Abstract
Background. Leptospira species cause leptospirosis, a zoonotic disease found worldwide. Current vaccines against leptospirosis provide protection only against closely related serovars.
Methods. We evaluated an attenuated transposon mutant of Leptospira interrogans serovar Manilae (M1352, defective in lipopolysaccharide biosynthesis) as a live vaccine against leptospirosis. Hamsters received a single dose of vaccine and were challenged with the homologous serovar (Manilae) and a serologically unrelated heterologous serovar (Pomona). Comparisons were made with killed vaccines. Potential cross-protective antigens against leptospirosis were investigated.
Results. Live M1352 vaccine induced superior protection in hamsters against homologous challenge. The live vaccine also stimulated cross-protection against heterologous challenge, with 100% survival (live M1352) versus 40% survival (killed vaccine). Hamsters receiving either vaccine responded to the dominant membrane proteins LipL32 and LipL41. Hamsters receiving the live vaccine additionally recognized LA3961/OmpL36 (unknown function), Loa22 (OmpA family protein, recognized virulence factor), LA2372 (general secretory protein G), and LA1939 (hypothetical protein). Manilae LigA was recognized by M1352 vaccinates, whereas LipL36 was detected in Pomona.
Conclusion. This study demonstrated that a live, attenuated vaccine can stimulate cross-protective immunity to L. interrogans and has identified antigens that potentially confer cross-protection against leptospirosis.
The spirochete Leptospira is a widespread zoonotic pathogen transmitted via the urine of carrier animals such as dogs, cattle, and rodents. Human disease varies greatly in severity from a mild flu-like illness to one with multiple organ failure, pulmonary hemorrhage, and death [1]. Infection rates remain significant, with more than 500,000 cases of severe leptospirosis reported each year, for which the mortality rate is >10% [2].
There are >250 serovars of Leptospira species, with multiple serovars being endemic in a given area. Protective immunity against Leptospira infection is mediated predominantly by antibodies directed against lipopolysaccharide (LPS) and is usually serovar specific. Most attempts to develop vaccines have used bacterin vaccines (killed whole cells). However, bacterins are usually reactogenic and confer short-term immunity. Protection may also be incomplete; for example, vaccination of dogs or cattle may prevent illness but not leptospiruria and transmission [3, 4]. More importantly, bacterin vaccines induce immunity that is restricted to closely related serovars. To overcome this problem, current vaccine research is aimed at identifying conserved protective antigens that may protect against a broad range of leptospiral serovars. Subunit vaccines have achieved some success against homologous challenge by use of antigens such as OmpL1 and LipL41 [5] and LigA [6], but conflicting results have been found with other antigens such as LipL32 (reviewed by Adler and de la Pena Moctezuma [1]).
The rational development of new leptospiral vaccines is hindered by the limited knowledge of pathogenesis and mechanisms of protective immunity against leptospirosis. Complete genome sequences of pathogenic and saprophytic Leptospira species [7–10] and advances in understanding of leptospiral pathogenesis through mutagenesis [11–16] may assist in identification of candidate vaccine antigens. We recently reported the identification of 2 Himar1 mariner transposon mutants (M895 and M1352) with altered LPS that did not cause disease in the hamster model of infection [13, 17]. In this study we evaluate the protective capacity of M1352 as a candidate vaccine for leptospirosis. The mutant was found to elicit protection against both homologous and heterologous challenge better than the equivalent killed whole-cell vaccine. Potential protective protein antigens were identified through analysis of serum samples from vaccinated hamsters.
METHODS
Bacterial Strains and Growth Conditions
Leptospira interrogans serovar Manilae strain L495 was obtained from N. Koizumi, National Institute of Infectious Diseases, Tokyo, Japan. The mutants M895 and M1352 were constructed from the parent strain L495 as described elsewhere using TnSC189-derived transposons carrying the respective antibiotic resistance marker [11, 13]. L. interrogans serovar Pomona (L523) was provided by Lee Smythe, World Health Organization/Food and Agricultural Organization/Office International des Epizooties Collaborating Centre for Reference and Research on Leptospirosis, Queensland Health Scientific Services, Australia. Leptospira borgpetersenii serovar Hardjobovis L664 was an Australian cattle isolate. All strains were cultured at 30°C in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (Becton Dickinson).
Preparation of Vaccines
Log phase bacteria were diluted in fresh EMJH medium to the desired concentration. The heat-killed vaccine was prepared by incubation at 100°C for 10 min. The formalin-killed vaccine was prepared by harvesting the bacteria by centrifugation (10,000 g for 5 min), washing in phosphate-buffered saline, resuspending in 10% neutral buffered formalin for 60 min, washing in phosphate-buffered saline, and then resuspending in fresh EMJH medium. The sterility of killed vaccines was confirmed by absence of bacterial growth on blood agar plates and EMJH medium at 37°C and 30°C, respectively.
Evaluation of Vaccines in the Hamster Model of Infection
Four-week-old Syrian golden hamsters (groups of 10) were immunized with a single dose of vaccine preparation in 100 μL of EMJH medium by intraperitoneal injection 14 d prior to intraperitoneal challenge with 100 μL of EMJH medium containing the desired challenge dose of Leptospira. Hamsters were monitored for 21 d after infection and euthanized if they were moribund, according to animal ethics requirements. At necropsy, lungs were inspected for gross pathology to identify macroscopic hemorrhages (each animal was scored as positive or negative for hemorrhage) and kidneys were taken for culture as described elsewhere [14]. Animal experiments were approved by the Khon Kaen University Animal Ethics Committee.
Blood Collection, RNA Isolation, cDNA Synthesis, and Cytokine Real-time Polymerase Chain Reaction (PCR)
Blood samples were collected on the day before vaccination, 18 h and 4 d after vaccination, and then 18 h and 4 d after challenge [18]. RNA was extracted within 1 h of collection for quantitative reverse-transcription PCR (RT-PCR). The gene expression levels for the cytokines interferon γ, interleukin 12p40, interleukin 4, and interleukin 10 were determined as described elsewhere [18, 19].
Two-dimensional Gel Electrophoresis
Leptospires were incubated overnight at physiological osmolarity (.15 mol/L sodium chloride) [20]. Total cell membranes were prepared by sonication and centrifugation at 100,000 g at 4°C for 20 min. Preparations were solubilized in rehydration buffer (7 mol/L urea, 2 mol/L thiourea, 4% wt/vol 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate, 13 mmol/L dithiothreitol (DTT), 2% vol/vol Bio-lyte 3/10 ampholyte [Bio-Rad], and .001% wt/vol bromophenol blue), and 50 μg of protein was loaded onto 7-cm Immobiline DryStrip pH 3–10 immobilised pH gradient strips (Amersham Biosciences) that were rehydrated passively overnight. Isoelectric focusing was performed at 18°C with use of an Amersham Biosciences Multiphor II according to the manufacturer's protocol. Focused strips were equilibrated in equilibration buffer (6 mol/L urea, 30% vol/vol glycerol, 2% wt/vol sodium dodecyl sulfate [SDS], .25% wt/vol DTT, and 50 mmol/L Tris-HCl [pH, 6.8]) for 15 min on a rocking table and then immersed for another 15 min in fresh equilibration buffer, with DTT replaced by 4.5% wt/vol iodoacetamide and .001% wt/vol bromophenol blue. Equilibrated strips were placed on 4% stacking per 10% resolving gels, and SDS polyacrylamide gel electrophoresis (SDS-PAGE) was conducted at 200 V in an XCell 6 electrophoresis tank (Invitrogen). Gels were fixed with 50% ethanol and 3% phosphoric acid and stained with colloidal Coomassie blue [21]. Immunoblotting and matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry were performed as described elsewhere [22, 23]. Immunoblots were probed with hamster serum samples pooled from groups of 5 animals, diluted 1:300, and detected with horseradish peroxidase–conjuated goat anti-hamster serum (Abcam).
Analysis of LPS
Analysis of LPS by SDS-PAGE and immunoblotting was conducted as described elswhere [17, 22]. Immunoblots were probed with individual hamster serum samples diluted 1:300.
Statistical Analysis
The Fisher exact test was used to determine differences, which were considered significant at P ≤ .05.
RESULTS
Live M1352 Vaccine Protects Against Homologous Challenge
Previously we reported 2 L. interrogans serovar Manilae transposon mutants with altered expression of LPS that were highly attenuated in the hamster model of infection [13, 17]. Because of the in vitro and in vivo stability of the TnSC189 transposon in L. interrogans [11, 13] and the clear attenuation of M1352, this strain was considered a potential candidate live vaccine. To assess protection against homologous challenge with serovar Manilae, hamsters were injected with a single dose of 103, 105, or 107 live M1352 leptospires. Controls of heat-killed wild-type (WT) bacteria and EMJH medium were included. After 14 d, hamsters were challenged with 103 WT Manilae leptospires (median infective dose [ID50], <10 leptospires [15]) and monitored for 21 d. Several criteria were used to assess vaccine efficacy, including hamster survival, colonization of hamster kidneys, and the presence of pulmonary hemorrhages in individual animals—the most critical clinical presentation of acute leptospirosis in hamsters [18, 24, 25].
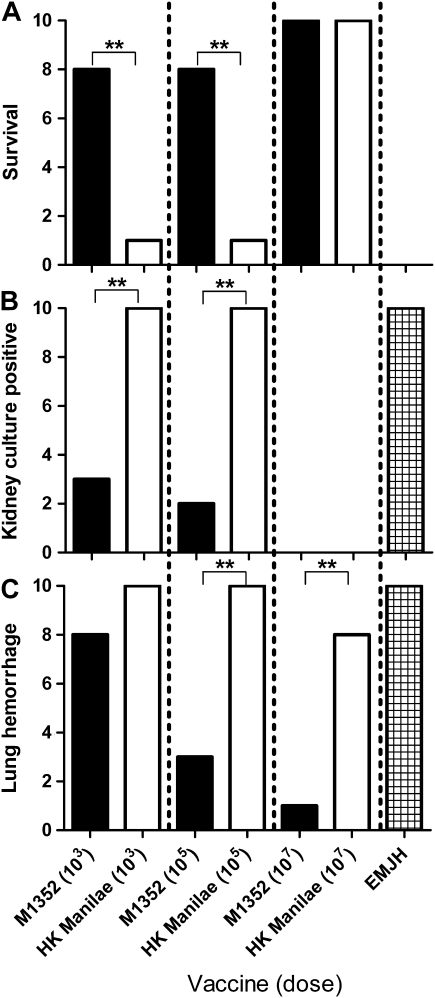
A single vaccination with live M1352 induced better protection in hamsters than that induced by the bacterin vaccine against homologous challenge with virulent Manilae WT (Figure 1). At vaccine doses of 103 and 105 leptospires, live M1352 stimulated better protection than the heat-killed WT vaccine against both acute lethality (Figure 1A) and kidney colonization (P < .01) (Figure 1B). At the higher vaccine dose (107 leptospires), there was equivalent protection against death and kidney colonization. The live vaccine also elicited superior protection against lung pathology (determined by the presence of macroscopic lung lesions) at the moderate and high vaccine dose (P < .01) (Figure 1C). The second attenuated LPS mutant (M895) was also tested as a live vaccine. M895 provided an intermediate level of protection between those of M1352 and the bacterin and was not used for further studies.
Figure 1.
Protection against challenge with Leptospira interrogans serovar Manilae. Hamsters (groups of 10) were vaccinated with a single intraperitoneal injection, with the dose indicated, of live M1352 vaccine, heat-killed (HK) wild-type (WT) Manilae vaccine, or EMJH medium. After 14 d, hamsters were challenged with 103 WT Manilae leptospires and monitored for 21 d. Vaccine efficacy was assessed by hamster survival (A), culture of leptospires from kidney tissue (B), and evidence of lung hemorrhage in each animal (C). **P < .01 (Fisher exact test).
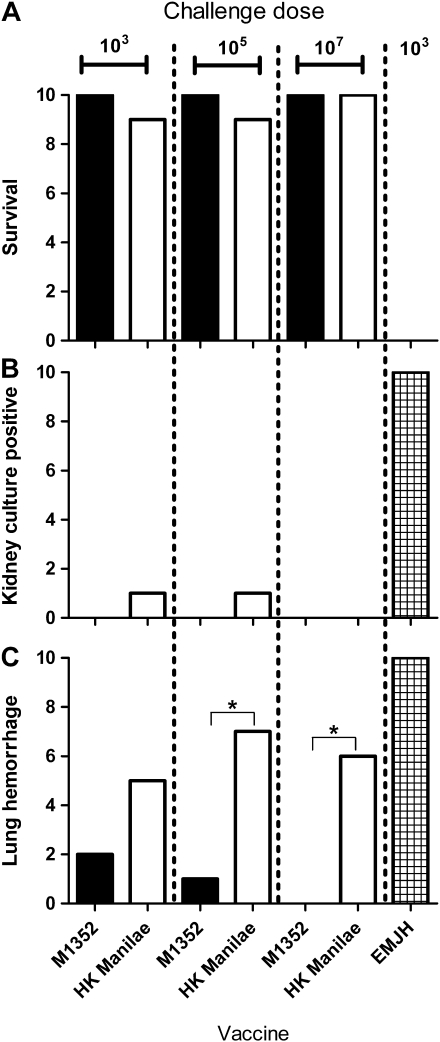
To determine the degree of protection afforded by the M1352 mutant, vaccinated hamsters were challenged with higher doses. Hamsters were immunized with a single dose of 107 leptospires (either live vaccine or heat-killed Manilae) and then challenged 14 d later with doses of 103, 105, or 107 leptospires. Both vaccines protected hamsters against death at all doses (Figure 2). At higher doses, M1352 provided superior protection to that of the bacterin against lung hemorrhage (P < .05) (Figure 2C).
Figure 2.
Protection against 3 different challenge doses with Leptospira interrogans serovar Manilae. Hamsters (groups of 10) were vaccinated with a single intraperitoneal injection of 107 live M1352 leptospires, 107 heat-killed (HK) Manilae leptospires, or EMJH medium. After 14 d, hamsters were challenged with the dose indicated of wild-type Manilae and monitored for 21 d. Vaccine efficacy was assessed by hamster survival (A), culture of leptospires from kidney tissue (B), and evidence of lung hemorrhage in each animal (C). * P < .05 (Fisher exact test).
Live M1352 Vaccine Protects Against Heterologous Challenge
Immunity to leptospires is predominantly humoral. Because the dominant immunogen is LPS, immunity is largely serotype specific, and previous attempts to construct cross-protective vaccines have had limited success. To investigate whether a live vaccine may overcome these limitations, hamsters were vaccinated with a single dose (107 leptospires) of either live M1352 or killed WT vaccine and then challenged with a dose of 103 L. interrogans serovar Manilae (serogroup Pyrogenes; ID50, <10), 104 L. interrogans serovar Pomona (serogroup Pomona; ID50, 103), or 106 L. borgpetersenii serovar Hardjobovis (serogroup Sejroe). The L. borgpetersenii strain was not hamster lethal and did not cause lung hemorrhage, so kidney colonization was used as a measure of infection. The experiment was performed up to 3 times using groups of 10 animals (some controls were only tested once). Pooled data are presented in Figure 3.
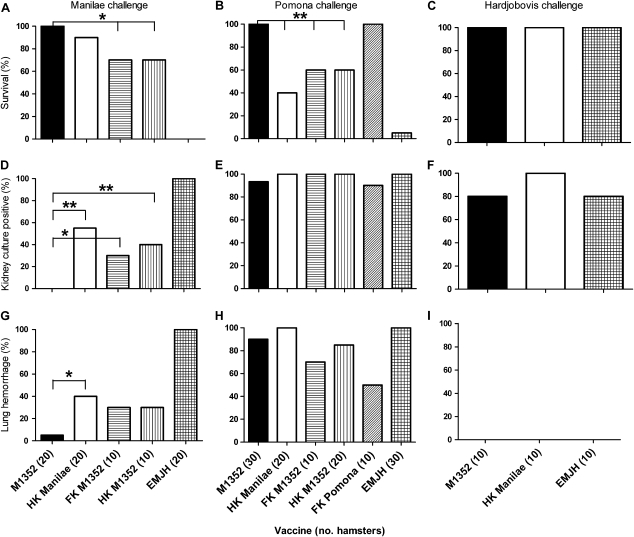
Figure 3.
Vaccine protection against heterologous leptospiral challenge. Hamsters (groups of 10) were vaccinated with a single intraperitoneal injection of 107 live M1352, heat-killed (HK) Manilae, formalin-killed (FK) M1352, or HK M1352 leptospires. EMJH medium was used as a negative control. After 14 d, hamsters were challenged with a dose of 103 Leptospira interrogans serovar Manilae (serogroup Pyrogenes) leptospires (A, D, and G), 104 serovar Pomona (serogroup Pomona) leptospires (B, E, and H), or 106 Leptospira borgpetersenii serovar Hardjobovis (serogroup Sejroe) leptospires (C, F, and I) and monitored for 21 d. The experiment was conducted up to 3 times and data were pooled (the total number of hamsters tested in each condition is indicated on the x-axis in parentheses). * P < .05; ** P < .01 (Fisher exact test).
M1352 elicited significantly better homologous protection than did the bacterin against kidney colonization (P < .01) (Figure 3D) and lung hemorrhage (P < .05) (Figure 3G).
In hamsters challenged with serovar Pomona, the M1352 vaccine conferred better survival than the heat-killed WT vaccine (P < .01) (Figure 3B), with all hamsters surviving for the duration of the experiment, compared with 40% survival for the bacterin vaccine. Disease pathology (lung hemorrhage) and kidney colonization did not differ significantly from those in hamsters receiving the Manilae bacterin vaccine. A Pomona bacterin vaccine positive control demonstrated homologous protection. Neither live M1352 nor heat-killed Manilae stimulated protection against renal colonization with L. borgpetersenii serovar Hardjobovis.
The enhanced protection observed with the M1352 vaccine over the heat-killed WT could be a result of the administration of live bacteria, or it may be due to inherent changes introduced by the mutation of lman_1408, for example, by exposing conserved LPS epitopes. Therefore, to determine whether M1352 had to be administered alive to confer significantly better protection than killed leptospires, the above experiments included M1352 as formalin or heat-killed bacterin vaccines (Figure 3). The live vaccine conferred significantly better protection than the equivalent killed vaccines against both homologous (P < .05 for hamster survival and P < .01 for kidney culture) and heterologous challenge (P < .01 for hamster survival) (Figure 3). The protection conferred by the M1352 bacterin vaccine did not significantly differ from that of the bacterin WT vaccine (P > .05).
Hamster Immune Response to Vaccination
Vaccination with live or killed vaccines may affect the balance of T-helper 1 (Th1) and T-helper 2 (Th2) immune responses. Accordingly, the induction of Th1 (interferon γ and interleukin 12p40) and Th2 (interleukin 4 and interleukin 10) cytokines was evaluated by quantitative RT-PCR in the blood of hamsters immunized with either 107 killed WT leptospires or 107 live M1352 leptospires or sham vaccinated with EMJH medium. Before immunization, all animals showed baseline levels of messenger RNA expression of all cytokines. After vaccination and after challenge, all hamsters responded with increased levels of each cytokine, but there was no difference between the responses of hamsters receiving the live vaccine and those of hamsters receiving the killed vaccine (data not shown). The trend in cytokine level changes after challenge was comparable to those found elsewhere [18].
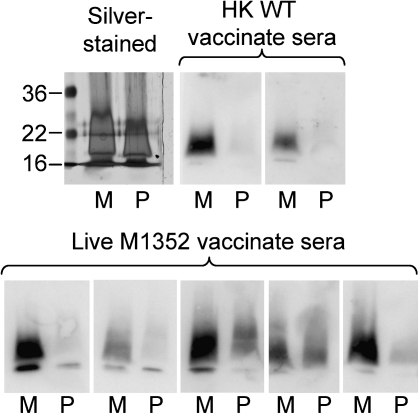
Immunity to leptospirosis is predominantly humoral [26], and recognition of different antigens may be responsible for the variation in protection against challenge. Therefore, agglutinating antibody was assessed by microscopic agglutination test as a potential indicator of immunity [27–29]. Each vaccinate group produced agglutinating antibody against serovar Manilae, but no reactivity was found against Pomona or Hardjobovis (Table 1). The lack of agglutinating antibodies against the heterologous serovar Pomona suggested that protection against serovar Pomona was unlikely to involve LPS antigen. Immunoblotting of proteinase K–digested whole-cell lysates of serovars Manilae and Pomona showed a strong response to L. interrogans serovar Manilae LPS but minimal response to Pomona LPS (Figure 4); we therefore conclude that the response was against non-surface-exposed, non-agglutinating LPS epitopes.
Table 1.
MAT Results for Various Vaccine Groups Against Different Leptospiral Serovars
| Geometric mean microscopic agglutination test titer against Leptospira interrogans or Leptospira borgpetersenii serovar (SD) |
|||
| Vaccine | Manilae | Pomona | Hardjobovis |
| Heat-killed Manilae | 46 (22) | <5 | <5 |
| M1352 | 86 (47) | <5 | <5 |
| EMJH medium | <10 | <10 | <10 |
NOTE. Example results for 10 animals from 1 experiment. Starting dilutions were 1:10 (EMJH-vaccinated group) or 1:5 (other groups).
Figure 4.
Western blot analysis of the immune response to leptospiral lipopolysaccharide. Proteinase K-digested whole-cell lysates of Leptospira interrogans serovar Manilae (M) or Pomona (P) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a membrane, and probed with serum samples from individual vaccinated hamsters.
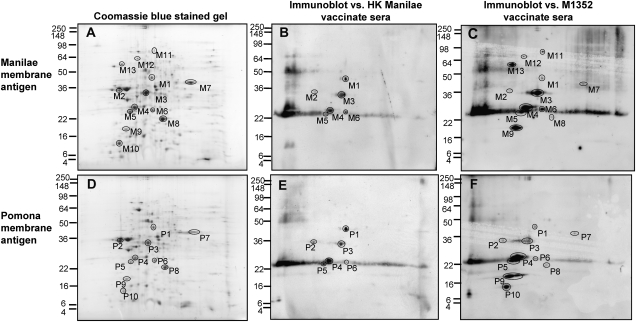
We therefore used immunoblotting to identify the protein antigens recognized in both the heterologous and homologous serovars by hamsters immunized with killed and live vaccines. Two-dimensional gel electrophoresis was conducted on total membrane preparations of serovars Manilae and Pomona, and either gels were stained or proteins were transferred to a membrane and probed with antiserum pooled from 5 hamsters from each vaccinate group. Relatively few of the proteins present in the membrane preparation were recognized in the corresponding immunoblot (Figure 5; Table 2).
Figure 5.
Humoral response to Leptospira interrogans after vaccination with the live or heat-killed (HK) vaccine. Membrane preparations from L. interrogans serovar Manilae (A-C) or Pomona (D-F) were separated by 2-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis. Gels were stained with colloidal Coomassie blue (A and D) or transferred and probed with pooled serum samples from 5 hamsters vaccinated with either HK serovar Manilae (B and E) or live M1352 (C and F).
Table 2.
Identity of Two-dimensional Gel Electrophoresis Spots and Recognition by Leptospiral Vaccinate Serum Samples
| Spot no. |
Detection by hamster serum samples |
|||||
| Protein IDa | Serovar Manilae | Serovar Pomona | Protein description | Cellular locationb | HK WT vaccinates | M1352 vaccinates |
| LA0616 | M1 | P1 | LipL41 | Outer membrane | Yes | Yes |
| LBL1323 | M2 | … | FlaB1, flagella core protein | Periplasm | Yes | Yes |
| LA0492 | … | P2 | LipL36 | Outer membrane | Yes | Yes |
| LA2637 | M3 | P3 | LipL32 | Outer membrane | Yes | Yes |
| LA2637 | M4 | P4 | LipL32 | Outer membrane | Yes | Yes |
| LA2637 | M5 | P5 | LipL32 | Outer membrane | Yes | Yes |
| LA2637 | M6 | P6 | LipL32 | Outer membrane | Yes | Yes |
| LA3961 | M7 | P7 | OmpL36 | Outer membrane | No | Yes |
| LA0222 | M8 | P8 | Loa22, OmpA family outer membrane protein | Outer membrane | No | Yes |
| LA2372 | M9 | P9 | General secretory pathway protein G (gspG) | Unknown | No | Yes |
| LA1939 | … | P10 | Hypothetical lipoprotein | Unknown | No | Yes |
| LA1244 | M11 | … | ATP synthase subunit α | Cytoplasm | No | Yes |
| LA2655 | M12 | … | GroEL, chaperone | Cytoplasm | No | Yes |
| LIC10465 | M13 | … | LigA, leptospiral immunoglobin-like protein | Outer membrane, secreted | No | Yes |
NOTE. ATP, adenosine triphosphate; HK, heat-killed; WT, wild-type.
From leptospiral genome sequences of Leptospira borgpetersenii serovar Hardjobovis L550 (GenBank accession no. CP000348.1), Leptospira interrogans serovar Lai strain 56601 (GenBank accession no. AE010300.1), and L. interrogans serovar Copenhageni strain Fiocruz L1-130 (GenBank accession no. AE016823.1).
Analyzed using pSortb (http://www.psort.org/psortb/) [35, 40, 42, 45].
Only LipL32 and LipL41 were recognized by hamsters receiving either vaccine. LipL36 (spot P2) was recognized in Pomona by hamsters receiving either vaccine. This protein may also have been recognized in serovar Manilae (at spot M2), but the presence of FlaB1 in the preparation may have masked the signal in mass spectrometric analysis. Some antigens were recognized in both Pomona and Manilae only by hamsters receiving the live vaccine and thus constitute potential cross-protective antigens. This group included LA3961 (hypothetical protein), Loa22 (OmpA family protein), and LA2372 (general secretory protein G [GspG]).
Interestingly, LigA was identified in Manilae but not Pomona and the size differed considerably from the expected >100 kDa, possibly as a result of proteolytic processing or degradation. The antigen LA1939 was recognized in serovar Pomona (spot P10) but not Manilae, despite being identified in the Coomassie-stained gel by mass spectrometry (spot M10).
DISCUSSION
Currently, the only vaccines available against leptospirosis consist of killed whole-cell bacterins. These vaccines have a narrow range of specificity, provide only short-term immunity, and are reactogenic, making them generally unsuitable for widespread use in humans [1]. To date, recombinant protein vaccines against leptospirosis have shown limited success (reviewed by Adler and de la Pena Moctezuma [1]). Undefined, live, attenuated leptospiral vaccines have been generated through passage in eggs, exposure to gamma radiation, and long-term subculture in the laboratory. Such vaccines show promise for prevention of leptospirosis but suffer the major drawback that the genetic basis of the attenuation is not defined. In the present study, we describe the first (to our knowledge) live, attenuated vaccine for leptospirosis that has a defined mutation and elicits cross-protective immunity.
Previously we identified 2 transposon mutants with LPS-associated mutations that were highly attenuated in the hamster model of infection. When administered live, the LPS mutant M1352 elicited dose-dependent better protection against homologous challenge than did killed vaccines (either heat or formalin killed). The M1352 vaccine protected even against very high challenge with no kidney colonization, 100% animal survival, and reduced evidence of lung hemorrhage. Under the same conditions, the bacterin vaccine failed to fully protect hamsters or prevent kidney colonization. It is significant that the protection could be achieved with only a single vaccine dose.
The M1352 vaccine conferred significant cross-protective immunity in hamsters against infection with serovar Pomona. All of the M1352-vaccinated hamsters survived challenge, whereas only 40% of those receiving the heat-killed Manilae vaccine survived. However, the vaccine did not confer sterilizing immunity. Significantly, protection against Pomona occurred in the absence of agglutinating antibodies, consistent with the lack of LPS involvement in cross-protection. Neither the live nor the bacterin vaccine stimulated protection against colonization with L. borgpetersenii serovar Hardjobovis, which suggests that there may be significant antigenic differences between the 2 Leptospira species or perhaps differences in pathogenic mechanisms. Alternatively, immunity against acute infection may differ from immunity that prevents establishment of the renal carrier state by either serovar Pomona or Hardjobovis.
Previous studies have used undefined attenuated live leptospiral vaccines. Two doses of live, attenuated serovar Icterohaemorrhagiae vaccine protected guinea pigs from death and renal infection after homologous challenge, whereas formalin-killed control vaccines did not protect against clinical disease [30]. Cattle immunized twice with live, attenuated serovar Pomona were protected against homologous infection and had a reduced rate of abortion [31], whereas a live serovar Pomona vaccine protected swine against leptospiruria and renal lesions [32]. Stalheim et al [33] reported that live, attenuated serovar Pomona protected hamsters and swine from death but did not confer sterilizing immunity. Only partial cross-protection by live, attenuated leptospiral vaccines has been reported elsewhere [34]. However, in all of these studies, the nature of the mutation or mutations was unknown and the basis for attenuation was therefore not defined.
Surprisingly, the serum of vaccinated hamsters recognized relatively few leptospiral membrane antigens (11 total). It should be noted that antigens expressed exclusively in vivo would not be detected. Both vaccine groups responded strongly to dominant antigens of the outer membrane, LipL32, LipL41, and LipL36 (although LipL36 was potentially masked by FlaB1 in the mass spectrometry analysis). LipL32, the most abundant surface-exposed protein, is expressed in vivo [35, 36] and plays a role in adhesion to extracellular matrix components [22, 37], but it is not required for virulence in acute or renal colonization infection models [15]. LipL32 has been used in various vaccine formulations in numerous studies, but results regarding protection are conflicting, falling on the side of limited or no protection (reviewed by Adler and de la Pena Moctezuma [1]). Response to LipL32 was not exclusive to the live vaccine group, which suggests that it is not a key or essential antigen in the immune response. However, it may contribute to immunity in combination with other antigens.
LipL41 is among the 3 most abundant proteins on the cell surface, is expressed in vivo [36], and is recognized by patient serum samples [38], although the function of LipL41 is unknown. LipL41 showed promise as a subunit vaccine component, conferring protection when administered in combination with OmpL1 in Escherichia coli cell membranes [5] and supporting the notion that LipL41 is a protective antigen. However, these results have not been reproduced.
The outer membrane protein LipL36 has an unknown function and does not appear to be recognized by human convalescent serum samples [38]. Indeed, the expression of LipL36 is downregulated during colonization of hamster kidneys [39].
A number of potential outer membrane proteins were recognized by hamsters receiving the live vaccine but not by those receiving the heat-killed vaccine. These proteins included the hypothetical protein LA1939 and outer membrane proteins LA3961, Loa22, and LigA. Interestingly, the second most abundant outer membrane protein, LipL21, which is expressed in vivo and is detected by serum from chronically infected rats [36], was not detected by serum from vaccinated hamsters. Loa22 is a surface-exposed, outer membrane protein containing an OmpA domain [40]. It is expressed during acute and chronic infection [36] and is recognized by serum from human patients [41]. Loa22 is also essential for virulence in the hamster model of infection [16]—a role compatible with a protective role in immunity; antibodies directed against Loa22 may bind to the protein, thereby neutralizing its role in pathogenesis. However, the precise function of Loa22 remains undefined.
LA1939 and LA3961 may have been overlooked in previous subunit vaccine studies in favor of better characterized proteins. LA1939 (annotated as LIC11966 in serovar Copenhageni, with a different start codon) encodes a putative lipoprotein of 160 amino acids that is restricted to the species L. interrogans. The most similar protein outside the Leptospira species occurs in the environmental gram-negative bacillus and opportunistic pathogen Sphingobacterium spiritivorum (157 amino acids, 35% identity, and 59% similarity). LA3961 (LIC13166/OmpL36 [42]) encodes a 307–amino acid protein found in both pathogenic and saprophytic leptospires (76% identity to that in Leptospira biflexa LBF_0259), but there are no proteins of significant similarity outside the Leptospira species. OmpL36 is recognized by serum samples from infected hamsters and humans [43]. While the presence of an orthologue of LA3961 in L. biflexa suggests that it is unlikely to be a virulence factor, its presence in the outer membrane [42] may make it a target for opsonising antibodies.
LigA, 1 of 3 leptospiral immunoglobulin-like proteins, is recognized during infection in humans [44] and may play a role in adhesion of leptospires to host tissues. LigA shows promise as a protective antigen [6], supporting a role for LigA in immunity to leptospirosis.
The set of proteins identified in our study adds to the list of antigens known to be expressed in vivo and contributes to our understanding of the immune response to acute L. interrogans infection. Previously, Guerreiro et al [38] examined the humoral immune response in serum samples from patients with leptospirosis and found strong reactivity to ∼7 antigens (including LipL32, LipL41, and GroEL, which were also identified in this study). Gamberini et al [41] screened serum samples from human patients with leptospirosis for reactivity to 150 recombinant proteins from L. interrogans serovar Copenhageni. Again, relatively few antigens (n = 16) were recognized by serum samples, and a response to Loa22 and LipL32 was identified. LipL41, LipL36, and LA3961/LIC13166 were included in the study, but it is not clear if these were among the 73% of proteins that were successfully expressed and tested. Comparisons with the present study are difficult because of differences in methods and host species.
This study has shown that vaccination with a defined, live, attenuated strain of L. interrogans serovar Manilae can confer a high level of protection against homologous and heterologous challenge. Analysis of the immune response highlighted potentially protective antigens, including several promising vaccine candidates and one known virulence factor. Further investigation may give insights into the basis for the protective immune responses to L. interrogans and lead to the identification of cross-protective protein antigens.
Funding
This work was supported by the Australian National Health and Medical Research Council (grant numbers 545826 and 465188, Peter Doherty Fellowship to G.L.M.); and the Australian Research Council (grant CE0562063).
References
- 1.Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–96. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–86. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 3.Bolin CA, Alt DP. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar Hardjo. Am J Vet Res. 2001;62:995–1000. doi: 10.2460/ajvr.2001.62.995. [DOI] [PubMed] [Google Scholar]
- 4.Feigin RD, Lobes LA, Jr, Anderson D, Pickering L. Human leptospirosis from immunized dogs. Ann Intern Med. 1973;79:777–85. doi: 10.7326/0003-4819-79-6-777. [DOI] [PubMed] [Google Scholar]
- 5.Haake DA, Mazel MK, McCoy AM, et al. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–82. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva EF, Medeiros MA, McBride AJ, et al. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine. 2007;25:6277–86. doi: 10.1016/j.vaccine.2007.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulach DM, Zuerner RL, Wilson P, et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A. 2006;103:14560–5. doi: 10.1073/pnas.0603979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimento AL, Ko AI, Martins EA, et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004;186:2164–72. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picardeau M, Bulach DM, Bouchier C, et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One. 2008;3:e1607. doi: 10.1371/journal.pone.0001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren SX, Fu G, Jiang XG, et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422:888–93. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 11.Bourhy P, Louvel H, Saint Girons I, Picardeau M. Random insertional mutagenesis of Leptospira interrogans, the agent of leptospirosis, using a mariner transposon. J Bacteriol. 2005;187:3255–8. doi: 10.1128/JB.187.9.3255-3258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray GL, Ellis KM, Lo M, Adler B. Leptospira interrogans requires a functional heme oxygenase to scavenge iron from hemoglobin. Microbes and Infection. 2008;10:791–7. doi: 10.1016/j.micinf.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Murray GL, Morel V, Cerqueira GM, et al. Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect Immun. 2009;77:810–6. doi: 10.1128/IAI.01293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray GL, Srikram A, Henry R, Puapairoj A, Sermswan RW, Adler B. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect. 2009;11:311–4. doi: 10.1016/j.micinf.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Murray GL, Srikram A, Hoke DE, et al. Major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans. Infect Immun. 2009;77:952–8. doi: 10.1128/IAI.01370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ristow P, Bourhy P, da Cruz McBride FW, et al. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 2007;3:e97. doi: 10.1371/journal.ppat.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray GL, Srikram A, Henry R, Hartskeerl RA, Sermswan RW, Adler B. Mutations affecting Leptospira interrogans lipopolysaccharide attenuate virulence. Mol Microbiol. 2010;78:701–9. doi: 10.1111/j.1365-2958.2010.07360.x. [DOI] [PubMed] [Google Scholar]
- 18.Srikram A, Wongratanacheewin S, Puapairoj A, Wuthiekanun V, Sermswan RW. Analyses of vaccination protocols for Leptospira interrogans serovar Autumnalis in hamsters. Am J Trop Med Hyg. 2008;79:779–86. [PubMed] [Google Scholar]
- 19.Jittimanee J, Sermswan RW, Puapairoj A, Maleewong W, Wongratanacheewin S. Cytokine expression in hamsters experimentally infected with Opisthorchis viverrini. Parasite Immunol. 2007;29:159–67. doi: 10.1111/j.1365-3024.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun. 2007;75:2864–74. doi: 10.1128/IAI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imin N, Kerim T, Weinman JJ, Rolfe BG. Characterisation of rice anther proteins expressed at the young microspore stage. Proteomics. 2001;1:1149–61. doi: 10.1002/1615-9861(200109)1:9<1149::AID-PROT1149>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Hoke DE, Egan S, Cullen PA, Adler B. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect Immun. 2008;76:2063–9. doi: 10.1128/IAI.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen PA, Xu X, Matsunaga J, et al. Surfaceome of Leptospira spp. Infect Immun. 2005;73:4853–63. doi: 10.1128/IAI.73.8.4853-4863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im JG, Yeon KM, Han MC, et al. Leptospirosis of the lung: radiographic findings in 58 patients. AJR Am J Roentgenol. 1989;152:955–9. doi: 10.2214/ajr.152.5.955. [DOI] [PubMed] [Google Scholar]
- 25.Zaki SR, Shieh WJ. Leptospirosis associated with outbreak of acute febrile illness and pulmonary haemorrhage, Nicaragua, 1995. The Epidemic Working Group at Ministry of Health in Nicaragua. Lancet. 1996;347:535–6. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]
- 26.Adler B, Faine S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect Immun. 1977;17:67–72. doi: 10.1128/iai.17.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler B, Faine S. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans serovar Pomona. Infect Immun. 1976;14:703–8. doi: 10.1128/iai.14.3.703-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler B, Faine S. The antibodies involved in the human immune response to leptospiral infection. J Med Microbiol. 1978;11:387–400. doi: 10.1099/00222615-11-4-387. [DOI] [PubMed] [Google Scholar]
- 29.Adler B, Faine S. Immunogenicity of boiled compared with formalized leptospiral vaccines in rabbits, hamsters and humans. J Hyg (Lond) 1980;84:1–10. doi: 10.1017/s0022172400026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbert WT, Miller JN. Studies on immunity in experimental leptospirosis: the immunogenicity of Leptospira icterohemorrhagiae attenuated by gamma-irradiation. J Immunol. 1965;95:759–64. [PubMed] [Google Scholar]
- 31.Kenzy SG, Gillespie RW, Lee JH. Comparison of Leptospira pomona bacterin and attenuated live culture vaccine for control of abortion in cattle. J Am Vet Med Assoc. 1961;139:452–4. [PubMed] [Google Scholar]
- 32.Fish NA, Kingscote B. Protection of gilts against leptospirosis by use of a live vaccine. Can Vet J. 1973;14:12–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Stalheim OH. Vaccination against leptospirosis: protection of hamsters and swine against renal leptospirosis by killed but intact gamma-irradiated or dihydrostreptomycin-exposed Leptospira pomona. Am J Vet Res. 1967;28:1671–6. [PubMed] [Google Scholar]
- 34.Babudieri B, Castelli M, Pisoni F. Comparative tests with formolized and irradiated vaccines against leptospirosis. Bull World Health Organ. 1973;48:587–90. [PMC free article] [PubMed] [Google Scholar]
- 35.Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun. 2002;70:2311–8. doi: 10.1128/IAI.70.5.2311-2318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nally JE, Whitelegge JP, Bassilian S, Blanco DR, Lovett MA. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect Immun. 2007;75:766–73. doi: 10.1128/IAI.00741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauk P, Macedo F, Romero EC, et al. LipL32, the major leptospiral lipoprotein, the C-terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect Immun. 2008;75:2642–50. doi: 10.1128/IAI.01639-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerreiro H, Croda J, Flannery B, et al. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect Immun. 2001;69:4958–68. doi: 10.1128/IAI.69.8.4958-4968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett JK, Barnett D, Bolin CA, et al. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–61. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koizumi N, Watanabe H. Molecular cloning and characterization of a novel leptospiral lipoprotein with OmpA domain. FEMS Microbiol Lett. 2003;226:215–9. doi: 10.1016/S0378-1097(03)00619-0. [DOI] [PubMed] [Google Scholar]
- 41.Gamberini M, Gomez RM, Atzingen MV, et al. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol Lett. 2005;244:305–13. doi: 10.1016/j.femsle.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Pinne M, Haake DA. A comprehensive approach to identification of surface-exposed, outer membrane-spanning proteins of Leptospira interrogans. PLoS One. 2009;4:e6071. doi: 10.1371/journal.pone.0006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinne M, Choy HA, Haake DA. The OmpL37 surface-exposed protein is expressed by pathogenic Leptospira during infection and binds skin and vascular elastin. PLoS Negl Trop Dis. 2010;4:e815. doi: 10.1371/journal.pntd.0000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsunaga J, Barocchi MA, Croda J, et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol Microbiol. 2003;49:929–45. doi: 10.1046/j.1365-2958.2003.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsunaga J, Sanchez Y, Xu X, Haake DA. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect Immun. 2005;73:70–8. doi: 10.1128/IAI.73.1.70-78.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]