Abstract
Functional characteristics of tuberculosis (TB)–specific CD4 T cells were studied in clinically active pulmonary TB (n = 21) and high TB exposure including LTBI (n = 17). Following tuberculin stimulation, activated CD4 T cells were identified by flow-cytometry (CD154 up-regulation, degranulation, interferon γ [IFN-γ], tumor necrosis factor α [TNF-α], and interleukin 2 [IL-2\ production). Interestingly, CD154 up-regulation accounted for ∼80% of activated CD4 T cells in the active TB group but just 40% in the controls, whereas IFN-γ accounted for only ∼50% of activated cells in each group. The frequencies of CD4 T cells displaying at least 1 activation marker discriminated better between the groups than those displaying degranulation or IFN-γ production alone.
Infection with Mycobacterium tuberculosis or other M. tuberculosis complex strains can be readily identified by interferon γ (IFN-γ) release assays (IGRAs). These tests identify either interferon γ (IFN-γ) production (Quantiferon) or the percentage of IFN-γ producing T cells (T-Spot TB). However, IGRAs do not discriminate between latent tuberculosis (TB) infection (LTBI) and active TB [1]. The control of LTBI by the immune system is the single most important factor in halting TB on a global scale. Improving discrimination between LTBI and active disease is a priority in areas where treating all infected cases is not possible. Interestingly, in TB contacts a positive Quantiferon tests is more predictive of subsequent active TB than a positive skin test [1]. How the immune system controls LTBI is not well understood, but CD4 T cells play an important role [2]. Likewise, it is well known that antagonizing tumor necrosis factor α (TNF-α) (produced by activated CD4 T cells) may result in reactivation of LTBI [3]. Here we explore the activation marker profiles of TB-specific CD4 T cells in order to identify differences between patients with acute pulmonary TB (apTB) and clinical staff with high TB exposure (hE) including cases of confirmed LTBI. The simultaneous production of TNF-α, IFN-γ and IL-2, has been widely discussed in the recent literature as important in the protection against microbes [4–6]. Our panel of activation markers for the detection of tuberculin-induced activated T cells included TNF-α, IFN-γ, interleukin 2 (IL-2), CD107-mobilization (degranulation), and CD154-up-regulation. The use of this panel exposed a marked difference in the frequency of tuberculin-specific T cells between apTB and hE, suggesting that a drop in overall numbers of tuberculin-specific T cells is associated with active TB.
MATERIALS AND METHODS
Patients and Patient Materials
TB patients were recruited from Lungenklinik Lostau (LL). Only patients with positive M. tuberculosis culture (sputum and/or bronchio-alveolar lavage fluid) were classified as having clinically active pulmonary tuberculosis (apTB) according to the criteria of the American Thoracic Society [7] (n = 21, 10 female, 11 male, age+/−standard deviation [SD] = 49+/−24 years). A tuberculin skin test >5 mm was reported in 15 of 18 tested patients (median 10 mm). BCG-vaccinated, long-term directly apTB exposed hospital staff (hE) were recruited at LL and Charité Campus Mitte (CCM) (n = 17, 12 female, 5 male, age+/− SD = 42+/−11 years), 14 were tested for LTBI by the commercial ‘Quantiferon Gold in-tube’ test, 7 tested positive. Donors with no known TB exposure but BCG-vaccinated (n = 7) or not (n = 7) were recruited additionally. Most TB patients were BCG-vaccinated, all of them tested HIV-negative. No other participants were known/suspected to be HIV-positive. There were no significant ethnic differences between the groups. TB treatment generally included isoniazid, ethambutol, and rifampicin; in some cases also pyrazinamide. Blood was anticoagulated with sodium citrate. Written informed consent was obtained as approved by the Charité Ethics Committee.
Reagents for Stimulation
Tuberculin (Statens Serum Institute, Copenhagen, Denmark) and SEB (Sigma, St. Louis, MO, USA) were dissolved in DMSO (Pierce, Rockford, IL, USA) prior to lyophilisation.
T Cell Activation and Flow-Cytometry
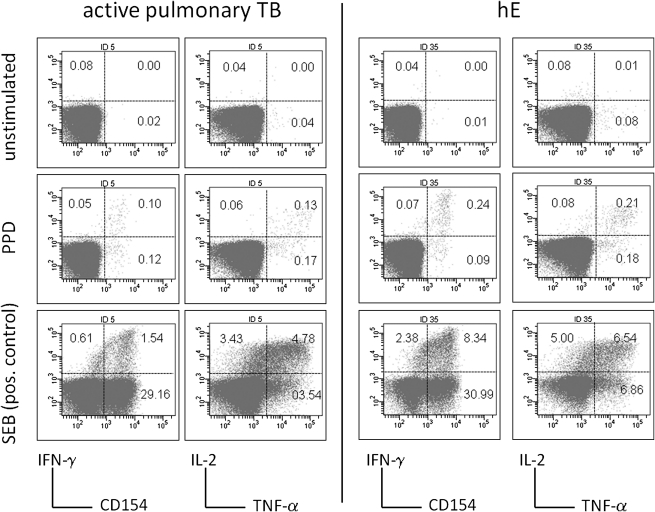
PBMCs were prepared within 4 h of blood collection, 200 mL aliquots (5 × 106 cells/mL) in complete RPMI culture media (Gibco/Invitrogen) were placed in 8 well-strips (part of 96-well strip-well plates) containing lyophilized stimulants (solvent alone for negative control, SEB for positive control, tuberculin), anti-CD107a [8], and Monensin (Sigma). Wells containing SEB also contained Brefeldin A (BFA; Sigma). All wells were reconstituted with 25 mL of PBS (Sigma) prior to PBMC addition. At 2 h, 25 mL of complete culture media was added to each well, tuberculin wells also received BFA at that time, allowing tuberculin processing during the first 2 h. Unstimulated controls were run for all conditions. Final stimulation volume was 250 mL, final concentrations of Monensin and BFA were 5 mg/mL each, final concentrations of tuberculin and SEB were 10 mg/mL and 1 microgram/mL, respectively. Plates were incubated for 16 h (37°C, 5% CO2). This was followed by the addition of 20 mL of 20 mmol/L EDTA (Sigma) in PBS/0.1% sodium azide (Sigma) to each well; extra- and intracellular staining followed the published protocol for lyoplates [9]. At least 200,000 lymphocytes were acquired per tube (LSR II flow-cytometer, BD Biosciences). Data were analyzed using FlowJo software (Treestar) and a standardized gating procedure. Frequencies of .01% positive CD4 T cells or more (with respect to each activation marker) were considered positive responses [10]. Examples are shown in Figure 1. Boolean gating created 31 nonoverlapping subsets covering all possible activation marker combinations. These were used to calculate the cumulative percentage of cells displaying each activation marker (eg, the sum of all IFN-γ producing subsets) and subsets of interest (the mean counts for subsets displaying at least 1 activation marker were 672 for apTB and 829 for hE).
Figure 1.
Detection of tuberculin-specific CD4 T cell responses. Cells were stimulated overnight with tuberculin or SEB. Unstimulated samples were run as controls. Activation was detected by CD154-up-regulation, degranulation (not shown), IFN-γ, TNF-α, and IL-2 production. SEB response size varied strongly between individuals and was not correlated with tuberculin response size. Percentages are indicated in quadrants, axes show log fluorescence intensity, CD4 T cells are displayed.
Monoclonal Antibodies and Beads
Pacific-blue (PB)-labeled anti-CD3, Peridine-Chlorophyll (PerCP)-Cy5.5-labeled anti-CD4, AmCyan-labeled anti-CD14, Fluorescein-Iso-Thio-Cyanate (FITC)-labeled anti-CD27, Phycoerythrin (PE)-labeled anti-CD154, PEAlexa 610-labeled CD107a, PE-Cy7-labeled IFN-γ, Allophyocyanine (APC)-labeled anti-IL-2, and AlexaFluor-700 labelled TNF-α, anti-Mouse Ig, κ/Negative Control (FBS), and compensation particles were provided by BD Biosciences. SPSS software version 15.0 was used for statistical analysis.
Interferon-γ Release Assay
The Quantiferon Gold in-tube test (Cellestis, Darmstadt, Germany) was performed according to the manufacturer's instructions.
RESULTS
Two different types of analysis were performed, first, “frequencies” of tuberculin activated CD4 T cells as a percentage of all CD4 T cells, second, the “proportions” of cells displaying certain activation markers among all activated CD4 T cells. CD8 T cell responses to tuberculin were exceedingly rare, very small, and therefore not analyzed further.
Tuberculin-Inducible CD4 T Cells in Peripheral Blood are Decreased in Active Tuberculosis
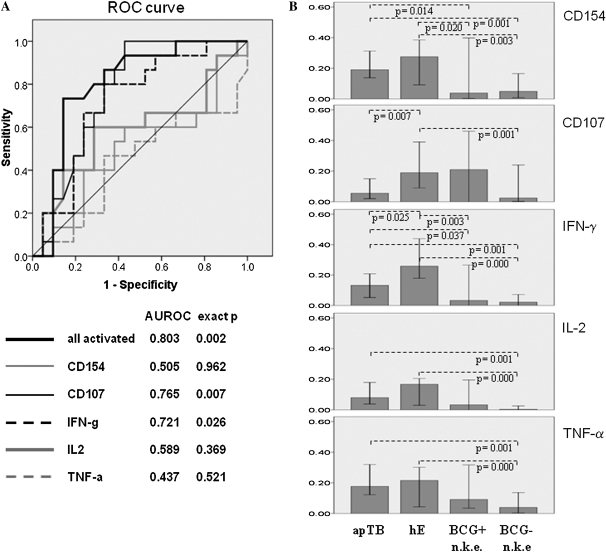
ROC curves in Figure 2A show that the frequency distribution of CD4 T cells bearing at least 1 of the 5 activation markers (all detectable activated cells) discriminated well between hE and apTB, degranulation (CD107) and IFN-γ reasonably well, and other single parameters less well. Figure 2B shows the corresponding frequencies of CD4 T cells bearing each individual activation marker. For comparison, results from BCG-vaccinated and non-BCG-vaccinated individuals with no known TB exposure are shown as well. Their responses were generally smaller or absent (especially in the nonvaccinated group). All displayed frequencies seem to be higher in hE than in apTB. These differences were significant for degranulation and IFN-γ only, in agreement with the ROC analysis (Figure 2A).
Figure 2.
Frequencies of activated CD4 T cells are higher in apTB than in hE, activation markers differ between groups. A, ROC-curves display sensitivity vs. specificity for discriminating apTB and hE by all tuberculin-induced activation markers combined and each activation marker alone. B, Bars (medians/95% confidence interval [CI]) indicate the frequencies of activated CD4 T cells per each activation marker alone. C, Bars (medians/95% CI) show proportions of activation marker positive cells among all activated cells. CD154 up-regulation was the most dominant single activation marker of tuberculin-specific T cells in apTB. D, Proportions of CD154, TNF-α (axes) and IFN-γ positive cells (bubble size) among all activated CD4 T cells are plotted against each other. The dotted line indicates an apparent division between hE and apTB. (“n.k.e”. = no known exposure to TB). Significant differences in B and C are indicated (Mann-Whitney U test).
We also evaluated whether the same or similar frequencies of activated cells were detectable when using only 2, 3, or 4 of the activation markers in our panel. The best combinations of 4 markers were those leaving out IL-2 or TNF-α, and, unsurprisingly, the best combination of 3 markers was that leaving out both. Such a panel including CD154, CD107, and IFN-γ would be able to identify 91.7+/−7.5% (mean+/− SD ) of all activated cells and still discriminate between the groups (AUROC .800, P = .002, not shown). Other combinations of 3 or 2 markers only would detect fewer activated cells and would not be able to discriminate apTB from hE. Interestingly, IFN-γ/TNF-α/IL-2 triple-positive ‘polyfunctional’ [4] CD4 T cells have been observed more frequently in active TB than in LTBI after stimulation with Ag85B, ESAT-6 and 16-kD [11] or tuberculin and an ESAT-6/CFP-10 fusion protein [12]. Following tuberculin stimulation, we found this subset in all apTB patients and hE controls, accounting for 14.7% and 8.3% (median, P = .191) of activated CD4 T cells, respectively. This finding seems to support the reported differences, but was not statistically significant and no acceptable discrimination of the groups was provided by this subset.
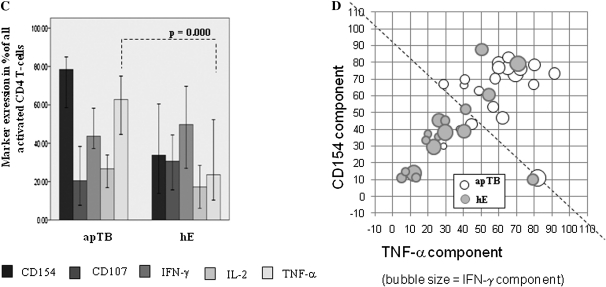
Proportions of T Cells Displaying CD154 Up-Regulation and TNF-α Production Differ Significantly between the Groups
Figure 2C shows the proportion of all activated cells detected by each of the single activation markers (cells displaying at least one marker = 100%). Of note, in apTB, CD154 up-regulation and TNF-α production occurred in much larger proportions of activated T cells than in hE. The functional implications of this are not clear, in particular for cells displaying only CD154 up-regulation. The data, however, show that marked functional changes can be found among tuberculin-specific CD4 T cells in active pulmonary disease. Of note, the markers that discriminated least between the groups in terms of CD4 T cell frequencies discriminated most in terms of their proportions among activated cells. The tuberculin-specific responses of BCG- vaccinated and non BCG-vaccinated groups with no known exposure are not shown, since the majority of responses were too small for this type of analysis. Figure 2D visualizes qualitative response differences between apTB and hE. The diagram conveys a sense of progression starting from the bottom left to the top right.
DISCUSSION
In this study the frequencies of tuberculin-specific CD4 T cells carrying at least 1 of the 5 measured activation markers were significantly higher in individuals with hE than apTB. Single activation markers were less good at discriminating the 2 groups than the combination of all. The fact that more degranulating and IFN-γ-producing cells were found in hE than apTB is in agreement with findings on IFN-γ release assays in The Gambia, where active TB cases had smaller responses than latently infected individuals [13]. It suggests that control of M. tuberculosis in LTBI and highly exposed individuals may be partly due to higher numbers of TB-specific CD4 T cells compared with active cases; however, an interpretation focused on response size would not consider potential differences in response “quality” [4, 14, 15]. Furthermore, a difference in response size in peripheral blood might result from activated T cells migrating to infected tissues.
Our data illustrate the important difference between studying activation marker positive cells in percent of CD4 T cells or as a proportion of the activated cell subset. There is no right or wrong in using frequencies of CD4 T cells or proportions of activated cells, or even absolute counts, as long as the use of data is clear and transparent. For example, median frequencies of CD154-up-regulating and TNF-α-producing CD4 T cells are higher in hE than apTB. However, in apTB ∼80% of all activated CD4 T cells on average up-regulate CD154 and >60% produce TNF-α compared with <40% and <30%, respectively, in hE.
Interestingly, IFN-γ production occurred in only half of the detectable, tuberculin-specific CD4 T cells (∼50% in each group). Adding relevant additional activation markers (to be identified) to the panel would decrease this proportion even more.
The proportion of T cells simultaneously producing IFN-γ, TNF-α and IL-2 among all activated cells seemed higher in apTB than hE, but unlike in other reports [11, 12] this was not a significant difference, probably owing to different stimulation conditions and activation marker panels.
In conclusion, individuals with LTBI or high exposure to TB possess higher frequencies of TB-specific T cells in the peripheral blood than individuals with apTB. Whereas TNF-α production and CD154 up-regulation are dominant components of this response in apTB, IFN-γ production is the most dominant component in highly exposed and latently infected individuals.
Funding
This work was funded in part by Charité – Universitätsmedizin Berlin and Brighton and Sussex Medical School
Acknowledgments
Thanks to Dr. Catherine Ross for proof-reading and discussions.
References
- 1.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–70. doi: 10.1164/rccm.200711-1613OC. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 3.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with Infliximab, a Tumor Necrosis Factor {alpha}-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 4.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 5.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 6.Lindenstrom T, Agger EM, Korsholm KS, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–55. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 7.Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 8.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 9.Maecker HT, Rinfret A, D'Souza P, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streitz M, Tesfa L, Yildirim V, et al. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS ONE. 2007;2:e735. doi: 10.1371/journal.pone.0000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4+T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. doi: 10.1002/eji.201040455. 2010; 40(8):2211-20. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39:723–9. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 13.Vekemans J, Lienhardt C, Sillah JS, et al. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in the Gambia. Infect Immun. 2001;69:6554–7. doi: 10.1128/IAI.69.10.6554-6557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 15.Soares AP, Scriba TJ, Joseph S, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]