Abstract
Antibodies are required to control blood-stage forms of African trypanosomes in humans and animals. Here, we report that intradermal infections by low numbers of African trypanosomes are controlled by innate resistance but prime the adaptive immune response to increase susceptibility to a subsequent challenge. Mice were found 100 times more resistant to intradermal infections by Trypanosoma congolense or Trypanosoma brucei than to intraperitoneal infections. B cell–deficient and RAG2−/− mice are as resistant as wild-type mice to intradermal infections, whereas inducible nitric oxide synthase (iNOS)−/− mice and wild-type mice treated with antibody to tumor necrosis factor (TNF) α are more susceptible. We conclude that primary intradermal infections with low numbers of parasites are controlled by innate defense mediated by induced nitric oxide (NO). CD1d−/− and major histocompatibility complex (MHC) class II−/− mice are more resistant than wild-type mice to primary intradermal infections. Trypanosome-specific spleen cells, as shown by cytokine production, are primed as early as 24 h after intradermal infection. Infecting mice intradermally with low numbers of parasites, or injecting them intradermally with a trypanosomal lysate, makes mice more susceptible to an intradermal challenge. We suggest that intradermal infections with low numbers of trypanosomes or injections with trypanosomal lysates prime the adaptive immune system to suppress protective immunity to an intradermal challenge.
African trypanosomes are extracellular hemoprotozoa. T. brucei gambiense and T. brucei rhodesiense cause sleeping sickness in humans. Sleeping sickness is an emerging disease in Africa [1]. There is no vaccine available against human African trypanosomiasis, and chemotherapy is less than satisfactory [2]. Trypanosoma congolense, T. brucei brucei, and Trypanosoma vivax are pathogens for livestock [3]. Although there are differences between the different species of African trypanosomes, they all share major features. African trypanosomes cause persistent infections of the blood. They survive in humans and animals by complex evasion mechanisms [4], including antigenic variation [5–7] and immunosuppression [8–12]. They are covered with a glycoprotein coat of a single molecular species, the variant surface glycoprotein (VSG) [5, 7]. The VSG is anchored into the membrane via a glycolipid, a glycosylphosphatidylinositol [13, 14]. Infections of mammalian hosts are associated with cycles of parasitemia and expression of new VSGs [5, 7, 15]. Each new VSG elicits a strong immunoglobulin (Ig) M anti-VSG response [5], which leads to phagocytosis of the trypanosomes by macrophages of the liver via complement receptor CR3 [16–18].
In nature, mammals become infected by bites from trypanosome-infected tsetse flies [3]. The labellar teeth of the proboscis of the tsetse fly cut small capillaries. Blood leaking from these capillaries forms a small pool under the skin and is sucked up through the proboscis into the pharynx [19]. The bite by the infected tsetse fly results in an infection in the skin of the bitten human or animal. It was found that the salivary gland of the tsetse fly had to contain at least 300–450 trypanosomes to establish infections in humans [20]. The trypanosomes migrate from the skin into the blood via the lymph system [15, 21].
Past research into the immunobiology of mice experimentally infected with African trypanosomes has mostly been based on the immune responses of mice infected via the intraperitoneal route [4, 8, 10, 22–25]. Although these studies have provided great insight into the host-parasite relationship, they have ignored the very early immunological events triggered by the infecting parasites. This situation prompted us to develop an animal model based on intradermal infections.
We have, for the first time, provided evidence that intradermal infections by low numbers of trypansomes are controlled by innate resistance and that the process of innate resistance is accompanied by the priming of the adaptive immune system resulting in increased susceptibility to a subsequent intradermal challenge. We propose that these findings have important bearings on the development of vaccines against African trypanosomiases.
MATERIALS AND METHODS
Mice
The study included 8–10-week-old female BALB/c, C57BL/6 and outbred Swiss mice (CD1), purchased from the Animal Resource Center, University of Saskatchewan; 6–8-week-old female CD1d−/−, MHC class II−/−, B cell–deficient (μMT), and iNOS−/− mice, with either BALB/c or B6 background, purchased from the Jackson Laboratory; and RAG2−/− B6 mice, purchased from Taconic. The mice were housed in polycarbonate cages on sawdust and allowed free access to food and water throughout the experiments, according to recommendations of the Canadian Council of Animal Care.
Parasites and Parasite Lysates
The origin of T. congolense, strain Trans Mara, clone TC13 used in this study has been described elsewhere [26]. T. congolense, clone TC14, a clone different from TC13, was obtained as described elsewhere [27]. T. brucei brucei, strain 10–26 and strain Whatat 1.1, were obtained from Dr Terry Pearson, University of Victoria, Victoria, British Columbia. Frozen parasite stabilates from liquid nitrogen were thawed and used to intraperitoneally infect outbred CD1 mice that had been immunosuppressed with cyclophosphamide (Cytoxan; 200 mg/body weight) 48 h earlier [26]. The parasites used for experimental infections were purified by diethylaminoethyl cellulose anion exchange columns [28] from the blood of infected CD1 mice. A known number of isolated parasites which had been frozen, thawed, and then sonicated, were used as lysate antigen.
Infection, Immunization, and Determination of Parasitemia and Survival Time
The parasites, enumerated with use of a hemocytometer, were injected in a volume of 20 μL intradermally into a hind footpad of mice. To make sure that low doses of parasites used for intradermal infections were potentially infectious, groups of control mice were infected intraperitoneally with these low doses. Parasitemia and survival time were determined as described elsewhere [29].
Antibodies and Enzyme-Linked Immunosorbent Assay
Purified monoclonal antibody (mAb) rat anti-mouse TNF-α, clone MP6-XT22 (IgG1, κ), was purchased from eBiosciense. OptEIA cytokine detection sets were obtained from BD Biosciences.
Ex Vivo Cytokine Production
Single cell suspensions of spleens and popliteal lymph nodes of mice were cultured at an optimal cell density (5 × 106 cells/mL/well for spleen cells and 2 × 106 cells/mL/well for lymph node cells) in 24-well plates (Becton Dickinson) for 48 h at 37°C. Culture supernatants were harvested and stored at −80°C until use. Cytokines in the culture supernatants were determined by enzyme-linked immunosorbent assay, as described elsewhere [30].
Statistical Analysis
Data are presented as means ± standard errors (SEs). Two-sample t tests comparing percentages were used to determine the significance of differences in infectivity data. Log-rank tests were used to determine the significance of differences in survival curves. Differences were considered statistically significant at P < .05.
RESULTS
Resistance to Parasite Challenge in Mice Infected Intradermally or Intraperitoneally with T. congolense or T. brucei
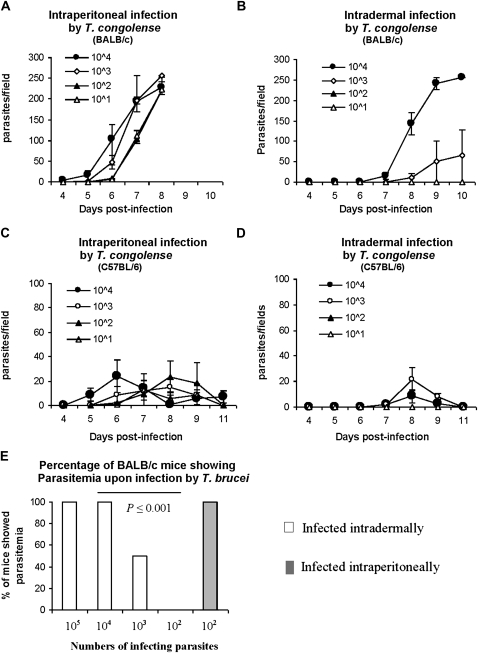
BALB/c mice are highly susceptible to T. congolense infections and die within 8–10 days after intraperitoneal infection with 103 T. congolense TC13, whereas relatively resistant C57BL/6 mice survive for >100 days [4]. BALB/c and C57BL/6 mice were infected either intraperitoneally or intradermally in a hind footpad with varying doses of T. congolense TC13. All mice infected intraperitoneally with 101–104 T. congolense TC13 developed parasitemia, regardless of the infecting dose (Figure 1. A, C), and all BALB/c mice died by day 8 after infection (Figure 1A). Neither BALB/c nor C57BL/6 mice infected intradermally with 101 or 102 T. congolense TC13 showed parasitemia (Figure 1 B, D). They survived with no signs of disease until day 30 when the experiment was terminated. All mice infected intradermally with 103 or 104 parasites developed parasitemia (Figure 1 B, D), but the prepatent periods of intradermal infections were, prolonged by ≥2 days, compared with intraperitoneal infectious (P < .05). All BALB/c mice infected with these higher numbers of parasites died by day 12 after infection (Figure 1B).
Figure 1.
The intradermal route of infection of mice by Trypanosoma congolense or Trypanosoma brucei requires a dose up to100-fold higher than does the intraperitoneal route to cause parasitemia and death. A–D, Groups of 4 mice were infected with varying doses of T. congolense, clone TC13. A, BALB/c mice were infected intraperitoneally, B, BALB/c mice were infected intradermally in a hind footpad, C, D, C57BL/6 mice were infected intraperitoneally (C) and C57BL/6 mice were infected intradermally in a hind footpad (D). E, Groups of 5–6 BALB/c mice were infected intradermally into a hind footpad with varying doses (102–105) of moderately virulent T. brucei brucei, strain 10–26, or intraperitoneally with 102 T. brucei brucei, strain 10–26. Parasitemia was monitored after the infection.
Intraperitoneal infections of BALB/c mice with 102 T. brucei brucei, strain 10–26, led to parasitemia in all mice (Figure 1E). In contrast, intradermal infections of BALB/c mice with 102 T. brucei brucei, 10–26, caused neither parasitemia nor disease. A dose of 103 T. brucei brucei, strain 10–26, led to parasitemia in ∼50% of the infected mice (Figure 1E). Intraperitoneal infections with 5 parasites of highly virulent T. brucei brucei, strain Whatat 1.1, caused parasitemia and disease in both BALB/c and C57BL/6 mice (not shown). BALB/c mice infected intradermally with highly virulent T. brucei brucei, strain Whatat 1.1, also showed lower susceptibility than when infected intraperitoneally. Intradermal infections with 5 or 10 parasites led to parasitemia in only 10% of the infected mice (not shown). C57BL/6 mice infected intradermally with 5 or 10 T. brucei, strain Whatat 1.1, did not develop parasitemia and only 80% of C57BL/6 mice infected intradermally with 102 T. brucei, strain Whatat 1.1 developed parasitemia (not shown). Although there are differences in the outcomes of infections, depending on the strain of mouse and the virulence of the trypanosomes, the results clearly show that responses to intradermal infections have an up to 100-fold higher probability of preventing parasitemia and disease compared with intraperitoneal infections.
Resistance to Intradermal Infections by Low Numbers of T. brucei or T. congolense without Antibodies or T Cells
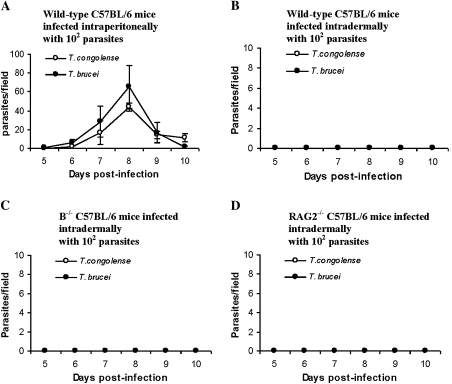
It is well established that in intraperitoneal infections by T. brucei [10, 31] or T. congolense [18, 23], there is an absolute requirement for antibodies to VSG for the control of parasitemia. To determine whether anti-VSG antibodies might be required for the resistance against intradermal infection with few parasites, wild-type C57BL/6 mice, B cell–deficient mice (μMT) and RAG2−/− C57BL/6 mice were used. All wild-type mice infected intraperitoneally with either 102 T. brucei brucei, strain 10–26, or 102 T. congolense TC13, develop parasitemia (Figure 2A). None of the wild-type mice develop parasitemia after intradermal infection with either 102 T. brucei brucei, strain 10–26, or 102 T. congolense TC13 (Figure 2B). Surprisingly, none of the B cell–deficient mice (Figure 2C) or the RAG2−/− mice (Figure 2D) develop parasitemia or become sick with this low-dose intradermal infection. We conclude that B cells, anti-VSG antibodies, and T cells are not required for the resistance to intradermal infection with low numbers of trypanosomes. These results indicate that the resistance to intradermal infections is due to innate defense mechanisms.
Figure 2.
B cell–deficient (μMT) and RAG2−/− mice are as resistant as wild-type mice when infected intradermally with low doses (102) of Trypanosoma brucei or Trypanosoma congolense. A, Wild-type C57BL/6 mice were infected intraperitoneally with 102 T. congolense, clone TC13 or 102 T. brucei brucei, strain 10–26. B–D, Groups of 5–6 wild-type C57BL/6 mice (B), B cell–deficient (μMT) (B−/−) mice (C), or RAG2−/− mice (D) were infected intradermally in a hind footpad with either 102 T. congolense, clone TC13 or 102 T. brucei brucei, strain 10–26. Parasitemia was monitored after the infection.
Induced NO as Major Mediator of Resistance to Intradermal Infections by Low Numbers of T. congolense
There is ample evidence that macrophage-derived NO has a detrimental effect on African trypanosomes [32, 33]. We examined whether induced NO might be a major mediator of the observed innate resistance to intradermal infections. Wild-type and iNOS−/− C57BL/6 mice were infected intradermally with 5 × 102 T. congolense TC13. None of the infected wild-type mice developed parasitemia (Figure 3A), but all infected iNOS−/− mice did (P < .001) (Figure 3B). The results indicate that iNOS−/− mice are more susceptible to T. congolense infection than wild-type mice. Thus, we conclude that induced NO is a major mediator controlling primary intradermal infections by T. congolense.
Figure 3.
Inducible nitric oxide synthase (iNOS)−/− mice and wild-type mice treated with monoclonal antibody to tumor necrosis factor (TNF) α are more susceptible than wild-type mice to intradermal infection with Trypanosoma congolense. A, B, Groups of 5 wild-type C57BL/6 (A) and B, iNOS−/− C57BL/6 (B) mice were infected intradermally into a hind footpad with 5 × 102 T. congolense, clone TC13. C, C57BL/6 mice were injected intradermally into a hind footpad with isotype control rat immunoglobulin G (25 μg/mouse) on day −1 and day 3 after infection. D, C57BL/6 mice were injected intradermally into a hind footpad with anti–TNF-α monoclonal antibodies (25 μg/mouse) on day −1 and day 3 after infection. On day 0, mice were infected intradermally into the same footpad with 5 × 102 T. congolense, clone TC13. Parasitemia was monitored after the infection. Parasitemia was significantly enhanced in the iNOS−/− mice (P < .001) and in anti–TNF-α–treated mice (P < .025).
TNF-α Mediation of Resistance to Intradermal Infections by Low Numbers of T. congolense
TNF-α enhances the induction of NO synthase [34] and has been shown, under certain conditions, to mediate control of intradermal infections by T. congolense [30]. C57BL/6 mice were injected intradermally with 25 μg/mouse of anti–TNF-α mAb or isotype control rat IgGs on the day before infection and day 3 after infection. Mice were infected in the same footpad on day 0 with 5 × 102 T. congolense TC13. None of the 5 control mice had detectable parasitemia (Figure 3C), but 4 of the 6 mice (67%) given anti–TNF-α mAb did develop parasitemia (P < .025) (Figure 3D). The results indicate that TNF-α is a mediator of protection against primary intradermal infections by T. congolense.
Effect of Adaptive Immune Responses on Resistance to Primary Intradermal Infections by T. congolense
We further considered whether T cells might have an influence on the outcome of intradermal infections by trypanosomes. We infected groups of 10 wild-type, CD1d−/−, and MHC class II−/− C57BL/6 mice intradermally into a hind footpad with 104 T. congolense TC13. All of the infected wild-type C57BL/6 mice developed parasitemia (Figure 4A). None of the infected CD1d−/− mice (P < .00001) (Figure 4B) and only 2 (22%) of the 9 MHC class II−/− mice (P < .001) (Figure 4C) developed parasitemia. CD1d−/− BALB/c mice infected intradermally with 5 × 102 T. congolense TC13 were also more resistant than wild-type BALB/c mice (not shown). Thus, both CD1d−/− and MHC class II−/− mice were more resistant than wild-type mice. The results indicate that CD1d-restricted natural killer T cells and MHC class II–restricted CD4+ T cells are induced by intradermal infections with T. congolense and exert a suppressive effect on the protective mechanism(s).
Figure 4.
CD1d−/− mice and major histocompatibility complex (MHC) class II−/− mice are more resistant than wild-type mice to intradermal infections by Trypanosoma congolense. Groups of 10 wild-type C57BL/6 mice (A), CD1d−/− C57BL/6 mice (B), or MHC class II−/− C57BL/6 mice (C) were infected intradermally with 104 T. congolense, clone TC13. Parasitemia was monitored after infection. Infected CD1d−/−mice (P < .00001) and MHC class II−/− mice (P < .001) showed no or significantly less parasitemia.
Systemic Adaptive Immune Response Within 24 h After Intradermal Infection
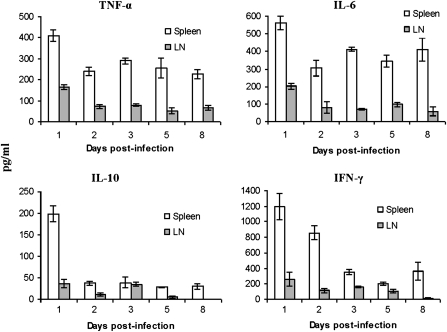
BALB/c mice were infected intradermally in a hind footpad with 102 T. congolense TC13. On days 1, 2, 3, 5, and 8 after infection, spleen cells and popliteal lymph node cells were cultured with or without lysates of T. congolense TC13 (equivalent to105 parasites/well) for 48 h. Cytokines (TNF-α, interleukin (IL) 6, IL-10, and interferon (IFN) γ) in the supernatants were determined by enzyme-linked immunosorbent assay (Figure 5). Significant trypanosome-specific production of IL-10 and IFN-γ were obtained with spleen cells collected at 24 h after intradermal infections. Thus, these observations show that priming of trypanosome-specific T cells in the spleen occurred as early as 24 h after intradermal infection.
Figure 5.
Kinetics of (parasite-specific) cytokine production by spleen cells and draining lymph node cells of BALB/c mice infected intradermally with a low dose of Trypanosoma congolense. Forty BALB/c mice were infected intradermally in a hind footpad with 102 T. congolense, clone TC13. At each time point (days 1, 2, 3, 5, and 8), 8 mice were euthanized. Spleen cells (Spleen) and popliteal lymph node cells (LN) of two mice were pooled. Four sets of pooled cells were cultured with/without TC13 lysates (equivalent to 105 parasites/well) for 48 h. Supernatants were collected and stored at −80°C until use. Concentrations of the cytokines were determined by enzyme-linked immunosorbent assay. Data represent means ± standard errors of the 4 sets of pooled cells at each time point. Cytokine values of cultures with antigen are shown. Cytokine values in cultures without antigen were negligible (not shown). Cytokine values in supernatants of cell cultures of spleens and popliteal lymph nodes of normal mice, cultured with or without antigen, also were negligible (not shown).
Intradermal Infections With 102 T. congolense Render BALB/c and C57BL/6 Mice More Susceptible to a Subsequent Intradermal Challenge With a Different Variant of T. congolense
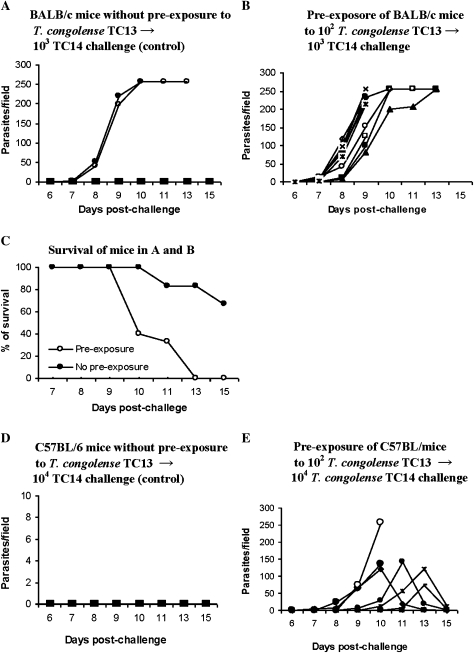
We considered that primary intradermal infections with low doses of trypanosomes might, after a certain lapse of time, induce a measure of protection against a subsequent challenge. Thus, both BALB/c and C57BL/6 mice were infected intradermally in a hind footpad with 102 T. congolense, clone TC13, 3 times, at weekly intervals. These mice did not develop any detectable parasitemia during this period of low-dose exposure. Ten days after the last infection, control (Figure 6A, D) and preexposed mice (Figure 6B, E) were challenged (103 and 104 TC14 for BALB/c and C57BL/6, respectively) intradermally in a hind footpad with T. congolense TC14. Parasitemia (Figure 6A, B, D, E) and survival time (Figure 6C) were monitored. To our great surprise, the preexposed mice developed a significantly higher incidence of parasitemia than the control mice (P < .05). The preexposed BALB/c mice had a significantly lower survival time (P < .05). All preexposed BALB/c mice died by day 13, whereas the control mice survived until day 30, when the experiment was terminated (Figure 6B). All of the preexposed C57BL/6 mice (Figure 6E) but none of the control mice (Figure 6D) developed parasitemia. Thus, the observations indicate that induction of a particular immune response on primary intradermal infection leads to enhanced susceptibility to intradermal reinfection, which is not variant specific.
Figure 6.
Intradermal infection with 102 Trypanosoma congolense, clone TC13, renders mice more susceptible to an intradermal reinfection with T. congolense, clone TC14. Mice were infected intradermally in a hind footpad with 102 T. congolense, clone TC13, 3 times at weekly intervals. These mice did not develop any detectable parasitemia during this period of low-dose exposure. Ten days after the last infection, the preexposed mice and unexposed control mice were infected intradermally in a hind footpad with T. congolense, clone TC14. Parasitemia and survival time (in BALB/c mice) was monitored after infection with T. congolense, clone TC14. A, 6 control BALB/c mice were infected intradermally in a hind footpad with 103 T. congolense TC14. B, 10 BALB/c mice were preexposed to 102 T. congolense TC13 and challenged with 103 T. congolense TC14. C, Survival times in control and preexposed mice challenged with 103 T. congolense TC14; the survival time in the preexposed mice was significantly (P < .02) shorter than that in the control mice. D, C57BL/6 control mice were infected with 104 T. congolense, TC14. E, Eight C57BL/6 mice preexposed to 102 T. congolense TC13 and challenged with 104 T. congolense TC14. *
Intradermal Injections of Lysates of T. congolense or T. brucei and Susceptibility to Subsequent Intradermal Challenges by T. congolense or T. brucei
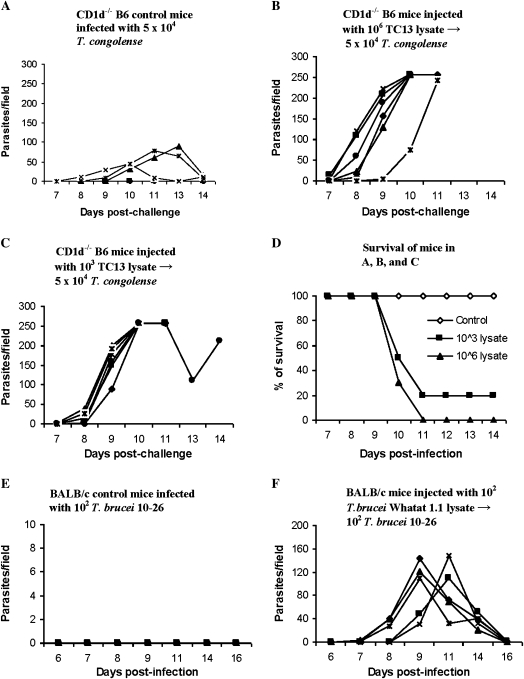
Because intradermal infections with low numbers of trypanosomes made the mice more susceptible to intradermal challenges, we wanted to know whether intradermal injections with killed trypanosomes might yield similar results. We chose to inject CD1d−/− mice rather than wild-type mice because CD1d−/− mice proved to be more resistant than wild-type mice to primary infections (Figure 4). CD1d−/− B6 mice were injected intradermally in a hind footpad with lysates of T. congolense, clone TC13, equivalent to 106 (Figure 7B) or 103 parasites (Figure 7C). Six weeks later, they were challenged intradermally in the footpad with 5 × 104 T. congolense, clone TC14. All unprimed control mice developed a moderate level of parasitemia (Figure 7A) and survived until day 30, when the experiment was terminated. All mice preexposed to lysate developed significantly higher parasitemia (Figure 7 A, B, C) (P < .05) and had significantly lower survival time than the control mice (Figure 7D) (P < .01).
Figure 7.
Intradermal preexposure of mice to lysates of Trypanosoma congolense or Trypanosoma brucei enhance susceptibility to a subsequent, intradermal T. congolense or T. brucei challenge. A–E, Groups of 4–6 CD1d−/− B6 mice were injected intradermally in a hind footpad with a single dose of a lysate of T. congolense, clone TC13. Six weeks later, these mice as well as a group of control mice were challenged with 5 × 104 T. congolense, clone TC14, intradermally in the footpad. Parasitemia and survival time were monitored. A, Control CD1d−/− mice infected with T. congolense, TC14. B, CD1d−/− mice preexposed to lysates of T. congolense, clone TC13 equivalent to 106 parasites and infected by T. congolense, TC14. C, CD1d−/− mice preexposed to lysates of T. congolense, clone TC13 equivalent to 103 parasites and infected by T. congolense, TC14. D, Survival of control and preexposed mice after challenge with 5 × 104 T. congolense, TC14. Survival in the preexposed mice was significantly (P < .005) shorter than that in the controls. E, Five control BALB/c mice were infected intradermally with 102 parasites of moderately virulent T. brucei, strain 10–26 (control). F, Five BALB/c mice were injected intradermally with parasite lysates (equivalent to 102 live parasites/mouse) of highly virulent T. brucei strain Whatat 1.1. Six months after this injection, they, together with the control mice (E), were infected intradermally with 102 T. brucei, strain 10–26. Parasitemia was monitored after infection.
BALB/c mice were also intradermally injected with lysate of highly virulent T. brucei brucei, strain Whatat 1.1, equivalent to 102 parasites. Six months later, the preexposed and control mice were intradermally infected with 102 T. brucei, strain 10–26. Control mice developed neither parasitemia nor disease by day 30, when the experiment was terminated (Figure 7E). All mice previously injected with the T. brucei lysate, however, developed parasitemia (Figure 7F) and died by day 30 (not shown). These results indicate that mice preexposed intradermally to a single dose of lysate of either T. congolense or T. brucei are rendered more susceptible to challenge by the respective trypanosomal species.
DISCUSSION
Experimental infections of mice with African trypanosomes have provided a wealth of information on the immunobiology of infections [4, 8–10, 22–25]. For no explicit reason, however, infections were usually carried out via the intraperitoneal route. Natural transmissions, by the bites of infected tsetse flies, result in dermal infections, which subsequently lead, via the lymph system, to infections of the blood. To our knowledge, there is only one published report on quantitative experiments of intradermal infections with African trypanosomes [20]. The authors found that low numbers of metacyclic T. brucei rhodensiense (<3.5–4 × 102 parasites) injected intradermally by tsetse fly bites did not establish an infection of the blood [20].
We have begun to examine how the change in route of infection might affect the conclusions drawn [30, 35, 36]. As reported in this study, we found a much higher susceptibility to experimental infections via the intraperitoneal route than via the intradermal route (Figure 1). The immunological environment in the peritoneal cavity is obviously profoundly different than in the dermis. Regarding the intraperitoneal versus intradermal routes, differences in the outcomes of other infections [37] or immunizations [38] have been reported.
Intraperitoneal infections of mice with either T. brucei [10, 31] or T. congolense [18, 23] lead to infection of the blood and absolutely require antibodies to VSG for the control of parasitemia. The surprising observation that B cell–deficient (μMT) as well as RAG2−/− mice were as resistant as wild-type mice to intradermal infection with low numbers of T. brucei or T. congolense (Figure 2) demonstrates that this resistance is due to innate mechanisms. We conclude that B cells, anti-VSG antibodies, and T cells are not required for this resistance. The enhanced susceptibility of iNOS−/− mice (Figure 3) and mice treated with anti–TNF-α antibody (Figure 3) to intradermal infections by low numbers of T. congolense indicates that induced NO as well as TNF-α contribute to this resistance. It has been reported elsewhere that NO produced by macrophages is detrimental to T. brucei [33] as well as to T. congolense [32]. There is also evidence that induced NO contributes to the control of the blood-stage forms of T. congolense [23].
What processes might lead to innate resistance to intradermal infections by low numbers of trypanosomes? It has been shown that T. brucei [39] and T. congolense [40] isolated from infected blood can have cleavage products of complement component C3 on their surface. T. brucei [41, 42] as well as T. congolense [43] have been observed to form filopodia. Immune complexes of anti-VSG antibody and complement have been shown to be shed via filopodia [43], which, in turn, can be taken up by macrophages [44]. Thus, it is conceivable that trypanosomes with iC3b on their surface will temporarily attach to macrophages via CR3 (CD11b/CD18) [17]. It might be possible that, even in the absence of anti-VSG antibodies, a certain threshold number of iC3b-CR3 interactions might induce formation of filopodia. We speculate that these filopodia might separate from trypanosomes and then are engulfed by macrophages, without harming the trypanosomes involved. This process might activate these macrophages to synthesize TNF-α and induce them to synthesize and secrete NO. Macrophage-derived TNF-α might, in an autocrine fashion, enhance synthesis of NO by the macrophages [34]. We suggest that all trypanosomes that subsequently attach to these activated macrophages might be killed by NO secreted by the activated macrophages.
The fact that CD1d−/− as well as MHC class II−/− mice are more resistant to primary intradermal infections (Figure 4) indicates that cells of the adaptive immune system act early to suppress protective responses. Moreover, local intradermal infection by few trypanosomes results in a very rapid systemic immune response, as shown by the priming for a trypanosome-specific cytokine response in the spleen within 24 h after intradermal infection (Figure 5). These results are in line with our previous observations [30], which led to a hypothesis of cross-regulation of natural killer T cells and CD4+ T cells after intradermal infection by T. congolense [36]. Primary intradermal infections by few African trypanosomes, associated with innate resistance, not only failed to generate a long-term protective immunological state but also resulted in enhanced susceptibility to intradermal challenges (Figure 6). Even more surprisingly, intradermal injection with a single dose of killed T. congolense or T. brucei could induce a suppressive immune response as demonstrated by enhanced susceptibility to intradermal challenge by T. congolense or T. brucei (Figure 7). These findings are novel.
The mechanisms underlying these observations are presently unknown. A complete picture of the trypanosome-induced immunosuppression has not yet emerged [8, 10, 12, 22]. In an extensive investigation into immunization studies against malaria, covering a period from 1965 to 2010, Guilbride et al [45] came to the conclusion that Plasmodium sporozoites induce a skin-stage–initiated immunosuppression inhibiting vaccine function. We see here a parallel to our results with intradermal infections by African trypanosomes. Intradermal vaccination against rabies, however, has been shown to be as safe and immunogenic as intramuscular vaccination [46]. Thus, there does not appear to be an intrinsic property of the skin that necessarily leads to immunosuppression when a pathogen enters. We propose that pathogenic Plasmodium and Trypanosoma share parameters, absent in rabies virus, that induce skin-stage–initiated immunosuppression.
Our observations have changed our perspective on whether an effective vaccination procedure might be realizable. There is a widely held concept that antigenic variation of the surface glycoprotein of African trypanosomes is the major hurdle for producing a vaccine [47–49]. On the basis of our present results, we do not share this view. We consider immunosuppression, induced by the trypanosomal infection, the major obstacle for producing a vaccine. Thus, the major question for the production of a potential vaccine is how one can most effectively prevent the induction of immunosuppression by the trypanosomes. To answer this question, it is crucial to clearly identify the mechanisms of immunosuppression induced by intradermal injections of trypanosomal lysates. In fact, we consider this line of investigation a path to developing a vaccination strategy against infections by African trypanosomes.
Funding
This work was supported by grants from the Canadian Health Research Institute (grant number 85167), the Regional Partnership Program, Saskatchewan Health Research Foundation (grant number ROP-85167), and the University of Saskatchewan: Bridge Funding from AVPR-Health and WCVM.
Acknowledgments
The authors thank Peter Bretscher for critical comments on the manuscript.
References
- 1.Welburn SC, Coleman PG, Maudlin I, Fevre EM, Odiit M, Eisler MC. Crisis, what crisis? Control of Rhodesian sleeping sickness. Trends Parasitol. 2006;22:123–8. doi: 10.1016/j.pt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Barrett MP, Burchmore RJ, Stich A, et al. The trypanosomiases. Lancet. 2003;362:1469–80. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan HW, Potts WH. The African trypanosomiases. New York: Wiley-INTERSCIENCES; 1970. [Google Scholar]
- 4.Tabel H, Kaushik RS, Uzonna JE. Susceptibility and resistance to Trypanosoma congolense infections. Microbes Infect. 2000;2:1619–29. doi: 10.1016/s1286-4579(00)01318-6. [DOI] [PubMed] [Google Scholar]
- 5.Barry JD, McCulloch R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol. 2001;49:1–70. doi: 10.1016/s0065-308x(01)49037-3. [DOI] [PubMed] [Google Scholar]
- 6.Borst P, Rudenko G, Blundell PA, et al. Mechanisms of antigenic variation in African trypanosomes. Behring Inst Mitt. 1997;18:1–15. [PubMed] [Google Scholar]
- 7.Cross GA. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 8.Askonas BA. Macrophages as mediators of immunosuppression in murine African trypanosomiasis. Curr Top Microbiol Immunol. 1985;117:119–27. doi: 10.1007/978-3-642-70538-0_6. [DOI] [PubMed] [Google Scholar]
- 9.Hudson KM, Byner C, Freeman J, Terry RJ. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976;264:256–8. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- 10.Roelants GE, Pinder M. Immunobiology of African trypanosomiasis. Contemp Top Immunobiol. 1984;12:225–74. doi: 10.1007/978-1-4684-4571-8_7. [DOI] [PubMed] [Google Scholar]
- 11.Sacks DL, Selkirk M, Ogilvie BM, Askonas BA. Intrinsic immunosuppressive activity of different trypanosome strains varies with parasite virulence. Nature. 1980;283:476–8. doi: 10.1038/283476a0. [DOI] [PubMed] [Google Scholar]
- 12.Uzonna JE, Kaushik RS, Zhang Y, Gordon JR, Tabel H. Experimental murine Trypanosoma congolense infections. II. Role of splenic adherent CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4+8- and CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4-8- cells in the production of IL-4, IL-10, and IFN-gamma and in trypanosome-elicited immunosuppression. J Immunol. 1998;161:6189–97. [PubMed] [Google Scholar]
- 13.Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 14.Gerold P, Striepen B, Reitter B, et al. Glycosyl-phosphatidylinositols of Trypanosoma congolense: two common precursors but a new protein-anchor. J Mol Biol. 1996;261:181–94. doi: 10.1006/jmbi.1996.0451. [DOI] [PubMed] [Google Scholar]
- 15.Gray AR, Luckins AG. The initial stage of infection with cyclically-transmitted Trypanosoma congolense in rabbits, calves and sheep. J Comp Pathol. 1980;90:499–512. doi: 10.1016/0021-9975(80)90099-7. [DOI] [PubMed] [Google Scholar]
- 16.Macaskill JA, Holmes PH, Whitelaw DD, McConnell I, Jennings FW, Urquhart GM. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology. 1980;40:629–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W, Ogunremi O, Wei G, Shi M, Tabel H. CR3 (CD11b/CD18) is the major macrophage receptor for IgM antibody-mediated phagocytosis of African trypanosomes: diverse effect on subsequent synthesis of tumor necrosis factor alpha and nitric oxide. Microbes Infect. 2006;8:1209–18. doi: 10.1016/j.micinf.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Shi M, Wei G, Pan W, Tabel H. Trypanosoma congolense infections: antibody-mediated phagocytosis by Kupffer cells. J Leukoc Biol. 2004;76:399–405. doi: 10.1189/jlb.1003500. [DOI] [PubMed] [Google Scholar]
- 19.Basic biology and anatomy of the tsetse fly. ftp://ftp.fao.org/docrep/fao/011/i0535e/i0535e01.pdf. Accessed 26 January 2009. [Google Scholar]
- 20.Fairbairn H, Burtt E. The infectivity to man of a strain of Trypanosoma rhodesiense transmitted cyclically by Glossina morsitans through sheep and antelope: evidence that man requires a minimum infective dose of metacyclic trypanosomes. Ann Trop Med Parasitol. 1946;40:270–313. doi: 10.1080/00034983.1946.11685286. [DOI] [PubMed] [Google Scholar]
- 21.Barry JD, Emery DL. Parasite development and host responses during the establishment of Trypanosoma brucei infection transmitted by tsetse fly. Parasitology. 1984;88:67–84. doi: 10.1017/s0031182000054354. [DOI] [PubMed] [Google Scholar]
- 22.Beschin A, Brys L, Magez S, Radwanska M, De Baetselier P. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J Leukoc Biol. 1998;63:429–39. doi: 10.1002/jlb.63.4.429. [DOI] [PubMed] [Google Scholar]
- 23.Magez S, Radwanska M, Drennan M, et al. Interferon-gamma and nitric oxide in combination with antibodies are key protective host immune factors during Trypanosoma congolense Tc13 infections. J Infect Dis. 2006;193:1575–83. doi: 10.1086/503808. [DOI] [PubMed] [Google Scholar]
- 24.Mansfield JM, Paulnock DM. Regulation of innate and acquired immunity in African trypanosomiasis. Parasite Immunol. 2005;27:361–71. doi: 10.1111/j.1365-3024.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 25.Dagenais TR, Freeman BE, Demick KP, Paulnock DM, Mansfield JM. Processing and presentation of variant surface glycoprotein molecules to T cells in African trypanosomiasis. J Immunol. 2009;183:3344–55. doi: 10.4049/jimmunol.0802005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabel H. Activation of the alternative pathway of bovine complement by Trypanosoma congolense. Parasite Immunol. 1982;4:329–35. doi: 10.1111/j.1365-3024.1982.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 27.Otesile EB, Tabel H. Enhanced resistance of highly susceptible Balb/c mice to infection with Trypanosoma congolense after infection and cure. J Parasitol. 1987;73:947–53. [PubMed] [Google Scholar]
- 28.Lanham SM, Godfrey DG. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28:521–34. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 29.Shi M, Pan W, Tabel H. Experimental African trypanosomiasis: IFN-gamma mediates early mortality. Eur J Immunol. 2003;33:108–18. doi: 10.1002/immu.200390013. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Tabel H. Regulatory T cells prevent control of experimental African trypanosomiasis. J Immunol. 2008;180:2514–21. doi: 10.4049/jimmunol.180.4.2514. [DOI] [PubMed] [Google Scholar]
- 31.Campbell GH, Esser KM, Weinbaum FI. Trypanosoma rhodesiense infection in B-cell-deficient mice. Infect Immun. 1977;18:434–8. doi: 10.1128/iai.18.2.434-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaushik RS, Uzonna JE, Gordon JR, Tabel H. Innate resistance to Trypanosoma congolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57Bl/6 mice. Exp Parasitol. 1999;92:131–43. doi: 10.1006/expr.1999.4408. [DOI] [PubMed] [Google Scholar]
- 33.Vincendeau P, Daulouede S, Veyret B, Darde ML, Bouteille B, Lemesre JL. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol. 1992;75:353–60. doi: 10.1016/0014-4894(92)90220-5. [DOI] [PubMed] [Google Scholar]
- 34.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Marcoux V, Wei G, Tabel H, Bull HJ. Characterization of major surface protease homologues of Trypanosoma congolense. J Biomed Biotechnol. 2010;2010:418157. doi: 10.1155/2010/418157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabel H, Wei G, Shi M. T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev. 2008;225:128–39. doi: 10.1111/j.1600-065X.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. 2006;74:4856–64. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478–82. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devine DV, Falk RJ, Balber AE. Restriction of the alternative pathway of human complement by intact Trypanosoma brucei subsp. gambiense. Infect Immun. 1986;52:223–9. doi: 10.1128/iai.52.1.223-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diffley P, Honigberg BM. Immunologic analysis of host plasma proteins on bloodstream forms of African pathogenic trypanosomes. II. Identification and quantitation of surface-bound albumin, nonspecific IgG, and complement on Trypanosoma congolense. J Parasitol. 1978;64:674–81. [PubMed] [Google Scholar]
- 41.Macadam RF, Herbert WJ. Fine structural demonstration of cytoplasmic protrusions (filopodia) in trypanosomes. Exp Parasitol. 1970;27:1–8. doi: 10.1016/s0014-4894(70)80002-9. [DOI] [PubMed] [Google Scholar]
- 42.Wright KA, Lumsden WH, Hales H. The formation of filopodium-like processes by Trypanosoma (Trypanozoon) brucei. J Cell Sci. 1970;6:285–97. doi: 10.1242/jcs.6.1.285. [DOI] [PubMed] [Google Scholar]
- 43.Frevert U, Reinwald E. Trypanosoma congolense bloodstream forms evade complement lysis in vitro by shedding of immune complexes. Eur J Cell Biol. 1990;52:264–9. [PubMed] [Google Scholar]
- 44.Shakibaei M, Frevert U. Cell surface interactions between Trypanosoma congolense and macrophages during phagocytosis in vitro. J Protozool. 1992;39:224–35. doi: 10.1111/j.1550-7408.1992.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 45.Guilbride DL, Gawlinski P, Guilbride PD. Why functional pre-erythrocytic and bloodstage malaria vaccines fail: a meta-analysis of fully protective immunizations and novel immunological model. PLoS One. 2010;5:e10685. doi: 10.1371/journal.pone.0010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. Rabies. http://www.who.int/immunization/topics/rabies/en/index.html. Accessed 6 August 2010. [Google Scholar]
- 47.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2009;375:148–59. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- 48.Gray AR. Immunological research and the problem of immunization against African trypanosomiasis. Trans R Soc Trop Med Hyg. 1976;70:119–21. doi: 10.1016/0035-9203(76)90167-x. [DOI] [PubMed] [Google Scholar]
- 49.Stuart K, Brun R, Croft S, et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–10. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]