Abstract
Background. Cell-mediated immune (CMI) responses to hepatitis C virus (HCV) antigens in adults without seroconversion or viremia are biomarkers for prior transient infection. We investigated HCV-specific CMI responses in seronegative children living with HCV-infected siblings.
Methods. Children 3–18 years of age living with HCV-infected siblings were screened for HCV antibodies and HCV RNA. Peripheral blood mononuclear cells (PBMCs) were evaluated for HCV-specific CMI responses by interferon γ (IFN-γ) enzyme-linked immunospot assay using 3 recombinant HCV protein antigens. Flow cytometry phenotypically characterized IFN-γ-secreting cells.
Results. Forty-five seronegative children and 5 seropositive viremic siblings had functionally viable PBMCs. Among the 45 seronegative siblings, 15 (33.3%) had positive HCV-specific IFN-γ responses, and subsequent RNA testing revealed that 3 were viremic. Compared with the 5 seropositive viremic children, the median number of HCV-specific spot-forming units was significantly higher in the 12 seronegative aviremic children (P = .002) and in the 3 seronegative viremic children (P = .025). Flow cytometric analysis revealed that IFN-γ was synthesized mainly by CD4+ T cells.
Conclusion. Strong HCV-specific CD4+ T cell responses were detectable in higher frequency among seronegative, aviremic children compared with persistently infected siblings. Further studies are needed to determine whether these immune responses are protective against HCV infection.
Infection with hepatitis C virus (HCV) can result in an HCV-specific cell-mediated immune (CMI) response in the absence of detectable HCV antibodies (anti-HCV) and RNA [1]. CMI responses to HCV antigens have been found in HCV-seronegative individuals who may have been exposed to the virus, including heath care workers [2], spouses [3], household contacts of persons with HCV infection [1, 4], children born to HCV-infected women [5, 6], prisoners [7], and injection drug users [8–10]. Low infective doses of HCV given to chimpanzees have resulted in induction of HCV-specific interferon γ (IFN-γ) synthesis in the absence of persistent infection as determined by enzyme-linked immunospot (ELISPOT) assay [11]. Our group previously reported on CMI responses to HCV antigens in anti-HCV-negative aviremic individuals [4] residing in a rural Egyptian community, where the prevalence of anti-HCV was 24% [12, 13]. Among individuals living with at least 2 anti-HCV household family members, 18% had positive HCV-specific IFN-γ responses, compared with only 2.9% of those living with no seropositive household members (P = .03) [4].
These findings suggest that an effective CMI response may accelerate clearance of HCV infection in the absence of persisting antibodies and that CMI response could be used as a surrogate marker for prior transient HCV infection. In the present study, we analyzed the HCV-specific CMI responses in seronegative children, and some of their chronically infected siblings, who lived in the same households and were being followed up in a natural history study of HCV infection in Egyptian children.
MATERIALS AND METHODS
Participants
Parents of known HCV-infected viremic children were invited to enroll their other uninfected children into this study to assess their HCV-specific CMI responses. Their HCV-infected viremic children were part of a natural history study of HCV infection in Egyptian children aged 1–9 years who were being followed up at the Cairo University Pediatric Hospital and the National Hepatology and Tropical Medicine Research Institute [14]. Siblings of these infected children who were 3–18 years of age were tested for anti-HCV. Only those who were seronegative, along with a group of their HCV-infected siblings, were further tested for HCV-specific CMI responses. Written informed consent from the parents, and an assent from the older children, was obtained. The institutional review board at the University of Maryland, Baltimore, and the relevant ethics review committee in Egypt approved the study protocol.
Serological Testing
Serum samples were collected in plain vacutainer tubes (BD) and tested for anti-HCV by a third-generation assay (Murex anti-HCV; version 4.0) according to the manufacturer's instructions. The assay utilizes antigens from the core, NS3, NS4, and NS5 regions of the HCV genome.
Virological Testing
Reverse-transcription polymerase chain reaction (RT-PCR) was performed on serum samples for detection and amplification of HCV RNA using an in-house nested RT-PCR assay developed at the Holding Company for Biological Products and Vaccines (VACSERA), Giza, Egypt. RT-PCR was performed on serum samples from anti-HCV-positive children to confirm persistent HCV infection and on serum samples from anti-HCV-negative children to determine whether underlying viremia was present in seronegative participants.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Approximately 5–8 mL of whole blood was drawn into an ethylenediaminetetraacetic acid vacutainer tube (BD), and PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation, as described elsewhere [4, 15].
Recombinant HCV Antigens
We used recombinant HCV antigens that were expressed as carboxy-terminal fusion proteins with human superoxide dismutase in yeast (Saccharomyces cerevisiae) or Escherichia coli (Chiron). These were derived from the HCV-1 sequence and encoded core C-22 protein (core; amino acids 2–120), C-100 protein (NS-3, NS4A, and NS4B; amino acids 1569–1931), and NS5 protein (NS5A and NS5B; amino acids 2054–2995).
IFN-γ ELISPOT Assay
The ELISPOT assay has the advantage of being able to specifically enumerate cells as well as quantify cytokine secretion patterns [16]. A modified mini-ELISPOT assay was developed in our laboratories in Cairo, Egypt, and Cincinnati, Ohio. In this assay, the mini-ELISPOT plates (Whatman Unifilter) used a lesser amount of blood (∼5–8 mL), which is appropriate for CMI studies in children. The sensitivity of our mini-ELISPOT assay is comparable to the standard ELISPOT assay (Shata T, Sherman K, Daef E, Abdelwahab S, and Strickland GT, unpublished data). The assay procedures are described elsewhere [4, 15]. Briefly, freshly prepared PBMCs (1 × 105 cells per well) were incubated in duplicate cultures in the mini-ELISPOT plates coated with anti-IFN-γ antibody and incubated for 16 h with or without recombinant HCV antigens at 10 μg/mL in complete Roswell Park Memorial Institute (RPMI) 1640 medium. Positive and negative controls were .1 μg/mL anti-CD3 (Mab Tech) and medium alone, respectively. The plate was incubated for 16–18 h and then developed until spots appeared in the wells, and then rinsed with tap water. The number of spots per well was scored using an automated ELISPOT reader (Cellular Technology). The functional viability of PBMCs was defined as >1,000 spot-forming units (SFUs) per 106 cells after stimulation with anti-CD3 [4]. Averaged numbers of SFUs in control wells were subtracted from the numbers of SFUs in antigen-stimulated wells to correct for spontaneous cytokine production and expressed as SFUs per 106 cells. Positive HCV antigen-specific responses were defined by a mean result of >55 SFUs per 106 PBMCs [4].
Flow Cytometric Analysis of Intracellular IFN-γ Synthesis by HCV-specific T Cells
PBMCs were stimulated with HCV antigens and the intracellular IFN-γ production was examined by flow cytometry as described elsewhere [17]. Briefly, 106 cells in complete RPMI 1640 medium were stimulated with the HCV antigen C-100 (at a concentration of 10 μg/mL) in a short-term assay of 6 h. Blockade of cytokine secretion and intracellular accumulation was achieved by treatment of the cells with brefeldin A (10 μg/mL) during the last 5 h of the assay. Negative and positive controls were medium alone or 50 ng/mL phorbol myristate acetate (PMA; Sigma), plus 1 μg/mL ionomycin (Sigma), respectively. After the stimulation period of 6 h, PBMCs were washed and stained with surface Fluorescein isothiocyanate–labeled anti–human CD4+ (BD Biosciences) and peridinin chlorophyll protein–labeled anti–human CD3 (BD Biosciences) for 30 min at room temperature in a dark place. The cells were then washed, fixed, and permeabilized for 10 min with fluorescence-activated cell sorting perm 2 solution (BD Biosciences), then washed and stained with allophycocyanin-labeled anti–human IFN-γ monoclonal antibody (BD Biosciences) for 30 min at room temperature in a dark place. The cells were then washed, fixed, and stored until data acquisition was performed on a FACSCalibur flow cytometer (BD Biosciences). A maximum of 100,000 total events were acquired, and cytokine-producing cells were analyzed using FlowJo software 4.5.2 (Tree Star). The cells were gated on small lymphocytes using side and forward light scatter profiles, then on CD4+CD3+ T cells, and then the synthesis of IFN-γ was expressed as a function of CD3 expression in contour plots. Results are expressed as the percentages of cytokine-positive cells. Negative controls and compensation tubes were used to verify the staining specificity of the assay. Unstimulated cells consistently gave <.02% IFN-γ-positive cells in the CD4+ cell population, whereas the PMA/ionomycin consistently gave >3% IFN-γ-positive cells in both populations (data not shown).
Data Analysis
Data analysis was performed using the Statistical Program for the Social Sciences (SPSS) software package (version 17.0; SPSS). The Student t test (or Mann-Whitney U test when appropriate) was performed for comparison of continuous data. Prism software 5.01 (commercial scientific 2-dimensional graphing and statistics software; GraphPad) was used when required for scientific graphing.
RESULTS
Study Population
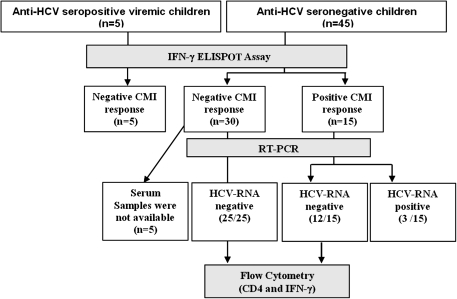
The study was conducted from June 2007 through August 2007. After parental consent and assent from older children was obtained, a 5–8-mL blood sample was drawn from each child, and fresh PBMCs were isolated and tested for functional viability (>103 SFUs per 106 cells) after anti-CD3 stimulation using ELISPOT immunoassays. Forty-five seronegative siblings (male sex, 55%; mean age [± SD], 10 ± 6 years) and 5 seropositive viremic children (mean age [± SD], 8 ± 2 years) qualified for the study and had functionally viable PBMCs (Figure 1).
Figure 1.
Study flow diagram. CMI, cell-mediated immune; ELISPOT, enzyme-linked immunospot; HCV, hepatitis C virus; IFN-γ, interferon γ; RT-PCR, reverse-transcription polymerase chain reaction.
HCV-specific CMI Responses Using ELISPOT Assay
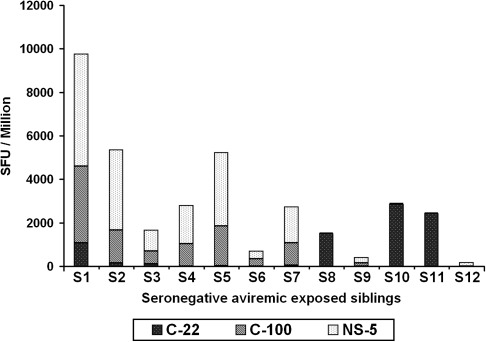
Of the 45 seronegative siblings, 15 (33.3%) had positive HCV-specific IFN-γ responses (>55 SFUs per 106 cells) against at least 1 of the 3 HCV protein antigens tested. Among the 5 HCV-infected seropositive viremic children, none of them elicited detectable HCV-specific IFN-γ responses to any of the HCV antigens (C-22, C-100, and NS5). We tested the seronegative children for HCV RNA using RT-PCR and found that 3 (20%) of the 15 seronegative children with positive HCV-specific IFN-γ responses were viremic, and the remaining 12 were HCV RNA negative. These 3 seronegative HCV-RNA-positive participants were retested 6 months after their initial evaluation and they remained viremic and seronegative, thus suggesting acute HCV infection with possible delayed seroconversion, a phenomenon previously noted only in adults [18]. Among the 30 children without HCV-specific IFN-γ, 25 were HCV RNA negative and 5 did not have sufficient serum samples available to test for viremia. Of the 12 seronegative aviremic siblings, 3 children (25%) responded to all 3 antigens, 5 children (42%) responded to 2 antigens, and 4 children (33%) to only 1 of the 4 antigens (Figure 2).
Figure 2.
γ enzyme-linked immunospot responses in 12 seronegative aviremic children after peripheral blood mononuclear cells (PBMCs) were stimulated with 3 hepatitis C virus antigens for 16 h. The bar chart represents the number of spot-forming units (SFUs) per 106 PBMCs in response to the C-22, C-100, and NS5 antigens.
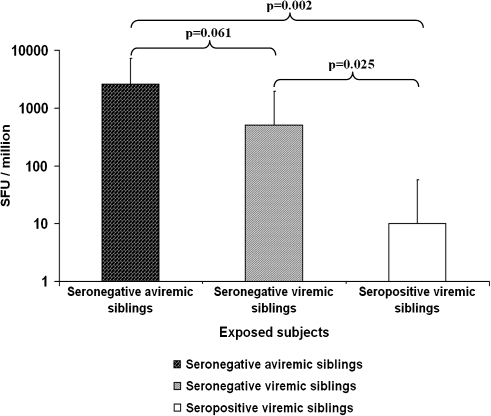
Figure 3 shows the total cumulative IFN-γ ELISPOT responses in the 12 seronegative aviremic siblings, the seronegative viremic siblings, and the seropositive viremic siblings. The magnitude of the HCV-specific CMI response in the 12 seronegative aviremic siblings was significantly higher than in the 5 seropositive viremic index case children (median, 2,585 SFUs per 106 cells; interquartile range [IQR], 906–4,636 SFUs per 106 cells; vs median, 10 SFUs per 106 cells; IQR, 2.5–47.5 SFUs per 106 cells; P = .002) and higher (but not statistically significantly) than in the 3 seronegative viremic HCV-infected case children (median, 2,585 SFUs per 106 cells; IQR, 906–4,636 SFUs per 106 cells; vs median, 505 SFUs per 106 cells; IQR, 150–1,445 per 106 cells; P = .061). The HCV-specific CMI response was also significantly higher in the 3 seronegative viremic participants compared with those in the 5 seropositive viremic index case participants (median, 505 SFUs per 106 cells; IQR, 150–1,445 SFUs per 106 cells; vs median, 10 SFUs per 106 cells; IQR, 2.5–47.5 SFUs per 106 cells; P = .025).
Figure 3.
Total cumulative hepatitis C virus (HCV)–specific interferon γ enzyme-linked immunospot responses (in median no. of spot-forming units [SFUs] per 106 peripheral blood mononuclear cells) among seronegative aviremic, seronegative viremic, and seropositive viremic siblings.
HCV-specific CMI Responses to Individual HCV Antigens
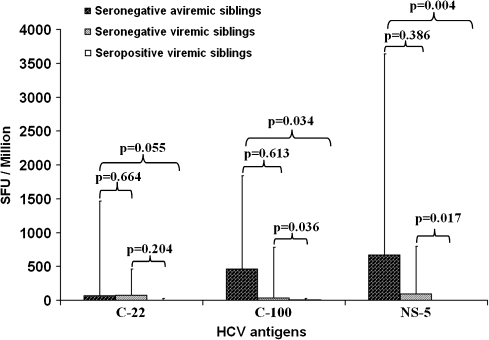
Among the 12 seronegative aviremic siblings, the frequency of positive IFN-γ ELISPOT response to NS5, C-100, and C-22 antigens was 75%, 66.6%, and 50% respectively. The highest magnitude of response among the 12 seronegative aviremic siblings was to NS5 followed by C-100 and C-22. Figure 4 shows that the magnitude of the median HCV-specific IFN-γ response in the 12 seronegative aviremic children compared with that in the 5 seropositive viremic children was significantly higher for NS5 (median, 670 SFUs per 106 cells, IQR, 51–2,970 SFUs per 106 cells; vs median, 0 SFUs per 106 cells; IQR, 0–2.5 SFUs per 106 cells; P = .004), for C-100 (median, 460 SFUs per 106 cells; IQR, 14–1,383 SFUs per 106 cells; vs median, 10 SFUs per 106 cells; IQR, 2.5–20 SFUs per 106 cells; P = .034), and for C-22 (median, 70 SFUs per 106 cells; IQR, 6–1,394 SFUs per 106 cells; vs median, 0 SFUs per 106 cells; IQR, 0–25 SFUs per 106 cells; P = .055). Similarly, the magnitude of these responses in the 3 seronegative viremic children was significantly higher when compared with that in the 5 seropositive viremic children for NS5 (median, 95 SFUs per 106 cells; IQR, 40–700 SFUs per 106 cells; vs median, 0 SFUs per 106 cells; IQR, 0–2.5 SFUs per 106 cells; P= .017) and for C-100 (median, 35 SFUs per 106 cells; IQR, 25–745 SFUs per 106 cells; vs median, 10 SFUs per 106 cells; IQR, 2.5–20 SFUs per 106 cells; P= .036) and higher, but not statistically significantly, for C-22 (P = .204).
Figure 4.
Magnitude of hepatitis C virus (HCV)–specific interferon γ enzyme-linked immunospot responses (in median no. of spot-forming units [SFUs] per 106 peripheral blood mononuclear cells) to each of 3 HCV antigens (C-22, C-100, and N-S5) among seronegative aviremic, seronegative viremic, and seropositive viremic children.
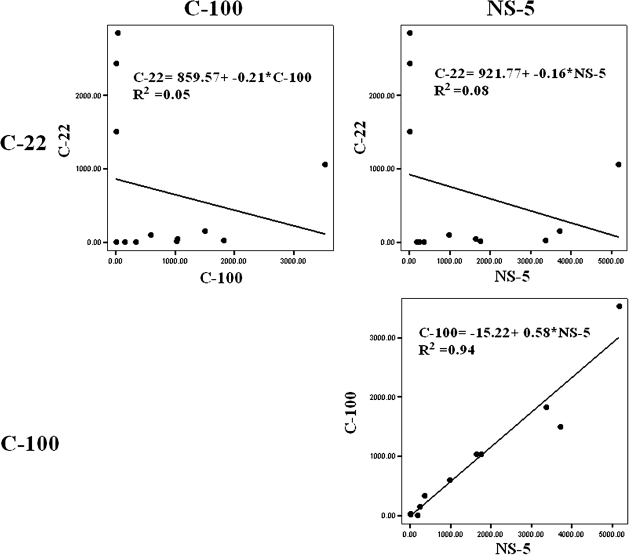
When we correlated immune responses among the 12 seronegative aviremic siblings to the individual antigens (Figure 5), a negative correlation was observed in the CMI response between the structural protein antigen C-22 and the nonstructural protein antigens C-100 and NS5 (R = .05 and R = .08, respectively). Conversely, a strong positive correlation in the strength of the CMI response was observed between the C-100 and NS5 antigens (R = .94).
Figure 5.
Correlation of cell-mediated immune (CMI) responses elicited among the 12 seronegative aviremic siblings in response to the 3 hepatitis C virus antigens (C-22, C-100, and NS5). A negative correlation in CMI responses was observed between the C-22 antigen and the C-100 and NS5 antigens (upper panels). Conversely, a strong positive correlation was observed between the C-100 and NS5 antigens (lower panel).
Flow Cytometric Analysis of the HCV-specific CD4+ T Cell Responses
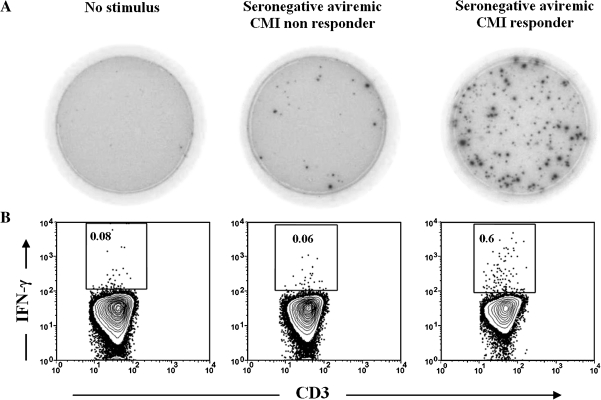
To assess the phenotype of the HCV-specific IFN-γ-producing cells for the responses elicited in seronegative aviremic children, we tested PBMCs from 6 of the 12 seronegative aviremic CMI responder siblings (>55 SFU per 106 cells) and 3 of the 5 CMI nonresponder siblings who had sufficient PBMCs available to conduct flow cytometry. PBMCs were stimulated with HCV protein (C-100) and then analyzed by flow cytometry for the synthesis of intracellular IFN-γ. PMA and ionomycin were used as a positive control. Figure 6 is representative of the flow cytometry data, showing the synthesis of IFN-γ versus CD3 expression of CD4+ T cells in the presence of the C-100 HCV protein antigens. Approximately .6% of CD4+ T cells were secreting IFN-γ upon stimulation with HCV protein C-100 in a responder. In contrast only .06% of CD4+ T cells were secreting IFN-γ in a nonresponder. In addition, the cumulative percentage of IFN-γ-producing cells for the HCV protein antigen (C-100) in all 6 responders confirms that the IFN-γ-producing cells were mainly CD4+ T cells. The percentage of HCV-specific CD4+ cells producing IFN-γ in response to C-100 protein antigen was greater in the seronegative aviremic siblings when compared with that in the seropositive viremic index case children, who failed to produce detectable amounts of IFN-γ beyond the background levels. These results are in agreement with our IFN-γ ELISPOT data.
Figure 6.
Flow cytometric analysis demonstrating that hepatitis C virus (HCV)–specific CD4+ T cells are the main source of interferon γ (IFN-γ). Peripheral blood mononuclear cells were stimulated with the HCV protein antigen short-term of 6 hours in the presence of brefeldin A in the last 5 h in the presence of brefeldin A in the last 7 h and then stained for surface markers and intracellular IFN-γ as described in the Materials and Methods. A, Representative enzyme-linked immunospot wells with samples from different participants. B, Synthesis of IFN-γ versus CD3 expression by cells that were gated on small lymphocytes using side and forward light scatter profiles and then on CD4+ cells in the presence of medium (negative control) (left panel) or in response to C-100 antigen for a nonresponse to C-100 antigen for a nonresponder (middle panel) and a responder (right panel). All of the gated small CD4 cells express CD3 (100%) and hence are CD3+CD4+ T cells. The cumulative percentage of IFN-γ-producing cells in all responders confirms that the IFN-γ-producing cells were mainly CD4+ T cells (data not shown). CMI, cell-mediated immune.
DISCUSSION
Previous studies have shown that HCV-specific CMI responses can be detected in adults who resolve infection but very rarely in chronically infected patients, supporting the view that HCV-specific CMI responses play a major role in controlling infection [19–27]. This observation has been previously reported in other infections such as with human immunodeficiency virus (HIV), in which CMI responses to subclinical infection without seroconversion were documented in commercial sex workers in Nairobi, Kenya, who were supposedly heavily exposed, but not infected, with HIV [28–32]. Although other studies have demonstrated HCV-specific CMI responses in children born to HCV-positive mothers, no studies, to our knowledge, have examined the CMI responses of uninfected siblings of HCV-infected children [5, 6]. Understanding these responses is necessary to accurately assess past exposure, predict future chronicity, and plan for adequate follow-up and care. This is particularly important now that HCV is rapidly becoming the leading cause of chronic blood-borne viral infection in Egyptian children, since the precipitous drop in hepatitis B virus (HBV) infection rates after the introduction of universal HBV infant immunization in 1991 [33].
In this study, 12 of 45 (27%) of our seronegative children living in the same household with their HCV-infected Egyptian siblings were aviremic and had positive HCV-specific IFN-γ T cell responses, indicating probable prior HCV exposure. Two-thirds (8 of 12) of those children responded to ≥2 of the HCV antigens tested, which probably represents a true immune reactivity to HCV rather than cross-reactivity to other antigens (eg, influenza virus), which we would have expected to be similar in all groups [34]. The frequency of children responding to the NS-5 antigen was the highest, followed by the frequency of children responding to the C-100 and C-22 antigens, and the magnitude of the response detected was highest to NS5 followed by C-100 and C-22. Hence, it appears that NS5 protein antigen represents highly immunogenic, possibly protective, regions of the HCV genome in this group of children.
Phenotypic characterization of the IFN-γ-producing cells was assessed by flow cytometry and confirmed that CD4+ T cells were the main cellular source of IFN-γ in those HCV-exposed aviremic children. Furthermore, our results suggest that the HCV-specific immune responses observed in aviremic, seronegative siblings were qualitatively and quantitatively different from those observed in their HCV chronically infected seropositive viremic siblings.
Our results are similar to the results demonstrated by Rosen et al [27] and Diepolder et al [22] that participants who had resolved HCV infection produced T-helper 1 (Th1)–type cytokines following stimulation with nonstructural antigens (NS3, NS4, and NS5). The broad specificity of the virus-specific CD4+ T-helper cell response observed in our series in response to nonstructural viral proteins (NS3, NS4A/B, and NS5A/B) has been reported in the majority of individuals with resolved HCV infection, whereas individuals with chronic infection typically have few to no detectable responses [17, 19–25, 27, 35]. Moreover, this broad HCV-specific effector T cell response against several viral protein antigens shown in our study was elicited when PBMCs were cocultured with recombinant HCV antigens without the addition of any particular cytokines in vitro for differentiation into effector cells. This suggests that an immunological memory in these siblings was established upon a prior exposure to the virus and that a subsequent exposure to the antigen is capable of inducing a prompt HCV-specific effector function ex-vivo, possibly providing antiviral protection. The maintenance of these effector T cell responses may be sustained by repeated low-level exposure to HCV and stimulation of memory cells. Furthermore, we found that seronegative viremic children have a significantly higher magnitude of CMI responses compared with that of chronically infected children, although it is lower than the magnitude of those of seronegative aviremic children. Although we had only 3 children in this group, it poses an interesting question as to whether their CMI responses will result in eventual viral clearance, or whether this is a transitional phase before chronicity in which HCV-specific memory cells will be destroyed, resulting in eventual loss of HCV-specific CMI. Further follow-up of these 3 and other similar children is likely to yield interesting results.
As with adults exposed to HCV [1–4, 36] and to HIV [28–32, 37], the evaluation of T cell reactivity in children may represent a useful biomarker in revealing previous exposures of the host to a viral agent, which could be identified as CMI conversion. In a recently published study, HCV-specific CD4+ T cell reactivity was demonstrated in the majority (71%) of a cohort of children born to HCV-infected mothers, and the proliferation of HCV-specific CD4+ T cells was more frequent and vigorous in children than in their mothers [5].
One of the limitations of our cross-sectional study was that these detectable HCV-specific IFN-γ T cell responses were determined at only 1 time point. Also, in this study, we did not investigate other Th1- or Th2-type cytokines following stimulation with HCV antigens or use major histocompatibility complex class I–restricted peptide antigens to examine the CD8+ T cell responses. However, our study was designed to determine whether HCV-specific CMI responses existed in seronegative, aviremic children in contact with HCV-infected siblings in the absence of known past infection, similar to those responses seen in adults.
In conclusion, we present evidence that a substantial percentage of HCV-seronegative aviremic Egyptian children who are potentially exposed to the virus by living in the same household with HCV-infected family members develop broad HCV-specific CD4+ T cell responses, which may have protected them from persistent infection and possibly protects against subsequent exposure. Characterization of these CMI responses could have important implications for developing vaccines that assist in the clearance of HCV following infection.
Funding
This work was supported by the Wellcome Trust/Burroughs Wellcome Fund (grant numbers 059113/z/99/a and 059113/z/99/z); and by the National Institutes of Health (grant number RO3 A1058971).
Acknowledgments
We thank Hoda Mansour and Amal Abdel-Baky for their support while conducting the study at VACSERA and the dedicated assistance of their professional and technical staff. We thank Nabiel Mikhail for his help with data management and statistical analysis of the results, and Chiron for their donation of the recombinant HCV antigens.
References
- 1.Scognamiglio P, Accapezzato D, Casciaro MA, et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–9. [PubMed] [Google Scholar]
- 2.Koziel MJ, Wong DK, Dudley D, Houghton M, Walker BD. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J Infect Dis. 1997;176:859–66. doi: 10.1086/516546. [DOI] [PubMed] [Google Scholar]
- 3.Bronowicki JP, Vetter D, Uhl G, et al. Lymphocyte reactivity to hepatitis C virus (HCV) antigens shows evidence for exposure to HCV in HCV-seronegative spouses of HCV-infected patients. J Infect Dis. 1997;176:518–22. doi: 10.1086/517279. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sherbiny M, Osman A, Mohamed N, et al. Exposure to hepatitis C virus induces cellular immune responses without detectable viremia or seroconversion. Am J Trop Med Hyg. 2005;73:44–9. [PubMed] [Google Scholar]
- 5.Della Bella S, Riva A, Tanzi E, et al. Hepatitis C virus-specific reactivity of CD4+-lymphocytes in children born from HCV-infected women. J Hepatol. 2005;43:394–402. doi: 10.1016/j.jhep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 6.El-Kamary S, Hashem M, Saleh D, et al. 13th International Symposium on Viral Hepatitis and Liver Disease. Washington, DC: 2009. Clearance of hepatitis C viremia in children born to, and living with, HCV-infected mothers is associated with long-lasting HCV-specific cell mediated immune responses [Abstract 10, Poster 109] [Google Scholar]
- 7.Post JJ, Pan Y, Freeman AJ, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846–55. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- 8.Freeman AJ, French RA, Post JJ, et al. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–7. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 9.Mizukoshi E, Eisenbach C, Edlin BR, et al. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J Infect Dis. 2008;198:203–12. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeremski M, Shu MA, Brown Q, et al. Hepatitis C virus-specific T-cell immune responses in seronegative injection drug users. J Viral Hepat. 2009;16:10–20. doi: 10.1111/j.1365-2893.2008.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shata MT, Tricoche N, Perkus M, et al. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–16. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Aziz F, Habib M, Mohamed MK, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology. 2000;32:111–5. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 13.Habib M, Mohamed MK, Abdel-Aziz F, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–53. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 14.Hashem M, El-Karaksy H, El-Naghi S, et al. 15th International Symposium on Hepatitis C Virus and Related Viruses. San Antonio, Texas: 2008. Epidemiological, virological characteristics and disease progression of hepatitis C virus (HCV)-infection in Egyptian children. [Google Scholar]
- 15.Shata MT, Barrett A, Shire NJ, et al. Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. 2007;328:152–61. doi: 10.1016/j.jim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer S, Laumer M, Mackensen A, Andreesen R, Krause SW. Analysis of the immune response against tetanus toxoid: enumeration of specific T helper cells by the Elispot assay. Immunobiology. 2002;205:282–9. doi: 10.1078/0171-2985-00131. [DOI] [PubMed] [Google Scholar]
- 17.Lauer GM, Ouchi K, Chung RT, et al. Comprehensive analysis of CD8(+)-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J Virol. 2002;76:6104–3. doi: 10.1128/JVI.76.12.6104-6113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–8. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 19.Chang KM, Thimme R, Melpolder JJ, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 20.Ciurea A, Hunziker L, Klenerman P, Hengartner H, Zinkernagel RM. Impairment of CD4(+) T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J Exp Med. 2001;193:297–305. doi: 10.1084/jem.193.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CL, Lauer GM, Robbins GK, et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584–95. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diepolder HM, Zachoval R, Hoffmann RM, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–7. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 23.Gerlach JT, Diepolder HM, Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 24.Missale G, Bertoni R, Lamonaca V, et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–14. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penna A, Missale G, Lamonaca V, et al. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology. 2002;35:1225–36. doi: 10.1053/jhep.2002.33153. [DOI] [PubMed] [Google Scholar]
- 26.Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 27.Rosen HR, Miner C, Sasaki AW, et al. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology. 2002;35:190–8. doi: 10.1053/jhep.2002.30293. [DOI] [PubMed] [Google Scholar]
- 28.Alimonti JB, Kimani J, Matu L, et al. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol Cell Biol. 2006;84:482–5. doi: 10.1111/j.1440-1711.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 29.Clerici M, Shearer GM. Correlates of protection in HIV infection and the progression of HIV infection to AIDS. Immunol Lett. 1996;51:69–73. doi: 10.1016/0165-2478(96)02557-6. [DOI] [PubMed] [Google Scholar]
- 30.Dittmer U, Spring M, Petry H, et al. Cell-mediated immune response of macaques immunized with low doses of simian immunodeficiency virus (SIV) J Biotechnol. 1996;44:105–10. doi: 10.1016/0168-1656(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 31.Fowke KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–51. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 32.Kaul R, Rutherford J, Rowland-Jones SL, et al. HIV-1 Env-specific cytotoxic T-lymphocyte responses in exposed, uninfected Kenyan sex workers: a prospective analysis. AIDS. 2004;18:2087–9. doi: 10.1097/00002030-200410210-00015. [DOI] [PubMed] [Google Scholar]
- 33.Zakaria S, Fouad R, Shaker O, et al. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis. 2007;44:e30–6. doi: 10.1086/511074. [DOI] [PubMed] [Google Scholar]
- 34.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham CS, Curry M, He Q, et al. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology. 2004;40:125–32. doi: 10.1002/hep.20258. [DOI] [PubMed] [Google Scholar]
- 36.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 37.Mazzoli S, Trabattoni D, Lo Caputo S, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–7. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]