carcinogens or metabolites (eg, by bacterial enzyme conversion of pro-carcinogens), potentially facilitating oncogenesis, and host-specific polymorphisms such as IL-8 polymorphisms, which already have been shown to influence host responses to enteric bacteria [22]. We would suggest that other Alpha-bugs can be fitted to this model using their individualized mechanistic actions. Candidates for consideration include Streptococcus gallolyticus (also known as Streptococcus bovis), superoxide-producing Enterococcus fecalis, and Escherichia coli [23–27].
FUTURE DIRECTIONS: DOES COLON CANCER RESULT FROM A CONTINUUM OF DIARRHEAL DISEASE TO ONCOGENESIS?
Testing the components of our model offers exciting challenges to investigators in infectious diseases as well as other disciplines and indicates the importance of cross-disciplinary studies to identify the points in this putative cascade that may optimally allow the development of preventive and therapeutic interventions to diminish the morbidity and mortality from colon cancer. One goal must be to determine if specific bacteria and/or their mucosal or even systemic molecular signatures can be established as worthy of routine clinical testing to identify individuals at high risk for colon oncogenesis. A new paradigm can be envisioned in which colonoscopy is no longer proposed for all individuals over 50 years of age but, rather, is precipitated by identification of individuals who carry Alpha-bugs and their bacterial collaborators (ie, a pro-oncogenic microbiota) and/or whose immune responses are identified as pro-oncogenic. This translation to human medicine will require defining the bacteria to be sought; the development of new diagnostic tests; and longitudinal and cross-sectional epidemiologic studies to demonstrate the feasibility, sensitivity, and specificity of such testing to identify those at risk for colon oncogenesis. A second key goal should be to better understand the putative intersection of global diarrheal disease epidemiology and colon cancer. Diarrheal illnesses remain a major global killer. We know that many diarrheal disease pathogens induce colon inflammatory responses and that prolonged, possibly chronic, colonization with these enteric bacteria occurs. We have come to understand that repeated bouts of diarrheal disease impacts later on the host with potential for the development of cognitive deficits and possibly even neurodegenerative disease. However, we know little about the impact of chronic colonization with specific enteric bacteria on mucosal immunology and oncogenesis. We know even less about the current epidemiology of cancer-including colon cancer in underresourced countries. Mapping the global impact of colon cancer and its relationship to diarrheal disease burden and specific enteric bacteria is essential to understand if living circumstances promote either protective or oncogenic microbiota or both. Identifying protective microbiota would open up the possibility suggested by studies in the 1980s that manipulation of the microbiota with probiotics may reduce colon cancer risk [28].
Fifteen years ago we could not have imagined the transformation in global health made possible by visionary investigators and the support of the President’s Emergency Plan for AIDS Relief and the Gates Foundation, among others. Understanding the global impact of cancer and its relationship to infectious agents will require similar resources and commitment. Studies in new experimental models of colon tumorigenesis such as the ETBF Min mouse model may enable us to design translational human studies to define the potential critical Alpha-bug cadre and their bacterial collaborators that we propose are essential triggers in the decades-long process leading to colon oncogenesis.
Funding
This work was supported by R01 DK45496 (to CLS) and R01 DK080817 (to CLS) and gifts from B. Schwartz (to CLS), W. and B. Topercer, D. Needle, and the Commonwealth Foundation (to DMP).
Acknowledgments
DMP is a Januey scholar and holds the Abeloff Chair in Oncology at Johns Hopkins University. We thank Dr. Shaoguang Wu for Figure 2. We thank the members of our laboratories for their dedication and inspiration in the development of this work.
References
- 1.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aries V, Crowther JS, Drasar BS, Hill MJ, Williams RE. Bacteria and the aetiology of cancer of the large bowel. Gut. 1969;10:334–5. doi: 10.1136/gut.10.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank DN, St.Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Pei Z. Bacteria, inflammation, and colon cancer. World J Gastroenterol. 2006;12:6741–6. doi: 10.3748/wjg.v12.i42.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope ME, Hold GL, Kain R, El Omar EM. Sporadic colorectal cancer—role of the commensal microbiota. FEMS Microbiol Lett. 2005;244:1–7. doi: 10.1016/j.femsle.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Rowland IR. The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des. 2009;15:1524–7. doi: 10.2174/138161209788168191. [DOI] [PubMed] [Google Scholar]
- 9.Finegold SM, Flora DJ, Attebery HR, Sutter VL. Fecal bacteriology of colonic polyp patients and control patients. Cancer Res. 1975;35:3407–17. [PubMed] [Google Scholar]
- 10.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. ClinMicrobiol Rev. 2009;22:349–69. doi: 10.1128/CMR.00053-08. Table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sears CL, Islam S, Saha A, et al. Enterotoxigenic Bacteroidesfragilis infection is associated with inflammatory diarrhea. Clin Infect Dis. 2008;47:797–803. doi: 10.1086/591130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Lim K-C, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonulaadherens protein, E-cadherin. ProcNatl Acad Sci USA. 1998;95:14979–84. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and {gamma}-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944–52. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee KJ, Wu S, Wu X, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–18. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riegler M, Lotz M, Sears CL, et al. Bacteroides fragilis toxin-2 damages human colonic mucosa in vitro. Gut. 1999;44:504–10. doi: 10.1136/gut.44.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterol. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of Stat3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 20.Lassmann S, Schuster I, Walch A, et al. Stat3 mRNA and protein expression in colorectal cancer: effects on Stat3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60:173–9. doi: 10.1136/jcp.2005.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 22.Jiang ZD, Okhuysen PC, Guo DC, et al. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J Infect Dis. 2003;188:506–11. doi: 10.1086/377102. [DOI] [PubMed] [Google Scholar]
- 23.Herrera P, Kwon YM, Ricke SC. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe. 2009;15:44–54. doi: 10.1016/j.anaerobe.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Swidsinski A, Khilkin M, Kerjaschki D, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterol. 1998;115:281–6. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 25.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterol. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 26.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–36. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 27.Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One. 2009;4:e5517. doi: 10.1371/journal.pone.0005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldin BR, Gorbach SL. Effect of Lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J Natl Cancer Inst. 1980;64:263–5. doi: 10.1093/jnci/64.2.263. [DOI] [PubMed] [Google Scholar]