Abstract
West Nile virus (WNV) causes an acute infection that is usually cleared by an effective immune response after several days of viremia. However, a recent study detected WNV RNA in the urine of 5 of 25 persons (20%) tested several years after their initial acute WNV disease. We evaluated an established cohort of 40 persons >6 years after initial infection with WNV. Urine collected from all participants tested negative for WNV RNA by reverse-transcription polymerase chain reaction and transcription-mediated amplification. Prospective studies are needed to determine if and for how long WNV persists in urine following WNV disease.
West Nile virus (WNV), a mosquito-borne flavivirus, was first detected in the United States in 1999. Since then, it has become the leading cause of arboviral disease in the United States. Approximately 80% of human WNV infections are asymptomatic. Most persons with symptoms experience an acute undifferentiated febrile illness. Less than 1% develop WNV neuroinvasive disease (eg, meningitis, encephalitis, or acute flaccid paralysis), which has a case fatality rate of ∼10% [1, 2].
WNV causes an acute infection that is thought to be cleared by an effective immune response after several days of viremia. Although the virus can persist for weeks to months in some experimentally infected animals [3–6], there is little evidence of chronic WNV infection in humans. Although damage caused by acute disease can result in prolonged symptoms and devastating sequelae, there has been only 1 report of human disease associated with persistent WNV infection in a severely immunocompromised patient [7]. Similarly, whereas WNV has been isolated from urine of experimentally infected hamsters [4–6], there have been few reports of WNV identified in human urine. In 1 case report, WNV RNA was detected in the urine of a 65-year-old man with WNV encephalitis on day 8 of illness; WNV RNA was not detected on subsequent days, and viral culture was negative [8]. However, in a recent study, WNV RNA was identified in the urine of 5 of 25 patients (20%) who had acute WNV disease 1–7 years earlier, including in 3 patients tested >6 years after their acute illness [9]. The virus could not be cultured from any of the patients. These results suggested that WNV RNA persists in the urine of some people for years after initial infection. If persistence of RNA is due to persistent live virus, this finding could have important implications regarding potential transmission of WNV through organ transplantation, as well as for long-term clinical evaluation and management of patients after acute WNV infection. We evaluated an established cohort of patients for evidence of persistent WNV RNA in the urine at >6 years after initial acute WNV disease.
METHODS
A cohort of 64 people hospitalized with laboratory-confirmed WNV disease in Colorado in 2003 has been followed up prospectively to assess the long-term effects of WNV disease [10]. Subjects enrolled in this cohort study were invited to participate in another follow-up in 2010. The study was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board. Each participant provided informed consent prior to enrollment.
At the 2010 visit, participants completed a survey about current subjective symptoms (headache, fatigue, or memory or concentration problems) and chronic medical conditions, underwent neurological examination, and had blood and urine collected. Serum was tested for WNV immunoglobulin M (IgM) antibodies by microsphere immunoassay [11]. Impaired renal function was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, obtained using the Modification of Diet in Renal Disease Study Group (MDRD) calculation [12].
Testing Participant Urine Samples for West Nile Virus RNA
Immediately following collection, clean catch urine specimens were frozen on dry ice and transported at the end of each study day to the CDC Arboviral Diseases Branch where they were stored at −20°C. Specimens were thawed within 2 weeks of collection and processed immediately. We extracted total RNA from 5 mL of each sample using the QIAGEN QIAamp Circulating Nucleic Acid kit, eluted in 150 μL of water, and 15 μL tested by reverse-transcription polymerase chain reaction (RT-PCR). We tested each urine specimen in duplicate using 2 previously published primer/probe sets located at 2 unique regions of the WNV genome (env gene and NS5 gene) [13]. The sensitivity of the assay is estimated to be [13] .1 plaque-forming units (pfu)/mL. In addition, we added an internal RNA control (Dengue virus type 2) to each urine specimen to assess for potential inhibitory components. Participant urine specimens that had been frozen, thawed, and stored at 4°C for 4 weeks were sent to Gen-Probe for testing using a transcription-mediated amplification (TMA) assay [14]. This assay has a 95% detection limit of 9.8 copies/mL for lineage 1 WNV in blood and uses a built-in control to identify any failure to amplify RNA due to inhibitory components in the specimen.
Evaluating for Degradation of West Nile Virus RNA in Participant Urine
We added WNV standards with known concentrations of WNV RNA to aliquots of urine specimens from 7 randomly selected participants and to buffered tissue culture (control) medium. These specimens were either tested within 5 min of preparation (without freezing) or frozen at −20°C, thawed, and tested. We ran the RT-PCR tests in triplicate and presented results as cycle threshold (Ct) values. We adjusted the Ct value of each participant by subtracting the Ct value of the control medium. The adjusted Ct values of participants were then modeled, using a linear mixed-effect model, as a function of whether the specimen was frozen.
Evaluating for Inhibition and Degradation of West Nile Virus RNA Using Negative Control Urine
To further examine for inhibitory components in urine, we added WNV standards to aliquots of negative control urine collected from a single WNV naïve donor and to control medium to the following final concentrations: 3240 pfu/mL, 324 pfu/mL, 32.4 pfu/mL, 16.2 pfu/mL, 5.4 pfu/mL, and 1.8 pfu/mL. We tested these urine specimens by RT-PCR prior to freezing, and we also tested urine and control medium specimens after freezing at −20°C. To look for degradation of RNA, we retested the thawed urine specimens by RT-PCR after being stored at 4°C for 14 d and by TMA after being stored at 4°C for 4 weeks. We constucted a linear fixed-effect model using log concentration of WNV standard added, specimen type, and specimen handling as predictors of Ct values.
RESULTS
In 2010, 40 persons who were hospitalized with WNV disease in 2003 participated in this follow-up study. The median time between onset of acute WNV disease and the 2010 evaluation was 6 years 7 months (range, 6 years 6 months to 6 years 8 months). The median age at follow-up was 58 years (range, 24–82 years), and 22 of 40 participants (55%) were female (Table 1). WNV neuroinvasive disease was documented in 31 of 40 participants (78%) in 2003. Of the 40 participants in the 2010 evaluation, 25 (63%) reported current symptoms, and 21 (53%) had an objective neurological deficit identified on examination. In 2010, 16 of 40 participants reported a diagnosis of hypertension (40%; 9 preexisting before WNV disease), 7 of diabetes mellitus (18%; 4 preexisting), and 3 of kidney dysfunction (8%; 1 preexisting). In 2010, 3 participants declined blood draw and specimens were insufficient for creatinine testing for another 2 participants. Impaired renal function was detected in 5 of 35 participants tested (14%), of whom 5 (100%) had diagnoses of hypertension, 4 of diabetes (80%); and 2 of kidney dysfunction (40%). Anti-WNV IgM antibodies were detected in 1 (3%) of 37 participants tested.
Table 1.
Characteristics of Participants >6 Years Following Hospitalization With West Nile Virus (WNV) Disease (N = 40)
| Characteristic | No. | (%) | |
| Sex | |||
| Female | 22 | (55) | |
| Male | 18 | (45) | |
| Age at 2010 evaluation, years | |||
| 0–19 | 0 | (0) | |
| 20–39 | 4 | (10) | |
| 40–59 | 17 | (43) | |
| 60–79 | 15 | (38) | |
| ≥80 | 4 | (10) | |
| Clinical syndrome with acute WNV disease | |||
| Meningitis | 13 | (33) | |
| Encephalitis | 9 | (23) | |
| Acute flaccid paralysis | 9 | (23) | |
| Fever | 9 | (23) | |
| Admission to intensive care unit with acute WNV disease | |||
| Yes | 5 | (13) | |
| No | 35 | (88) | |
| Current symptoms at 2010 evaluation | |||
| Fatigue | 22 | (55) | |
| Problems with memory or concentration | 17 | (43) | |
| Headache | 11 | (28) | |
| None of the above | 15 | (38) | |
| Neurological examination findings at 2010 evaluation | |||
| Normal examination | 16 | (40) | |
| Decreased or absent limb reflexes | 13 | (33) | |
| Tremor in upper limb | 9 | (23) | |
| Muscle atrophy | 9 | (23) | |
| Flaccid paralysis | 8 | (20) | |
| Gait abnormality | 8 | (20) | |
| Not examined | 3 | (8) | |
| Medical conditions present at 2010 evaluation | |||
| Hypertension | 16 | (40) | |
| Diabetes mellitus | 7 | (18) | |
| Kidney dysfunction | 3 | (8) | |
| None of the above | 23 | (58) | |
Testing Participant Urine Samples for West Nile Virus RNA
Urine collected from all 40 participants tested negative for WNV RNA by RT-PCR and TMA (95% confidence interval [CI], 0%–7.2%). Testing of participants’ urine samples following addition of the dengue virus RNA control yielded positive results for every participant.
Evaluating for Degradation of West Nile Virus RNA in Participant Urine
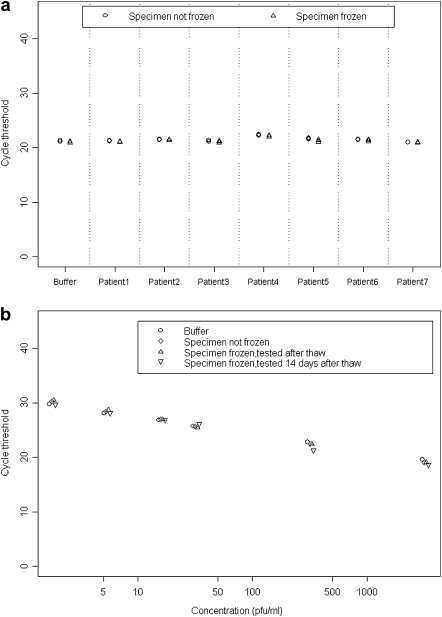
Following addition of WNV standards, the mean difference detected between Ct values for the urine specimens of 7 randomly selected participants and the control medium was .30 (95% CI, −.02 to .61; P = .06) (Figure 1a). Furthermore, no statistically significant difference was detected between mean adjusted Ct values in urine specimens that were not frozen compared with those that were frozen (mean difference, 0; 95% CI, −.11 to .12; P = .89).
Figure 1.
Reverse-transcription polymerase chain reaction (RT-PCR) cycle threshold (Ct) values for detection of West Nile virus (WNV) RNA following addition of WNV standards to buffered tissue culture medium, participant urine, and negative control urine. Lower Ct values indicate a greater concentration of WNV RNA in the specimen. A, Ct values following addition of a single concentration of WNV standard to buffered tissue culture medium (buffer) and participant urine (patients 1–7). RT-PCR tests were run in triplicate on specimens that were not frozen and specimens that were frozen at −20°C. B, Ct values following addition of 6 concentrations of WNV standard (3240 plaque-forming units [pfu]/mL, 324 pfu/mL, 32.4 pfu/mL, 16.2 pfu/mL, 5.4 pfu/mL, and 1.8 pfu/mL) to buffered tissue-culture medium (buffer) and negative control urine. Buffered medium specimens were frozen at −20°C and tested immediately upon thawing. Urine specimens were tested without being frozen and immediately upon thawing after freezing at −20°C. Urine specimens were also tested using a separate RT-PCR run after being frozen at −20°C, thawed, and stored at 4°C for 14 d.
Evaluating for Inhibition and Degradation of West Nile Virus RNA Using Negative Control Urine
At each concentration of WNV standard added, we detected no difference in Ct values between the control medium and urine specimens, even after the urine specimens were frozen, thawed, and stored at 4°C for 14 d prior to testing (P = .12) (Figure 1b). Furthermore, we detected WNV RNA by TMA in all spiked control urine specimens after the samples were frozen, thawed, and stored at 4°C for 4 weeks prior to retesting.
DISCUSSION
WNV RNA was not detected in urine of any of the 40 persons tested >6 years following initial acute WNV disease. This contrasts with a recent study in which WNV RNA was detected in the urine of 5 of 25 patients (20%) at 1–7 years after initial acute WNV disease [9]. Although we cannot rule out persistence of WNV RNA in urine in some people for years following WNV disease, our results indicate this does not occur as frequently as previously suggested [9].
In the study by Murray et al [9], all 5 patients in whose urine WNV RNA was detected were male, had previous WNV encephalitis, and had preexisting hypertension; 4 reported persistent symptoms attributed to WNV infection, and 1 developed renal failure following his acute illness. No information was provided regarding age, WNV clinical syndrome, comorbidities, or chronic symptoms for the 20 patients in whose urine WNV RNA was not detected. Therefore, conclusions cannot be made about the potential relationship between WNV viruria and preexisting conditions, development of renal disease, or chronic symptoms. Furthermore, in our cohort, hypertension, diabetes, and kidney dysfunction were identified both before and after onset of WNV disease, and many participants also reported current symptoms, suggesting that these conditions were not necessarily related to WNV infection.
Although only 1 study has reported persistence of WNV RNA in human urine [9], several studies indicate WNV persistence in animals [3–6]; however, the relevance of these animal data to human infections is unknown. In 2 studies, WNV was cultured from urine in 56%–60% of hamsters tested ≤ 8 months after inoculation with WNV [5, 6]. Persistent WNV infection has been associated with neurological sequelae in hamsters [4]. In monkeys, WNV has been cultured from the central nervous system (CNS) in 7 of 18 tested (39%) at days 38–167 after inoculation [3]. In humans, WNV RNA has been documented by TMA in 4 of 175 asymptomatic viremic blood donors (2%) tested >40 d after index donation, although RNA concentrations were low and detection in 2 donors was intermittent [15]. There has been 1 case report of persistent WNV infection resulting in fatal encephalitis in a severely immunocompromised patient with lymphoma, in whom WNV RNA was detected, by RT-PCR, in the CNS at autopsy 99 d after hospital admission [7].
Several possible explanations for the different results between our study and those of Murray et al [9] exist. First, the cohorts may have undefined host factors that result in a different propensity to persistent WNV infection. Second, WNV persistence may be time-limited. In the Murray et al [9] study, the interval between acute WNV disease and WNV RNA being detected in the urine was 6–7 years for 3 of the 5 patients (60%) and 1–5 years for the remaining 2 patients; all participants in our study were 6–7 years from initial acute WNV disease. Third, shedding of WNV RNA may be intermittent, which would require repeated serial sampling of individuals to demonstrate a positive result. Fourth, testing procedures may have led to inhibition or degradation of WNV RNA in our urine specimens. For example, in the study by Murray et al [9], urine specimens were not frozen. However, our data suggest minimal possibility of inhibition or degradation of any WNV RNA present in the urine specimens and show that WNV particles are stable in urine. Fifth, the sensitivity of the assays used may be insufficient to detect a low level of circulating RNA in urine, but these assays are known to be very sensitive for detection of WNV RNA in serum and cerebrospinal fluid, and testing of control urine samples with WNV standards indicate adequate sensitivity in urine [13, 14]. Finally, small sample size may have precluded positive findings.
Although prior hamster data and a recent study in humans have shown persistent WNV RNA in urine, WNV RNA was not detected in urine in any of our 40 cohort members tested >6 years following initial WNV disease. The significance of detection of WNV RNA in urine remains unclear, and replication of these results is important. A prospective evaluation of persons from the time of WNV disease with serial testing of urine for WNV RNA would aid in this assessment. Further studies are needed to determine if and for how long WNV infection persists in humans and to evaluate the clinical relevance therein.
Funding
This work was supported by the the Centers for Disease Control and Prevention.
Acknowledgments
We thank the cohort participants for their contribution to this study, along with Amanda Panella and Olga Kosoy for assistance with serologic testing.
References
- 1. Centers for Disease Control and Prevention. Surveillance for West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ 2010; 59(SS-2):1–17. [Google Scholar]
- 2.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV A, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–9. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pogodina VV, Frolova MP, Malenko GV, et al. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75:71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- 4.Siddharthan V, Wang H, Motter NE, et al. Persistent West Nile virus associated with a neurological sequela in hamsters identified by motor unit number estimation. J Virol. 2009;83:4251–61. doi: 10.1128/JVI.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesh RB, Siirin M, Guzman H, et al. Persistent West Nile virus infection in the golden hamster: Studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287–95. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- 6.Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;72:320–4. [PubMed] [Google Scholar]
- 7.Penn RG, Guarner J, Sejvar JJ, et al. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42:680–3. doi: 10.1086/500216. [DOI] [PubMed] [Google Scholar]
- 8.Tonry JH, Brown CB, Cropp CB, et al. West Nile virus detection in urine. Emerg Infect Dis. 2005;11:1294–6. doi: 10.3201/eid1108.050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray K, Walker C, Herrington E, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sejvar JJ, Curns AT, Welburg L, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008;2:477–99. doi: 10.1348/174866407x218312. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AJ, Noga A, Kosoy O, Lanciotti RS, Johnson AAA, Biggerstaff BJ. Duplex microsphere-based immunoassay for detection of anti––West Nile virus and anti–St. Louis encephalitis virus immunoglobulin M antibodies. Clinic Diagn Lab Immunol. 2005;12:566–74. doi: 10.1128/CDLI.12.5.566-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D the Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lanciotti RS, Kerst AJ, Nasci RS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–71. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linnen JM, Deras ML, Cline J, et al. Performance evaluation of the PROCLEIX West Nile virus assay on semi-automated and automated systems. J Med Virol. 2007;79:1422–30. doi: 10.1002/jmv.20930. [DOI] [PubMed] [Google Scholar]
- 15.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–93. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]