Abstract
The presence of more than one human papillomavirus (HPV) genotype may influence the duration of prevalently detected infections. This analysis included 1,646 infections detected at enrollment in 980 women from the Guanacaste, Costa Rica, cohort who were actively followed up every 6–12 months for up to 8 years. We categorized HPV infections as single or multiple types. Persistence of infections was estimated using discrete-time survival analysis. The difference between the duration of single and that of concurrent multiple type-specific prevalent HPV infections was not significant (P = .9; log-rank test). Concurrent, prevalent detection of additional HPV types did not change the likelihood of viral persistence.
Persistent cervical infection by oncogenic human papillomavirus (HPV) types is a necessary condition for cervical carcinogenesis [1]. Although most infections clear rapidly, persistent infection with 1 of ∼12 genetically related oncogenic HPV genotypes strongly increases the risk of precancerous lesions, cervical intraepithelial neoplasia (CIN) grade 3, which, if untreated, may invade. The likelihood of persistence (and ultimately progression) is not uniform among oncogenic types [2–4], but the interplay of viral and host factors that causes some infections to clear and others to persist remains unknown.
Although one longitudinal study found that co-infection with at least 1 additional type during follow-up was associated with increased duration of incident oncogenic infections [5], other large prospective studies have found no association between concurrency and persistence [6–8]. Several studies have found an association between the presence of multiple infections and CIN grade 2 or higher (CIN2+) or CIN grade 3 or higher [9–11]. Because of the difficulties of attributing lesions to a particular HPV type [12], however, it has not been demonstrated whether associations are due to simple additive risk or synergistic interaction between multiple infections. Thus, it is unclear at what stage of cervical carcinogenesis multiple HPV infections exert their effects on the risk of CIN2+.
To examine the impact of multiple HPV types on viral persistence, which strongly predicts the development of CIN2+, we studied the duration of prevalently-detected single and multitype infections among women in a population-based cohort study in Guanacaste, Costa Rica.
METHODS
During 1993–1994, a population-based cohort of 10,049 women ≥18 years old was assembled in Guanacaste, Costa Rica, to study the natural history of HPV infection and cervical neoplasia. Details regarding the study design, methods, and written informed consent have been described elsewhere [13]. The study protocol was reviewed and reapproved annually by the National Cancer Institute and a Costa Rican institutional review board.
We defined a risk-stratified subset of 2,655 sexually active women to investigate the origins of incident CIN2+, as described elsewhere [2]. This subcohort was actively followed up and screened every 12 months for 5–7 years, except for women presenting with low-grade intraepithelial lesion or CIN grade 1 at any visit, who were shifted to a 6-month screening interval. For some women, data were available through 8 years of follow-up, so we included data from these visits in our analysis. At each visit, women were screened with conventional and liquid-based cytology, and samples were collected for HPV testing for >40 HPV types by means of a MY09/MY11 L1 degenerate-primer polymerase chain reaction–based method [9, 14].
In the present analysis, we considered persistence of each HPV infection separately, and herein refer to an index infection (rather than a woman) as the unit of analysis. We restricted our sample to 1,731 prevalently detected HPV index infections in 1,013 women who were actively followed up. We further excluded 85 (4.9%) index infections with ≥2 subsequent, consecutive intervening negative results for that type due to uncertainty as to their meaning. The final analysis included 1,646 prevalently detected HPV infections among 980 women.
To standardize time among infections and account for delayed visits, each clinic visit was assigned to 1 of 16 time bins from enrollment through year 8 of follow-up (0, enrollment; 1, 0 to <9 months; 2, 9 to <15 months; and so on in 6-month intervals). If >1 visit occurred within a bin and the HPV results were discordant, the overall result was assumed to be positive to acknowledge the possibility of HPV measurement error (73 changes were made). Missing or negative HPV results flanked by positive results for the same type were recategorized as positive (n = 160 infections). We assumed clearance occurred at the first negative result if no subsequent positive results were observed. Missing results between positive and negative results were resolved using the following algorithm (n = 777 infections): if there were 1–3 missing bins, the first was imputed as positive while remaining bins were made negative; if there were 4–5 missing bins, the first 2 were imputed as positive while remaining bins were made negative; and so on. After imputing HPV results as described, we collapsed the duration of each infection into yearly intervals to minimize differences in duration that could arise from differing follow-up schedules (ie, 6 vs 12 months). The first interval (year 1) included HPV results from the first <15 months of the study, and subsequent 12-month intervals began at 15 months (15 to <27 months, etc).
We classified prevalent index infections as either single or multiple at enrollment. Among multiple infections, each concurrently detected type was analyzed as a separate index infection. Persistence of single and multiple infections was estimated using discrete-time survival analysis, with censoring of infections at clearance, diagnosis of CIN2+, or loss to follow-up. We used the log-rank test to assess the significance of the difference of persistence between groups; in addition to log-rank P values, we also report the probability of 8-year viral persistence in each group. We repeated the analysis but restricted index infections to oncogenic types, detected as single infections or concurrently with any other HPV type. We stratified infections on the total number of types present at enrollment to assess trend in persistence. To examine potential effect modification by age, we stratified observations into quartiles according to the woman's age at enrollment (18–24, 25–31, 32–46, and ≥47 years old). Because there was evidence that prevalent infections are more likely to persist in older women than in younger women [15], we excluded the oldest age quartile in a sensitivity analysis. We performed a separate sensitivity analysis to examine the impact of excluding HPV type 16 (HPV-16) infections. Although all infections present at the time of CIN2+ diagnosis were censored thereafter from the study, we performed a sensitivity analysis in which we categorized these infections as positive through year 8 to capture the tendency of these lesion-associated infections to persist.
We estimated the hazard ratio for clearance of multiple versus single prevalent infections, after adjusting for confounders, with a discrete-time logistic regression (complementary log-log link), using generalized estimating equations (GEE) to obtain standard errors robust to an independence correlation structure.
RESULTS
The main analysis compared 574 single and 1,072 multiple prevalent infections (median, 3 types; range, 2–7 types; n = 406 women) in 980 women total. In a multivariate analysis, women with multiple infections were more likely to (1) be followed up every 6 months versus every 12 months (34.7% vs 26.3%; P = .008); (2) be in the youngest age quartile (28.1% vs 15.5%; P < .001); and (3) have >1 sexual partner in their lifetime (69.2% vs 59.4%; P = .001). At the infection level, there were no differences between groups of single and multiple infections for (1) the prevalence of definite and possible oncogenic HPV genotypes (defined as 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82; 53.1% vs 51.3%, respectively; P = .5); (2) the prevalence of HPV-16 (8.9% vs 6.5%; P = .08); and (3) the risk of CIN2+ (4.2% vs 3.3%; P = .3).
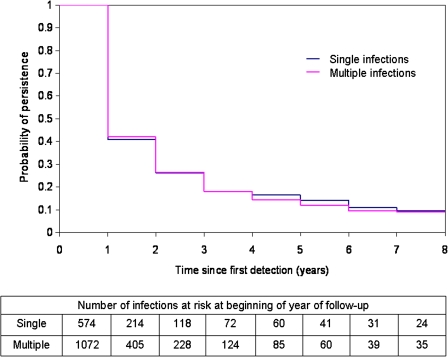
Figure 1 displays the discrete-time survival curves for time to clearance of discrete HPV types from women with single or multiple prevalent infections. The probability of type-specific viral persistence was not statistically different between groups (P = .9; 8-year persistence for single infections, 9.5%; 8-year persistence for multiple infections, 8.9%). Restriction to oncogenic index infections did not impact findings (P = .9; 8-year persistence for single infections, 13.9%; 8-year persistence for multiple infections, 10.9%). The time to clearance was not significantly different whether 1, 2, or ≥3 types were concurrently detected (8-year persistence, 9.5%, 7.2%, and 10.7%, respectively; P = .5 for trend).
Figure 1.
Discrete-time survival estimates for single versus multiple prevalent infections, and number of infections at risk at beginning of each year of follow-up.
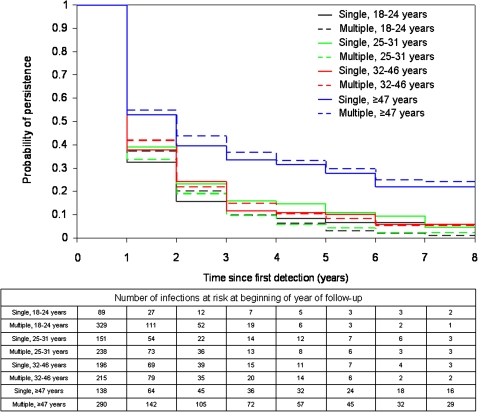
Figure 2 displays discrete-time survival curves for single versus multiple infections stratified by age quartile. The probability of 8-year persistence was not statistically different between single and multiple infections in 18–24-year-olds (4.5% vs 1.0%; P > .999), 25–31-year-olds (4.7% vs 2.2%; P = .07), 32–46-year-olds (5.7% vs 5.5%; P = .9), or ≥47-year-olds (21.9% vs 24.2%; P = .6). After infections in women ≥47 years old were excluded, there was no significant difference in the likelihood of HPV infection persistence between single and multiple infections (P = .2; 8-year persistence, 5.0% vs 2.6%, respectively).
Figure 2.
Discrete-time survival estimates by age quartile for single versus multiple prevalent infections, and number of infections at risk at beginning of each year of follow-up.
Excluding HPV-16 index infections from the analysis did not change results (P = .9; 8-year persistence for single infections, 8.1%; 8-year persistence for multiple infections, 7.7%). If index infections associated with CIN2+ were assumed to persist through year 8, the probability of persistence was statistically the same between groups (P = .9; 8-year persistence for single infections, 11.9%; 8-year persistence for multiple infections, 11.1%).
The GEE model corroborated the lack of association between the presence of multiple HPV infections and clearance. The hazard ratio for clearance of multiple versus single prevalent infections was 1.02 (95% confidence interval, 0.91–1.15) after we adjusted for age quartile, age at first sexual contact, number of sexual partners in a woman's lifetime, 6-month versus 12-month follow-up, and carcinogenic versus noncarcinogenic HPV type.
DISCUSSION
We found no difference in duration between single and multiple prevalently detected HPV infections over 5–7 years of follow-up. There are limitations to the present analysis. Infections were followed up every 6–12 months, and we collapsed data into approximate yearly intervals to avoid a potential bias in time to clearance between comparison groups. Thus, our estimates of time to clearance are crude and unlikely to capture subtle differences in duration between single and multiple index infections. Furthermore, we imputed HPV results for missing visits according to the algorithms described above. This process may have further masked small differences in duration of infections between groups that are unlikely to be clinically significant.
Statistical power was limited because persistence was uncommon. We considered a large number of infections, but once we stratified by possible effect modifiers such as age, our power to detect small differences between survival curves was limited.
One study did not find any association between the presence of HPV-16 at enrollment and the persistence of other prevalent infections (with either oncogenic or low-risk HPV types) over an average follow-up time of 16.6 months [6]. A study of incident infections with longer follow-up time (median, 6.4 years) also did not find any association between co-infection or prior infection with any other HPV type and viral clearance [7]. Plummer et al [8] found that infections with oncogenic HPV types were no more or less likely to persist over a 6-month period given the presence of another HPV type within the same species or within the Hybrid Capture 2 (Qiagen) probe set. Our results contrast with findings by Trottier et al [5], who found that the presence of additional HPV types during follow-up was associated with slightly longer mean duration of incident high-risk HPV infections than single HPV infections, even when HPV-16 was excluded. Disparate results may be attributable to shorter intervals between visits and greater power in the Trottier et al [5] study and to different definitions of co-infection. Alternatively, Trottier and colleagues defined multiple infections as those that were detected in co-infection with other types during follow-up [5]. By defining comparison groups based on the detection or absence of other types in the future, Trottier and colleagues do not consider the inherent dependence between persistence and acquisition of other infections—the longer an infection persists, the greater the likelihood of acquiring infections with other types—and are thus more likely to classify persistent infections as co-infections. Studies that define comparison groups based on detection at >1 point in time may be more likely to ascertain a difference in persistence between groups of single and multiple infections.
Viral persistence with oncogenic HPV is a critical step on the pathway to cervical cancer. Although studies have documented an increased risk of high-grade precancerous lesions among women with multiple infections [9–11], results may be attributable to additive risk presented by individual oncogenic types, rather than synergistic interaction. In the present study, prevalent multitype HPV infections did not appear to impact each other's natural history.
Funding
This work was supported by the National Cancer Institute, National Institutes of Health (grant number R01 CA93435); and the Intramural Research Program.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–7. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;229:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffman M, Herrero R, DeSalle R, et al. The carcinogenicity of human papillomavirus reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Trottier H, Mahmud S, Prado JCM, et al. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1236–47. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liaw K, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz N, Hernandez-Suarez G, Méndez F, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–90. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 9.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papipllomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 10.Trottier H, Mahmud S, Costa M, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler CM, Hunt WC, Schiffman M, Castle PE Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Study Group. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. J Infect Dis. 2006;194:1291–9. doi: 10.1086/507909. [DOI] [PubMed] [Google Scholar]
- 12.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrero R, Schiffman MH, Bratti C, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste project. Rev Panam Salud Publica. 1997;1:362–75. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 14.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:1–10. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]