Abstract
Background. Staphylococcal enterotoxin B (SEB) may be associated with the exacerbation of atopic dermatitis. We investigated whether SEB causes proliferation of sensory C-fibers and subsequent enhancement of plasma leakage induced by sensorineural stimulation in rat skin.
Methods. SEB was applied intracutaneously to the abdomen of preweaning and adult rats. Evans blue dye leakage into the skin induced by topical 10% formalin was measured as an index of neurogenic skin vascular permeability. Local expression of substance P, tachykinin NK1 receptors, and nerve growth factor was assessed immunohistochemically. In addition, we assessed the effects of topical tacrolimus on these skin responses induced by SEB.
Results. Increased neurogenic skin plasma leakage was seen 7 days after SEB treatment in 2 different age groups. Innervation of substance P-immunoreactive nerves and expression of tachykinin NK1 receptors and nerve growth factor were also promoted by SEB, peaking at 7 days, 7 days, and 56 h after SEB treatment, respectively. Tacrolimus markedly inhibited these skin changes.
Conclusions. SEB increased the innervation of sensory C-fibers and tachykinin NK1 receptors in rat skin, probably because of upregulated production of neurotrophins, including nerve growth factor, leading to enhancement of neurogenic skin inflammation. T cell activation induced by SEB may initiate these changes.
Patients with atopic dermatitis have an increased susceptibility to infection or colonization with bacteria, fungi, and viruses, most notably Staphylococcus aureus [1, 2]. The main consequence of increased infection or colonization with S. aureus in the skin may be the development and exacerbation of eczematous symptoms [1, 2]. However, the contribution of superantigens released from S. aureus, such as staphylococcal enterotoxins A, B, and C [1, 3, 4], to the pathogenesis of atopic dermatitis is controversial. There are multiple lines of evidence supporting such an etiological role of these superantigens: staphylococcal enterotoxin B (SEB) applied to intact human skin induces eczematous lesions [5]; in animal models, epicutaneously applied SEB induces a strong skin inflammatory response that is T cell-dependent [6]; and repeated epicutaneous exposure to SEB induces allergic skin inflammation [7, 8]. In addition, SEB potently promotes polyclonal activation of SEB-reactive TCR Vβ families of T cells in the skin of patients with atopic dermatitis [9, 10]. Further potential mechanisms by which staphylococcal superantigens can enhance skin inflammation need to be investigated.
Neurogenic inflammation is defined as inflammatory responses mainly mediated by neuropeptides, such as substance P, that are released from sensory C-fibers [11–13]. The released neuropeptides lead to pain, pruritus, vascular modulation (ie, plasma leakage, vasodilation, and endothelial cell activation), and activation of immune cells, including T and B cells, and mast cells [11–13]. Therefore, exaggerated neurogenic skin inflammation, which is based on pathophysiological changes, including hyperinnervation of sensory C-fibers, an increase in tachykinin receptors, and impairment of neutral endopeptidases [11–13], is likely to be associated with several inflammatory skin diseases, such as atopic dermatitis. In fact, the production of neurotrophins, such as nerve growth factor (NGF), increases in the skin of patients with atopic dermatitis [14, 15]. Toyoda et al [16] showed that increased plasma concentrations of substance P and NGF reflect an increased severity of atopic dermatitis. However, to our knowledge, there are no data indicating that microbial superantigens augment skin neurogenic inflammation. We therefore determined whether SEB causes the proliferation of sensory C-fibers and subsequent enhancement of neurogenic inflammation in rat skin.
Topical tacrolimus is an immunosuppressive agent that inhibits T cell activation and T cell–derived cytokine production [17, 18] and is highly efficacious for the treatment for atopic dermatitis [19, 20]. We therefore used tacrolimus ointment to investigate the role of T cell activation in rat skin neurogenic mechanisms that are affected by SEB treatment.
Atopic dermatitis often presents within the first 2 years of life [21]. There is a strong association between infection with S. aureus and acute and subacute skin symptoms, such as impetigized lesions, that are commonly seen in infants with atopic dermatitis [1, 2], suggesting that staphylococcal infection, partially involving superantigen-mediated mechanisms, may also potentially contribute to exaggerated neurogenic inflammation in atopic dermatitis during infancy. However, we previously showed that innervation of sensory C-fibers and tachykinin NK1 receptors were relatively sparse in the normal skin of pups, compared with those in adults [22]. We therefore investigated age-related differences in the effects of SEB on mechanisms associated with neurogenic skin inflammation between preweaning and adult rats.
METHODS
Animals
All animal experimentation was conducted with the prior approval of the Animal Ethics Committee of the Institute for Laboratory Animal Research, Nagoya University Graduate School of Medicine. This committee operates in accordance with the Guide for the Care and Use of Laboratory Animals of Nagoya University (2007; Nagoya, Japan). Pathogen-free male Wistar rats aged 14 days (weight, 22–25 g) and 8 weeks (weight, 240–260 g) were purchased from Japan SLC 3 days before the experiments. Rat pups that had not been weaned were housed with their dams in polycarbonate cages with polyester filter covers.
Materials
SEB (Toxin Technology), capsaicin, and 37% (W/V) formaldehyde solution (formalin) were dissolved in phosphate-buffered saline (PBS), neat ethanol, and .9% saline, respectively. Tacrolimus ointment (.03% and .1%; Protopic), its base (ingredients: mineral oil, paraffin, propylene carbonate, white petrolatum, and white wax), and fluocinonide ointment (.05%; Topsym) were donated by Astellas Pharmaceuticals.
Immunofluorescence Staining Methods
Localization of substance P, transient receptor potential channel vanilloid 1 (TRPV1), tachykinin NK1 receptors, endothelial cells, NGF, brain-derived neurotrophic factor (BDNF), and tumor necrosis factor (TNF)–α were examined using immunofluorescence staining methods, and fluorescence image analyses were performed according to our previous methods [22]. PBS containing 1.5% nonimmune goat serum (Vector Laboratories) or mouse serum (Vector Laboratories) was used for blocking unoccupied sites. Rabbit antiserum to substance P (Chemicon International), goat immunoglobulin (Ig) G polyclonal antibodies to TRPV1 (Santa Cruz Biotechnology), BDNF (Santa Cruz Biotechnology) and TNF-α (R&D Systems), rabbit IgG polyclonal antibodies to tachykinin NK1 receptors (Sigma Aldrich) and NGF (Chemicon International), and mouse IgG1 monoclonal antibody to rat endothelial cells (Hycult Biotechnology) were used as a primary antibody. Goat IgG polyclonal antibody to rabbit IgG (Jackson ImmunoResearch Laboratories), mouse IgG polyclonal antibody to goat IgG (Chemicon International), and goat IgG polyclonal antibody to mouse IgG1 (Invitrogen) conjugated with Cy3 were used as a second step reagent for indirect immunofluorescent staining. Nonimmune goat IgG (Chemicon International) or nonimmune rabbit serum (Pierce Biotechnology) was used as a negative control for a primary antibody. Substance P and TRPV1 are widely distributed not only in nervous systems but also in nonneural cells [23, 24]. However, nervous systems are undoubtedly the major source of these molecules in the skin [11, 12, 23]. In addition, our preliminary experiments showed that substance P- and TRPV1-immunoreactive sites were almost all localized in PGP9.5-immunoreactive sites, suggesting that substance P- and TRPV1-immunoreactivities may mainly represent nerve fibers (data not shown). Therefore, the substance P- and TRPV1-immunoreactivites in the skin were defined as nerve fibers. For all experiments, the immunohistochemical results were evaluated in a blind manner and were repeated twice.
Experimental Protocols
Time-Course Changes in Histological and Immunohistochemical Features in the Skin Induced by Intracutaneous SEB
Rats from both age groups were anesthetized using gaseous diethyl ether. SEB (2–200 μg/mL) or PBS was injected intracutaneously in volumes of 20 μL (pups) or 80 μL (adults) in 4 areas marked on the shaved abdomen in random order. Skin samples were collected at several times after injection to assess histological and immunohistochemical changes induced by intracutaneous SEB.
Enhancement of Neurogenic Plasma Leakage in the Skin by Intracutaneous SEB
Neurogenic microvascular leakage in the skin was measured by quantifying the extravasation of Evans blue dye induced by topical formalin, according to our previous study [22, 25]. SEB (200 μg/mL) or PBS was administered intracutaneously to anesthetized rats of both age groups (6 per group), as described above. Seven or 14 days later, after induction of anesthesia and close shaving of the skin, Evans blue dye (20 mg/kg) was injected via the caudal vein, and 8 μL of 10% formalin and .9% saline were placed on the sites treated with SEB or PBS. Each site was then obliquely punctured and lifted with the apical port (2 mm) of a 21 G injection needle (Terumo). Thirty min later, skin samples were collected to measure leakage of the dye. All rats tested were pretreated with phosphoramidon (2.5 mg/kg intravenously) to prevent interference with neutral endopeptidases in the skin, according to a previous study [26].
Assessment of the Ability of Adult Rat Serum to Block the Effect of SEB in Pups
Pooled serum obtained from rat adults was incubated at 56°C for 30 min to inhibit complement activities. SEB was mixed with the inactivated serum 30 min before SEB injection. Histological changes in the skin of rat pups that were induced by intracutaneous SEB (100 μg/mL) alone or SEB (100 μg/mL) mixed with the serum (10% or 30%) were assessed.
Effects of Tacrolimus Ointment on SEB-Induced Enhancement of Neurogenic Plasma Leakage and Immunohistochemical Changes in the Skin
Tacrolimus ointment (.03% or .1%), fluocinonide ointment (.05%), or the tacrolimus ointment base was topically applied on the shaved abdomen of rat pups (6 per group) at 8 h before and immediately after intracutaneous injection of SEB (200 μg/mL) or PBS. Seven days later, dye leakage in the skin induced by topical 10% formalin or .9% saline was assessed in the groups pretreated with tacrolimus ointment (.1%) or its base. Substance P content in the skin was also measured according to our previous methods [22]. In addition, histological and immunohistochemical changes induced by SEB were evaluated in all 3 pretreated groups. The concentration of tacrolimus ointment (.03% and .1%) used in this study was based on previous studies showing that .1% topical tacrolimus reduces plasma leakage induced by topical m-xylene in rat skin [27] and delays allergic responses in mouse skin [28]. The fluocinonide ointment (.05%) used was a high-potency (class II) glucocorticosteroid.
Statistical Analysis
Statistical analysis was performed using StatView software, version 4.0 (Abacus Concept). Values are expressed as the means ± standard error of the mean. To evaluate the significance of the difference between 2 independent groups with equal variance, which was originally assessed using the F test, we performed an unpaired Student's t test (2-tailed). When the variance was unequal, we used Welch's test (2-tailed). A level of P < .05 was considered to be statistically significant.
RESULTS
Effects of Intracutaneous SEB on Histological Features of the Skin of Rats of 2 Different Ages
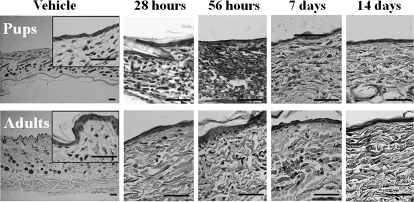
In both pups and adults, SEB caused infiltration of lymphocytes in the dermis within 28 h, peaking at 56 h after treatment (Figure 1). The response then gradually returned to the basal level by day 14 (Figure 1). The SEB response was concentration-dependent for both groups of rats (data not shown) and was clearly observed in pups at concentrations of SEB as low as 20 μg/mL. However, higher SEB concentrations were required for the SEB response in adults (ie, ≥80 μg/mL) and only a weak response was observed in adults when SEB was injected at the same concentration as in pups. Hardly any infiltration of macrophages, neutrophils, or eosinophils in the dermis was observed in either of the 2 age groups during the period of observation.
Figure 1.
Histological changes in the skin in 2- and 8-week-old rats after staphylococcal enterotoxin B (SEB) or vehicle injection. Twenty or 80 μL of SEB (200 μg/mL) or vehicle per site was injected intracutaneously into the shaved abdomen of rat pups or adults, respectively. Skin samples were stained with hematoxylin and eosin 28 or 56 h or 7 or 14 days after injection. Bars=50 μm. The photographs are representative of at least 6 independent experiments.
Effects of Intracutaneous SEB on Skin Innervation with Substance P- and TRPV1-immunoreactive Nerves Using Immunofluorescent Staining
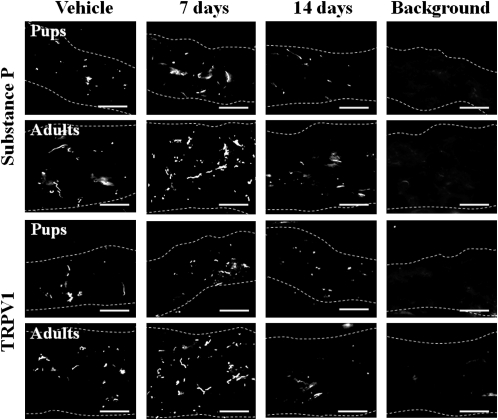
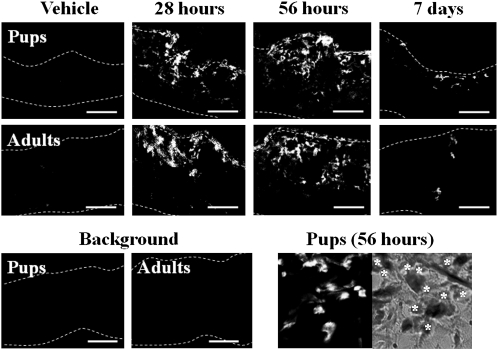
In adults, innervation of the skin with substance P- and TRPV1-immunoreactive nerves increased at 7 days after SEB injection but had returned to the basal level by day 14 (Figure 2). Both immunoreactive nerve types were distributed throughout the dermis, and parts of their endings reached into the epidermis. These proliferative changes in nerve fibers were SEB concentration–dependent for both groups of rats (data not shown) and were less marked in the pups than in the adults (Figure 2). However, the threshold concentration for induction of these changes was lower in pups (2 μg/mL) than in adults (80 μg/mL).
Figure 2.
Innervation of sensory C-fibers in the skin of 2- and 8-week-old rats after intracutaneous injection of staphylococcal enterotoxin B (SEB). Sensory C-fibers in skin sections of rat pups and adults were analyzed by immunofluorescent staining using substance P and transient receptor potential channel vanilloid 1 (TRPV1) antibodies or using control, nonimmune serum (Background) 7 and 14 days after SEB injection as in Figure 1. Dotted lines outline the borders of the dermis. Bars=20 μm. The photographs are representative of at least 6 independent experiments.
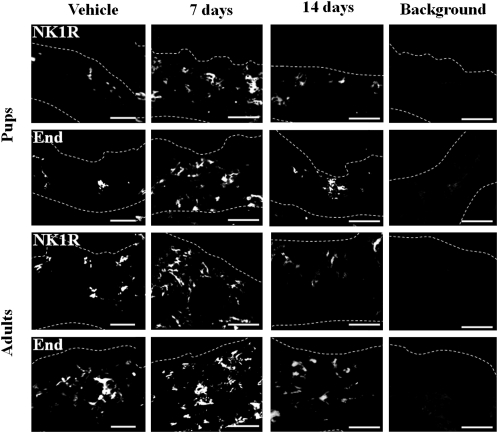
Tachykinin NK1 receptors were detected mainly on endothelial cells of blood vessels in the dermis and were markedly upregulated in both groups of rats at 7 days after SEB injection (Figure 3). The level of these receptors had almost reverted to the basal level by day 14 (Figure 3). These changes were concentration-dependent for both groups of rats (data not shown); the threshold concentration was 2 μg/mL in pups and 80 μg/mL in adults. In the rats from both age groups, the observed changes in endothelial cell distribution induced by SEB closely paralleled those induced in the expression of tachykinin NK1 receptors (Figure 3).
Figure 3.
Local expression of tachykinin NK1 receptors and endothelial cells in the skin of 2- and 8-week-old rats after intracutaneous injection with staphylococcal enterotoxin B (SEB). Skin sections were analyzed by immunofluorescent staining using specific antibodies against tachykinin NK1 receptors (NK1R) and endothelial cells (End) or using control, nonimmune serum (Background) 7 and 14 days after SEB injection as in Figure 1. Dotted lines outline the borders of the dermis. Bars=50 μm. The photographs are representative of at least 6 independent experiments.
Effects of Intracutaneous SEB on Immunohistochemical Localization of NGF, BDNF, and TNF-α in the Skin of Rats of 2 Different Ages
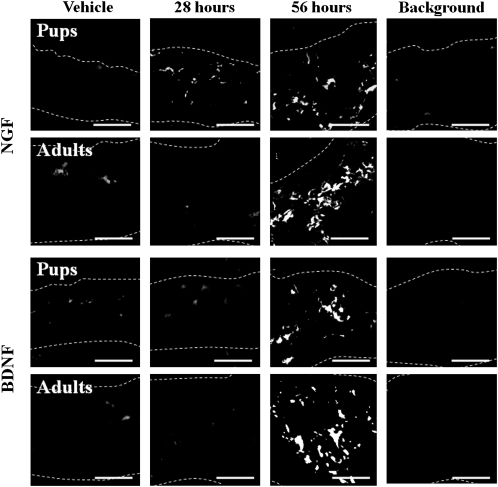
In the 2 age groups, NGF and BDNF were constitutively expressed by fibroblasts in the dermis based on immunofluorescence analysis, although the signals were very faint (Figure 4). SEB markedly enhanced the expression of NGF and BDNF at 56 h after treatment (Figure 4), and this response had disappeared by day 7 (data not shown). In pups, increased expression of NGF was also detected at 28 h after treatment with SEB (Figure 4). These responses were concentration dependent for both groups of rats (data not shown); the threshold concentration was 2μg/mL in pups and 80 μg/mL in adults.
Figure 4.
Effect of staphylococcal enterotoxin B (SEB) injection on the local expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in rat skin. Skin sections of rat pups and adults were analyzed by immunofluorescent staining using specific antibodies against NGF and BDNF or using control, nonimmune serum (Background) 28 or 56 h after SEB injection as in Figure 1. Dotted lines outline the borders of the dermis. Bars=50 μm. The photographs are representative of at least 6 independent experiments.
SEB enhanced the expression of TNF-α mainly in lymphocytes that were sparsely localized in the dermis at 14 h after treatment of both age groups of rats (data not shown). Subsequently, TNF-α expression was enhanced in keratinocytes and fibroblasts as well as in infiltrating lymphocytes and reached a peak at 56 h after SEB injection (Figure 5). The level of TNF-α expression returned to the basal level by day 7 (Figure 5). In the rats from both age groups, the observed changes in TNF-α expression were concentration dependent (data not shown); the threshold concentration of SEB for this effect was 2 μg/mL in pups and 80 μg/mL in adults.
Figure 5.
Effect of staphylococcal enterotoxin B (SEB) injection on the local expression of tumor necrosis factor (TNF)–α in the skin. Skin sections of rat pups and adults were analyzed by immunofluorescent staining using specific antibodies against TNF-α or using control, nonimmune serum (Background) 28 or 56 h or 7 days after SEB injection as in Figure 1. Dotted lines outline the borders of the dermis. Bars=50 μm. The photographs are representative of at least 6 independent experiments. Infiltrating lymphocytes are indicated by asterisks in the photo at bottom right.
Enhancement of the Effect of Intracutaneous SEB on Neurogenic Skin Plasma leakage
Dye leakage in the skin induced by topical formalin was significantly enhanced at 7 days after treatment with SEB in pups (15.7 ± 1.3 vs 22.8 ± 2.1 μg/mg of wet tissue; P < .05; n = 6; analyzed by an unpaired t test) and in adults (49.0 ± 4.9 vs 82.7 ± 5.6 μg/mg of wet tissue; P < .01; n = 6; analyzed by an unpaired t test). The enhanced response induced by SEB was not observed in pups or adults after 7 days (data not shown).
Assessment of the Ability of Adult Rat Serum to Block the Effect of SEB
There were no differences in histological changes, particularly in lymphocyte infiltration, in the skin of the 3 groups of rat pups injected with SEB alone or with SEB mixed with serum from rat adults (10% or 30%; data not shown). Intracutaneous injection of the serum (30%) alone had no effect.
Effects of Tacrolimus and Fluocinonide Ointments on Histological and Immunohistochemical Changes Induced by Intracutaneous SEB in the Skin of Rat Pups
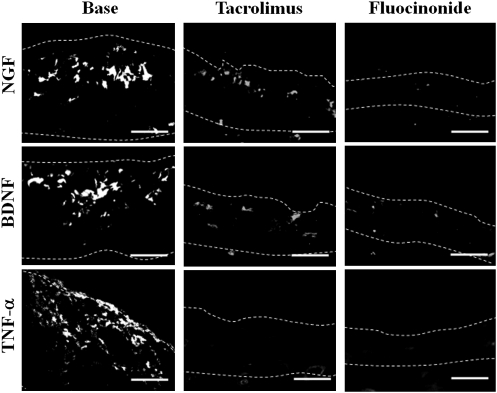
Tacrolimus (.03 and .1%) and fluocinonide (.05%) almost completely inhibited lymphocyte infiltration in the skin that was induced by SEB injection (data not shown), as well as the SEB-induced increased skin innervation with substance P- and TRPV1-immunoreactive nerves (Figure 6 and data not shown, respectively, and the SEB-induced increased expression of tachykinin NK1 receptors and endothelial cells in the skin (Figure 6). The base of the tacrolimus ointment, used as a control, had no effect on these parameters. Furthermore, tacrolimus (.03 and .1%) and fluocinonide (.05%), but not the base, markedly inhibited the enhanced expression of NGF, BDNF, and TNF-α that was induced in the skin after SEB injection (Figure 7).
Figure 6.
Effect of topical tacrolimus, fluocinonide, and base on staphylococcal enterotoxin B (SEB) enhancement of sensory C-fiber proliferation, expression of tachykinin NK1 receptors and endothelial cells in rat pups. Tacrolimus (.1%), fluocinonide (.05%), and base were topically applied to the skin of rat pups treated with SEB as in Figure 1. Seven days after injection, skin sections were analyzed by immunofluorescence for proliferation of sensory C-fibers (SP, upper panels, using substance P antibody), expression of tachykinin NK1 receptors (NK1R, middle panels) and endothelial cells (End, lower panels). Dotted lines outline the borders of the dermis. Bars=50 μm. The photographs are representative of 6 independent experiments.
Figure 7.
Effect of topical tacrolimus, fluocinonide, and base on staphylococcal enterotoxin B (SEB) enhancement of neurotrophins and tumor necrosis factor (TNF)–α in the skin of 2-week-old rats. Tacrolimus (.1%), fluocinonide (.05%) and base were topically applied to the skin of rats intracutaneously injected with SEB (200 μg/mL). The expression of neurotrophins (NGF and BDNF) and TNF-α was assayed by immunofluorescence using specific antibodies 56 and 28 h, respectively, after injection. Dotted lines outline the borders of the dermis. Bars=50 μm. The photographs are representative of 6 independent experiments.
SEB significantly increased substance P content in the skin (21.15 ± 1.07 vs 26.83 ± .85 ng/g of wet tissue; P < .01; n = 6; analyzed by an unpaired t test). Tacrolimus (.1%) and fluocinonide (.05%) completely suppressed the increased substance P content (26.83 ± .85 vs 20.41 ± .87 and 21.29 ± .87 ng/g of wet tissue, respectively; P < .01; n = 6; analyzed by an unpaired t test). In addition, SEB significantly enhanced the dye leakage induced by topical formalin in the skin from 18.3 ± 2.0 to 26.6 ± 2.5 μg/mg of wet tissue (P < .05; n = 6; analyzed by an unpaired t test). Tacrolimus (.1 %) decreased this response to the basal level (26.6 ± 2.5 vs 15.9 ± 1.8 μg/mg of wet tissue; P < .01; n = 6; analyzed by an unpaired t test).
DISCUSSION
We showed that intracutaneous SEB enhances neurogenic skin microvascular leakage 7 days after treatment of rat pups and adults. Using immunohistochemical methods, we revealed that intracutaneous SEB increases innervation of substance P- and TRPV1-immunoreactive nerves in the skin of rat pups and adults at 7 days after treatment with SEB. Because substance P- and TRPV1-immunoreactive nerves mainly represent sensory C-fibers [11, 12], our data indicate that SEB promotes the proliferation of sensory C-fibers in both age groups. This finding was confirmed by the SEB-induced increase in substance P content in the skin of rat pups. These results suggest that innervation of sensory C-fibers may be positively related to the amount of endogenously released tachykinins in the skin.
In the present study, there were significant age-dependent differences in the increased innervation of sensory C-fibers induced by SEB. Thus, the SEB-induced increase was less in pups than it was in adults at 7 days after treatment with SEB. In contrast, the expression of tachykinin NK1 receptors was strongly upregulated by SEB in both age groups. Hu et al [29] also showed that exogenous NGF doubles the expression of tachykinin NK1 receptors in the lungs of weaning and adult rats. SEB increased neurogenic skin microvascular leakage to a similar extent in rat pups and adults. Thus, the enhanced expression of tachykinin NK1 receptors may play a more important role in induction of the enhanced neurogenic skin inflammation in rat pups [30, 31] than in the increased innervation of sensory C-fibers.
An increased level of both endogenous and exogenous NGF has been demonstrated to increase the innervation of sensory C-fibers in the skin [32–34]. The effects of NGF on sensory C-fibers appear to be similar to those of BDNF in the skin [11, 35]. These neurotrophins also increase the level of tachykinin NK1 receptors, as shown in the rat airway [29]. We found that the local expression of NGF and BDNF was strongly upregulated by SEB predominantly in fibroblasts in both age groups and that this upregulation was followed by increased proliferation of sensory C-fibers and the upregulation of tachykinin NK1 receptors. These data suggest that NGF and BDNF, released from fibroblasts by SEB, may promote the observed SEB-induced neurogenic mechanisms in the skin. However, our data only provide indirect evidence for this hypothesis, because we did not evaluate the effect of blockade of these neurotrophins on the responses induced by SEB.
In the present study, in rats of 2 different ages, SEB rapidly increased expression of TNF-α in infiltrating lymphocytes in the skin, followed by increased expression of NGF and BDNF in dermal fibroblasts and TNF-α in keratinocytes and fibroblasts. TNF-α and IL-1β are known to promote the production of neurotrophins, such as NGF and BDNF, from epithelial cells and fibroblasts in the airway [36–38]. TNF-α also induces the production of NGF in human foreskin fibroblasts [39] and increases the expression of NGF in mouse skin [40]. Therefore, it is possible that, in our study, primarily and secondarily released TNF-α triggered the production of NGF and BDNF in the skin and maintained their production at high levels.
We revealed that the threshold concentrations of SEB required for increases in innervation of substance P- and TRPV1-immunoreactive nerves, for expression of NGF, BDNF, and TNF-α, and for induction of lymphocyte infiltration in rat skin are much lower in pups than in adults. To our knowledge, there is no report regarding age-dependent differences in these skin responses induced by SEB. The reason for these age-dependent differences is not clear. However, in the present study, addition of rat adult serum to SEB did not alter the time course or the extent of histological changes induced by SEB in rat pups. Thus, these age-dependent differences are unlikely to depend on an antibody that blocks SEB effects that was acquired by adult rats.
We revealed that tacrolimus completely suppresses the SEB-induced upregulation of TNF-α expression in infiltrating lymphocytes in the skin by strongly inhibiting T cell activation [17, 18]. In addition, tacrolimus likely blocked the release of other T cell–derived cytokines or mediators, such as IL-1β, that promote production of neurotrophins, because previous data showed that tacrolimus largely inhibited the release of TNF-α and IL-1β from human T cells stimulated with monoclonal antibodies to CD3 and CD28 [41, 42]. In our study, tacrolimus strongly suppressed the SEB-induced enhancement of expression of NGF and BDNF, innervation of substance P- and TRPV1-immunoreactive nerves, and neurogenic inflammation in the skin. Therefore, SEB-induced T cell activation may initiate these changes. This possibility is supported by our finding that fluocinonide had the same suppressive effect as tacrolimus, because glucocorticoids are known to suppress activation of T cells [43]. However, we cannot exclude the possibility that something other than the superantigenic property of SEB contributed to these skin changes, because we have not shown that SEB expands T cell subsets in the skin that are specifically responsive to SEB as a superantigen.
In conclusion, SEB enhanced the innervation of sensory C-fibers and the expression of tachykinin NK1 receptors in the skin of rat pups and adults. These changes were likely caused by the upregulated production of neurotrophins, such as NGF and BDNF, at least partially through a TNF-α–mediated pathway. T cell activation induced by SEB may initiate these changes. The duration of the SEB-induced enhancing effects was short but was of similar duration to enhancing effects in a skin-scratching mouse model [44]. However, SEB may have the potential to trigger a vicious circle of chronic skin inflammation, because the SEB-induced enhancement may be maintained by repeated stimulation with SEB and scratching, which increase in the skin of patients with atopic dermatitis. Finally, further investigation is required to assess whether other superantigens, such as SEA and SEC, also promote mechanisms associated with increased neurogenic skin inflammation.
Funding
This work was supported by the National Institute of Occupational Safety and Health, Japan (KIBAN37610).
References
- 1.Cardona ID, Cho SH, Leung DY. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am J Clin Dermatol. 2006;7:273–9. doi: 10.2165/00128071-200607050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozman A, Yao Y, Bina P, et al. Encoding a superantigen by Staphylococcus aureus does not affect clinical characteristics of infected atopic dermatitis lesions. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.09966.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkwright PD, Cookson BD, Haeney MR, Sanyal D, Potter MR, David TJ. Children with atopic dermatitis who carry toxin-positive Staphylococcus aureus strains have an expansion of blood CD5- B lymphocytes without an increase in disease severity. Clin Exp Immunol. 2001;125:184–9. doi: 10.1046/j.1365-2249.2001.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- 6.Saloga J, Leung DY, Reardon C, Giorno RC, Born W, Gelfand EW. Cutaneous exposure to the superantigen staphylococcal enterotoxin B elicits a T-cell-dependent inflammatory response. J Invest Dermatol. 1996;106:982–8. doi: 10.1111/1523-1747.ep12338479. [DOI] [PubMed] [Google Scholar]
- 7.Savinko T, Lauerma A, Lehtimaki S, et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J Immunol. 2005;175:8320–6. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- 8.Laouini D, Kawamoto S, Yalcindag A, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–7. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Skov L, Olsen JV, Giorno R, Schlievert PM, Baadsgaard O, Leung DY. Application of Staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T cells by a superantigen-mediated mechanism. J Allergy Clin Immunol. 2000;105:820–6. doi: 10.1067/mai.2000.105524. [DOI] [PubMed] [Google Scholar]
- 10.Davison S, Allen M, Vaughan R, Barker J. Staphylococcal toxin-induced T cell proliferation in atopic eczema correlates with increased use of superantigen-reactive Vbeta-chains in cutaneous lymphocyte-associated antigen (CLA)-positive lymphocytes. Clin Exp Immunol. 2000;121:181–6. doi: 10.1046/j.1365-2249.2000.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 13.Peters EMJ, Ericson ME, Hosoi J, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006;126:1937–47. doi: 10.1038/sj.jid.5700429. [DOI] [PubMed] [Google Scholar]
- 14.Dou YC, Hagstromer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006;298:31–7. doi: 10.1007/s00403-006-0657-1. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009;53:48–54. doi: 10.1016/j.jdermsci.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71–9. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 17.Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718–26. [PubMed] [Google Scholar]
- 18.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797–803. [PubMed] [Google Scholar]
- 19.Breuer K, Werfel T, Kapp A. Safety and efficacy of topical calcineurin inhibitors in the treatment of childhood atopic dermatitis. Am J Clin Dermatol. 2005;6:65–77. doi: 10.2165/00128071-200506020-00001. [DOI] [PubMed] [Google Scholar]
- 20.El-Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. 2009;54:76–87. doi: 10.1016/j.jdermsci.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Development of atopic dermatitis in the DARC birth cohort. Pediatr Allergy Immunol. 2010;21:307–14. doi: 10.1111/j.1399-3038.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohshima M, Miyake M, Takeda M, et al. Development of mechanisms associated with neurogenic-mediated skin inflammation during the growth of rats. Pediatr Res. 2010;67:363–8. doi: 10.1203/PDR.0b013e3181d026a5. [DOI] [PubMed] [Google Scholar]
- 23.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 24.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology. 2006;44:128–34. [PubMed] [Google Scholar]
- 25.Futamura M, Goto S, Kimura R, et al. Differential effects of topically applied formalin and aromatic compounds on neurogenic-mediated microvascular leakage in rat skin. Toxicology. 2009;255:100–6. doi: 10.1016/j.tox.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Chakir M, D'Orléans-Juste P, Plante GE. Neutral endopeptidase inhibition, a new approach in the exploration of diabetic vasculopathy in rats. Eur J Pharmacol. 1995;285:11–8. doi: 10.1016/0014-2999(95)00315-c. [DOI] [PubMed] [Google Scholar]
- 27.Goto S, Kondo F, Ikai Y, et al. Tacrolimus hydrate ointment inhibits skin plasma extravasation in rats induced by topical m-xylene but not capsaicin. Eur J Pharmacol. 2009;608:91–6. doi: 10.1016/j.ejphar.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Geba GP, Ptak W, Askenase PW. Topical tacrolimus and cyclosporin A differentially inhibit early and late effector phases of cutaneous delayed-type and immunoglobulin E hypersensitivity. Immunology. 2001;104:235–42. doi: 10.1046/j.0019-2805.2001.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L494–502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 30.Baluk P, Bowden JJ, Lefevre PM, McDonald DM. Upregulation of substance P receptors in angiogenesis associated with chronic airway inflammation in rats. Am J Physiol. 1997;273:L565–71. doi: 10.1152/ajplung.1997.273.3.L565. [DOI] [PubMed] [Google Scholar]
- 31.Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am J Physiol. 1999;277:L831–40. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- 32.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–32. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387:489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Diamond J, Holmes M, Coughlin M. Endogenous NGF, nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:1454–66. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeMaster AM, Krimm RF, Davis BM, et al. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–31. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olgart C, Frossard N. Human lung fibroblasts secrete nerve growth factor: effect of inflammatory cytokines and glucocorticoids. Eur Respir J. 2001;18:115–21. doi: 10.1183/09031936.01.00069901. [DOI] [PubMed] [Google Scholar]
- 37.Fox AJ, Patel HJ, Barnes PJ, Belvisi MG. Release of nerve growth factor by human pulmonary epithelial cells: role in airway inflammatory diseases. Eur J Pharmacol. 2001;424:159–62. doi: 10.1016/s0014-2999(01)01138-4. [DOI] [PubMed] [Google Scholar]
- 38.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117:787–94. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 39.Hattori A, Tanaka E, Murase K, et al. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. J Biol Chem. 1993;268:2577–82. [PubMed] [Google Scholar]
- 40.Blasing H, Hendrix S, Paus R. Pro-inflammatory cytokines upregulate the skin immunoreactivity for NGF, NT-3, NT-4 and their receptor, p75NTR in vivo: a preliminary report. Arch Dermatol Res. 2005;296:580–4. doi: 10.1007/s00403-005-0563-y. [DOI] [PubMed] [Google Scholar]
- 41.Sakuma S, Kato Y, Nishigaki F, et al. FK506 potently inhibits T cell activation induced TNF-alpha and IL-1beta production in vitro by human peripheral blood mononuclear cells. Br J Pharmacol. 2000;130:1655–63. doi: 10.1038/sj.bjp.0703472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumont FJ, Staruch MJ, Fischer P, DaSilva C, Camacho R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J Immunol. 1998;160:2579–89. [PubMed] [Google Scholar]
- 43.Schmidt J, Fleissner S, Heimann-Weitschat I, et al. Effect of corticosteroids, cyclosporin A, and methotrexate on cytokine release from monocytes and T-cell subsets. Immunopharmacology. 1994;27:173–9. doi: 10.1016/0162-3109(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka J, Di ZH, Sun W, Kawana S. Erratum to “changes in cutaneous sensory nerve fibers induced by skin-scratching in mice”. J Dermatol Sci. 2007;47:172–82. doi: 10.1016/j.jdermsci.2006.12.012. [DOI] [PubMed] [Google Scholar]