Abstract
In contrast to strong epidemiologic, preclinical, and secondary clinical evidence for vitamin E (tocopherols) in reducing cancer risk, large-scale clinical cancer-prevention trials of α-tocopherol have been negative. This vexing contrast helped spur substantial preclinical efforts to better understand and improve the antineoplastic activity of tocopherol through, for example, the study of different tocopherol forms. We previously showed that the γ-tocopherol–rich mixture (γ-TmT) effectively inhibited colon and lung carcinogenesis and the growth of transplanted lung-cancer cells in mice. We designed the present study to determine the relative activities of different forms of tocopherol in a xenograft model, comparing the anticancer activities of δ-tocopherol with those of α- and γ-tocopherols. We subcutaneously injected human lung cancer H1299 cells into NCr nu/nu mice, which then received α-, γ-, or δ-tocopherol or γ-TmT in the diet (each at 0.17% and 0.3%) for 49 days. δ-Tocopherol inhibited tumor growth most strongly. γ-Tocopherol and γ-TmT (at 0.3%) also inhibited growth significantly, but α-tocopherol did not. δ-Tocopherol also effectively decreased oxidative DNA damage and nitrotyrosine formation and enhanced apoptosis in tumor cells; again, γ-tocopherol also was active in these regards but less so, and α-tocopherol was not. Each supplemented diet increased serum levels of its tocopherol—up to 45 µM for α-tocopherol, 9.7 µM for γ-tocopherol, and 1.2 µM for δ-tocopherol; dietary γ- or δ-tocopherol, however, decreased serum α-tocopherol levels, and dietary α-tocopherol decreased serum levels of γ-tocopherol. Each dietary tocopherol also increased its corresponding side-chain–degradation metabolites, with concentrations of δ-tocopherol metabolites greater than γ-tocopherol and far greater than α-tocopherol metabolites in serum and tumors. The present study is the first in vivo assessment of δ-tocopherol in tumorigenesis and demonstrates that δ-tocopherol is more active than α- or γ-tocopherol in inhibiting tumor growth, possibly through trapping reactive oxygen and nitrogen species and inducing apoptosis; δ-tocopherol metabolites could contribute significantly to these results.
Keywords: Tocopherols, lung cancer cells, xenograft, tocopherol metabolites
Introduction
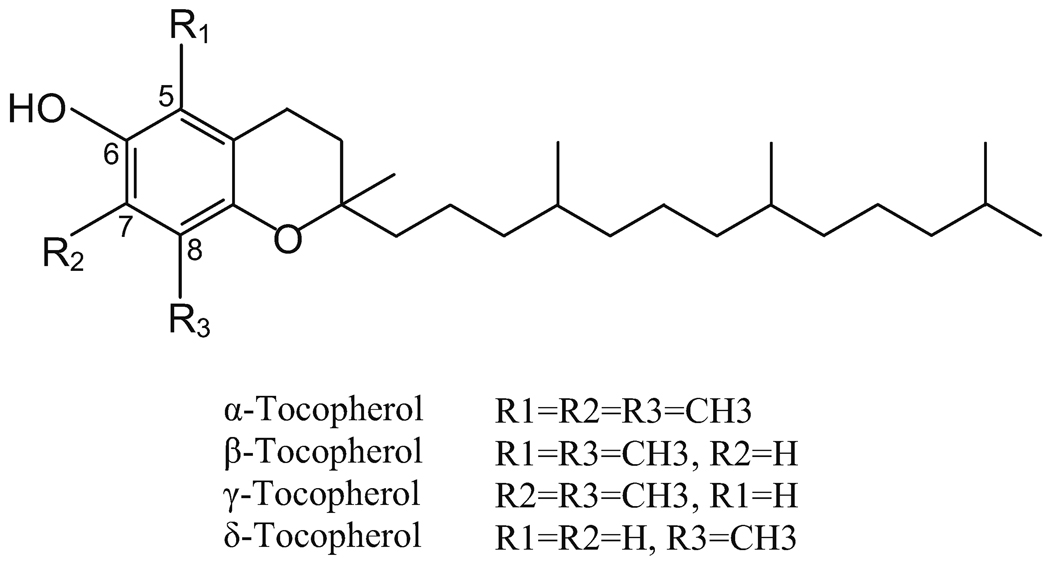
Tocopherols, collectively known as vitamin E, are important dietary antioxidants (1). They are a family of phenolic compounds; each contains a chromanol ring system and a 16-carbon side-chain. Depending upon the number and position of methyl groups on the chromanol ring, they exist as α-, β- γ- or δ-tocopherol (T). For example, α-T is trimethylated at the 5-, 7- and 8-positions; γ- is dimethylated at the 7- and 8-positions; and δ-T is methylated at the 8-position. The structures of these tocopherols are shown in Figure 1.
Fig 1.
Structures of tocopherols.
The relationship between vitamin E nutrition and human cancer risk as well as the activities of tocopherols in inhibiting cancer formation and growth have been studied extensively and reviewed recently (2). Many studies have shown that a lower vitamin E nutritional status is associated with increased risk of certain types of cancers (2–6). These results are consistent with the hypothesis that reactive oxygen and nitrogen species (RONS) are involved at different stages of carcinogenesis, and tocopherols as antioxidants can inhibit cancer formation and development. On the other hand, the results of some large-scale human trials with α-T have failed to demonstrate any cancer preventive effect (7–10). For example, the Alpha-Tocopherol, Beta-Carotene (ATBC) [lung] Cancer Prevention Study found that α-T did not reduce the overall risk of lung cancer in Finnish male smokers, although secondary ATBC results appeared to indicate that it might have reduced prostate-cancer risk. (β-Carotene actually increased lung-cancer risk.) The secondary ATBC finding on α-T led in part to the Selenium and Vitamin E [prostate] Cancer Prevention Trial (SELECT), which was launched with high expectations but ultimately showed that selenium (200 µg/day from L-selenomethionine) and α-tocopherol (400 IU/day of all rac-α-tocopherol acetate), taken alone or in combination for an average of five years, did not prevent prostate cancer (9). One possible interpretation is that supplementation of a nutrient to a population that is already adequate in this nutrient may not produce any beneficial effect. Another possibility, as will be discussed below, is that α-T may not have been the right form of tocopherol to use. The exact reasons for the negative results of these large trials are not known. Nevertheless, the outcome of these large-scale trials reflects our lack of understanding of the biological activities of tocopherols and illustrates to the need for systematic studies on the activities of the different forms of tocopherols against cancer formation and growth.
Because α-T is the most abundant form of tocopherol found in the blood and tissues and is usually considered to be “the vitamin E”, most of the human intervention studies have used synthetic α-tocopherol acetate, which is readily converted to free α-T (7–10). Nevertheless, a robust cancer preventive or anticancer activity of α-T has never been demonstrated in studies with animal models or cell lines (2). On the other hand, γ-T has been shown to have stronger anti-inflammatory and anticancer activities than α-T (11–17). Yu et al. also showed that α-T, not only failed to exhibit anticancer properties, but also reduced the anticancer action of γ-T in vivo (17). Recent studies from our research team at Rutgers University have demonstrated the inhibition of colon, prostate, mammary, and lung carcinogenesis by a tocopherol mixture that is rich in γ-T, known as γ-TmT 18–24). γ-TmT is a by-product in the refining of vegetable oil and usually contains 13% α-T, 1.5% β-T, 57% γ-T, and 24% δ-T. In these studies with tocopherol mixtures, although we think that the activity was mainly due to γ-T and δ-T, the actual activities of the individual tocopherols are unknown.
In a recent study, we have demonstrated that γ-TmT inhibits the growth of human lung cancer H1299 xenograft tumors in nude mice, and this activity is parallel to the inhibition of chemically-induced lung tumorigenesis in A/J mice (24). Mechanistically, in both systems, γ-TmT treatment caused reduction of oxidative stress and enhanced apoptosis (24). Cancer cell lines in culture and in xenograft models are convenient experimental systems to study the anticancer activities of tocopherols. Because these cancer cells retain many of the pro-growth and anti-apoptotic molecular pathways that have been activated in carcinogenesis, some of the information obtained from studies in xenograft models should be relevant to both cancer prevention and treatment. Using this xenograft tumor model, we compared the inhibitory activities of purified δ-T with those of purified γ- and α-tocopherol.
This model provides us with a convenient experimental system for studying the activities of individual tocopherols in comparison with γ-TmT. Such in-vivo studies also offer an opportunity to study the blood and tissue levels of different tocopherols and their metabolites. Such information is essential for a better understanding of the cancer-preventive activities of tocopherols, and very little information on δ-T and γ-T has been available to date.
We report here the first in-vivo study of δ-T activity in tumorigenesis and assessment of potential mechanisms for this activity, including oxidative DNA damage and nitrotyrosine formation and apoptosis. We also report the tissue levels of different tocopherols and their metabolites in response to tocopherol supplementation at different concentrations.
Materials and Methods
Tocopherols and other chemicals
γ-TmT, containing 57% γ-T, 24% δ-T, 13% α-T and 1.5% β-T, was from Cognis Corporation (Kankakel, IL). γ-T was purified from γ-TmT by silica gel chromatography to a purity of 97%, with no detectable α-T and δ-T. d-α-Forms of δ-tocopherol (containing 94% δ-T, 5.5% γ-T, and 0.5% α-T) and α-tocopherol (containing 69.7% α-T, 2.6% γ-T and 0.2% δ-T) were from Sigma-Aldrich (St Louis, MO). Other reagents were of the highest grade commercially available.
Studies with xenograft tumor model
Male NCr nu/nu mice (5-weeks old) were purchased from Taconic Farms, Inc. (Germantown, NY). The mice were randomly allocated into 9 groups (10 mice per group), housed in plastic cages (5 mice per cage) with filter tops and acclimated to the AIN93M diet for one week. Human lung cancer H1299 cells (from American Type Cell Collection, Manassas, VA) were cultured in RPMI-1640 medium with 10% FBS. H1299 cells (1 × 106 cells) in 100 µL of a 1:1 mixture of serum-free RPMI-1640 and matrigel (BD, Franklin Lakes, NJ) were injected subcutaneously to both flanks of the mice. On the same day, the mice in the 8 treatment groups were switched to AIN93M diets supplemented with γ-TmT, γ-T, δ-T or α-T at the level of 0.17% or 0.3%, which were prepared by Research Diets, Inc. (New Brunswick, NJ). Tumor volume, body weight, and food consumption were monitored twice a week. Tumor size (length and width) was measured by a caliper and calculated based on the formula (tumor volume = length × width2 × 0.5). When the average tumor volume of the mice reached 1000 mm3, the mice were sacrificed by CO2 asphyxiation. The tumors were dissected and weighed; one half of the tumor was fixed in 10% buffered formalin for histological and immunohistochemistry analysis, and the second half was snap-frozen for biochemical analysis.
Immunohistochemistry
The formalin-fixed tumor samples were embedded in paraffin and 5 µm sections were mounted on slides. Deparaffinized sections on slides were treated in an antigen-unmasking solution (DAKO, Copenhagen, Denmark) in a microwave oven for antigen retrieval. Endogenous peroxidase was quenched using 3% H2O2. The slides were incubated with primary antibody overnight at 4°C. Biotin-conjugated secondary antibody (1:200) and avidin-biotin peroxidase complex (ABC, 1:100) were then applied. Antibodies against 8-hydroxy-2′-deoxyguanosine (8-OHdG) (1:100, JaICA, Japan), anti-nitrotyrosine (1:200, Millipore, Bedford, MA), phosphorylated histone 2AX (γ-H2AX) (1:400, Cell signaling, Danvers, MA), and cleaved caspase-3 (1:300, Cell signaling, Danvers, MA) were used for the analysis. The slides were then washed with PBS, incubated with ABC reagent for 30 min, and stained with 3,3’-diaminobenzidine substrate reagent for 1 min at room temperature. Each experiment was performed with an IgG control. For each slide, 10 representative 200× power photomicrographs were taken, and positive stained cancer cells and total number of cells were analyzed with ImagePro+ image processing program.
Analysis of tocopherols and metabolites by high-performance liquid chromatography
Our previous method (18, 25), with some modifications, was used for the determination of the levels of tocopherols and their metabolites in serum and tissues. In brief, the serum sample (20 µL) was mixed with 130 µL deionized water and 150 µL ethanol, and fat-soluble materials were extracted with 1 mL hexane twice. Tissue samples were homogenized in 50% ethanol containing 0.05% ascorbic acid, and the supernatant was extracted with hexane twice. Both the aqueous phase and the hexane phase samples were saved. The material in the dried hexane extract was redissolved in 100 µL ethanol and then analyzed by high-performance liquid chromatography (HPLC) using a Supelcosil C18 reversed-phase column (150 × 4.6 mm; 5 µm particle size), isocratic mobile phase of 82% ethanol in water containing 20 mmol/L ammonium acetate (pH 4.4) at a flow rate of 1.2 mL/min. The eluant was monitored with an ESA 5600A Coulochem electrode array system (CEAS) from ESA, Inc. (Chelmsford, MA) with potentials set at 200, 300, 500, and 700 mV (18, 25). The tocopherol concentrations in mouse serum and tissues were determined by comparison with the peak heights of the standard serum (25). For the analysis of short-chain degradation products of tocopherols, such as carboxyethyl hydroxychromans (CEHC) and carboxymethylbutyl hydroxychromans (CMBHC), the aqueous phase samples were dried under vacuum. The residues were redissolved in 320 µL of 0.15 M sodium phosphate buffer (pH 5.0) containing 0.03% ascorbic acid, 25 U of sulfatase and 300 U of β-glucuronidase. The mixture was incubated at 37 °C for 90 min. After cooling on ice, the samples were extracted with 1 mL of ethyl acetate twice. The dried ethyl acetate extracts were reconstituted in 100 µL of 40% aqueous ethanol. The metabolites were analyzed by HPLC as described above.
Statistical analysis
One-way Analysis of Variance (ANOVA) followed by Dunnett’s test was used for the comparison of the differences between treatment groups and the control group. Student’s t- test was used to determine the difference between two groups. Statistical significance was indicated by p < 0.05 in the two-tailed comparison.
Results
Effects of different tocopherols on the growth of H1299 xenograft tumors
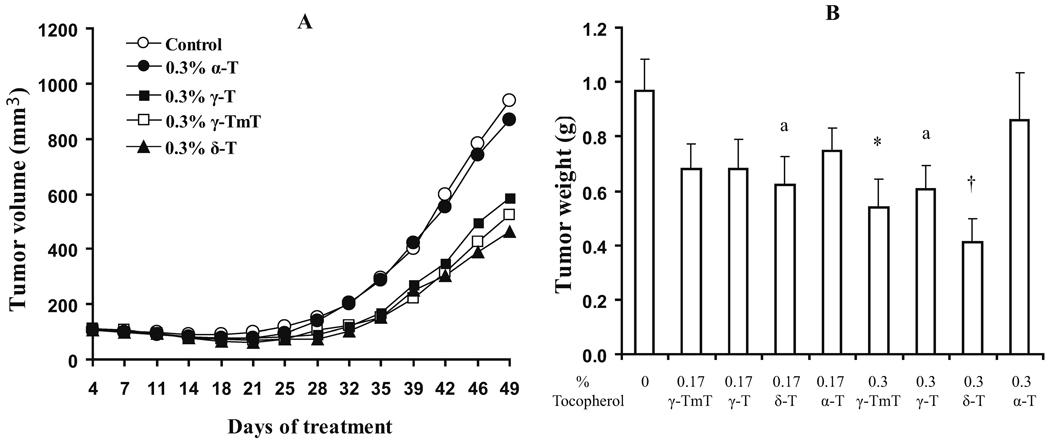
In order to determine the relative potency of different tocopherols in the inhibition of tumor growth in vivo, NCr nu/nu mice were injected with H1299 cells and treated with pure δ-T, γ-T and, α-T and γ-TmT, at levels of 0.17% or 0.3% in AIN93M diet for a 49-day experiment. No difference in food intake and body weight gain was observed among the different tocopherol treatment groups (data not shown). Retardation of tumor growth by tocopherols was observed starting on Day 28 (Fig. 2). The growth inhibition effectiveness appears to follow the ranking order of δ-T > γ-TmT > γ-T, but α-T was not effective. The final tumor volumes of the δ-T, γ-TmT, and γ-T groups were significantly lower than that of the control group (p<0.05), but the differences among the three treated groups were not statistically significant. One-way ANOVA followed by Dunnett’s test showed that final tumor weight in the 0.3% δ-T and 0.3% γ-TmT groups were significantly lower than that in the control group. In addition, two-tailed t-test indicated that the tumor weights in the 0.17% δ-T and 0.3% γ-T groups were also lower than those in the control group (p<0.05).
Fig 2.
Effects of dietary tocopherol supplementation on xenograft tumor growth and tumor weight. Experimental conditions are described in Materials and Methods. In the growth curve (A), the growth of the groups on 0.17% of different tocopherols are not shown to avoid overcrowding the figure. The group with 0.17% δ-T overlaps the group with 0.3% γ-T. The curves for groups with 0.17% γ-TmT, γ-T and α-T are superimposed and they showed less inhibitory activity than 0.17% δ-T for the measurements on Days 46 and 49. For tumor weight (B), the values are mean ± S.E. (n=10). * (p < 0.05) and † (p < 0.01) in ANOVA-Dunnett’s test; a (p < 0.05) in two-tailed t-test when compared with the control.
Effects of tocopherols on oxidative stress, nitrotyrosine formation, and apoptosis in xenograft tumors
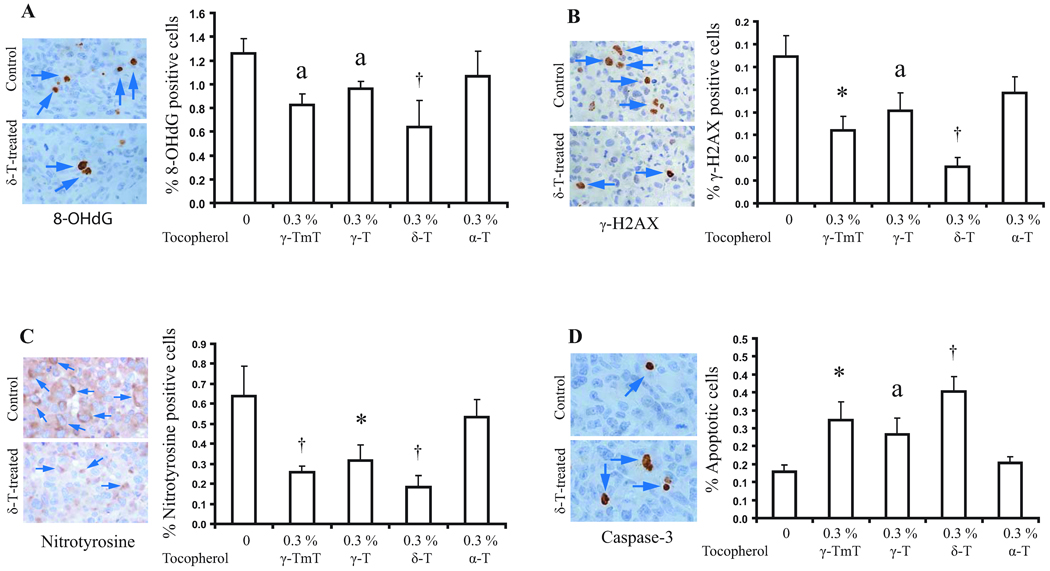
The effects of different tocopherols on oxidative and nitrosative stress and apoptosis were determined by immunohistochemistry; only the 0.3% tocopherol supplemented groups were analyzed (Fig. 3). δ-T significantly reduced the number of 8-OHdG positive cells, γ-H2AX positive cells, and nitrotyrosine positive cells (by 49.2%, 76.9%, and 70% respectively, p<0.01), and increased apoptosis as indicated by cleaved caspase-3 staining (by 2.7 fold, p<0.01) as compared with the control group. γ-TmT and γ-T also showed similar effects and their effectiveness appeared to follow the order of δ-T > γ-TmT > γ-T. On the other hand, α-T had no effect on apoptosis. α-T appeared to slightly inhibit the formation of 8-OHdG, γ-H2AX, and nitrotyrosine; however, the differences were not statistically significant.
Fig 3.
Effects of tocopherol supplementation on 8-OHdG positive cells (A), γ-H2AX positive cell (B), nitrotyrosine positive cells (C), and apoptosis (D) in xenograft tumors. Arrows indicate immunopositive stained cells. Designations for statistical analysis are the same as Fig 2, except n=5.
Effects of tocopherol supplementation on the levels of different tocopherols and their metabolites in serum
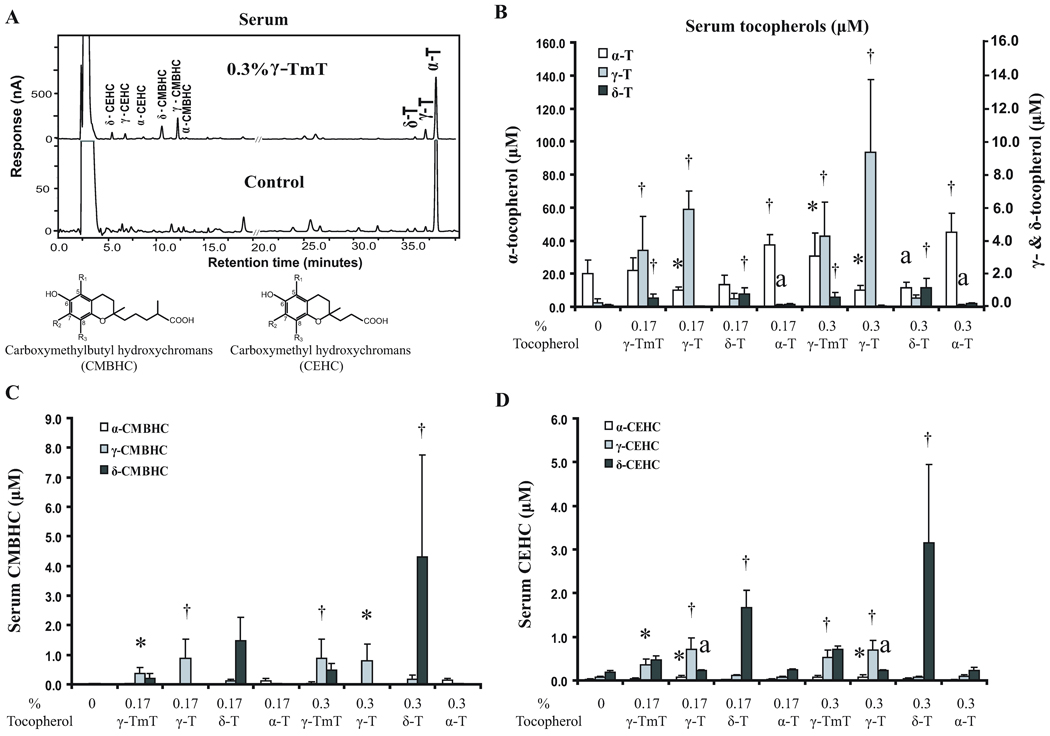
Serum and tissue levels of tocopherols and their metabolites were analyzed by HPLC and a representative chromatogram is shown in Fig. 4A. The serum α-T levels were significantly increased from 20 µM (in control group) to 30–45 µM by dietary supplementation with α-T or 0.3% γ-TmT. Interestingly, α-T levels were decreased by 0.17% or 0.3% γ-T and 0.3% δ-T (Fig. 4B). Serum γ-T levels were, as expected, significantly increased (from 0.3 µM to 3.5–9.2 µM) by γ-T or γ-TmT; but γ-T levels were decreased by α-T. The serum δ-T levels were significantly increased (from 0.08 µM to 0.5–1.2 µM) by δ-T or γ-TmT.
Fig 4.
Effects of tocopherol supplementation on serum levels of tocopherols and their metabolites. (A) HPLC chromatogram and structures of CMBHCs and CEHCs; the α-, γ- and δ-designations are the same as in Figure 1. (B) Serum levels of α-, γ- and δ-tocopherols shown on different scales. (C) Serum levels of CMBHCs. (D) Serum levels of CEHCs. Designations for statistical analysis are the same as Fig 3.
The major metabolites observed in the serum were the side-chain degradation products CMBHCs and CEHCs. δ-CMBHC was the most prominent metabolite, at levels of 1.5 and 4.3 µM in mice supplemented with 0.17% and 0.3% δ-T, respectively (Fig. 4C). γ-TmT, which contained 24% δ-T, also significantly increased the δ-CMBHC level. γ-CMBHC was observed at levels of about 0.9 µM in the mice supplemented with γ-T or 0.3% γ-TmT. α-CMBHC was observed at much lower levels, 0.12–0.15 µM, even with α-T supplementation. The serum levels of CEHCs were slightly lower than the corresponding CMBHCs (Fig. 4D). For example, the highest δ-CEHC level observed was 3.2 µM. The highest γ-CEHC level was approximately 0.7 µM. An interesting observation is that γ-T supplementation appeared to increase the levels of δ-CEHC.
Effects of tocopherol supplementation on levels of tocopherols and their metabolites in xenograft tumors and lung tissues
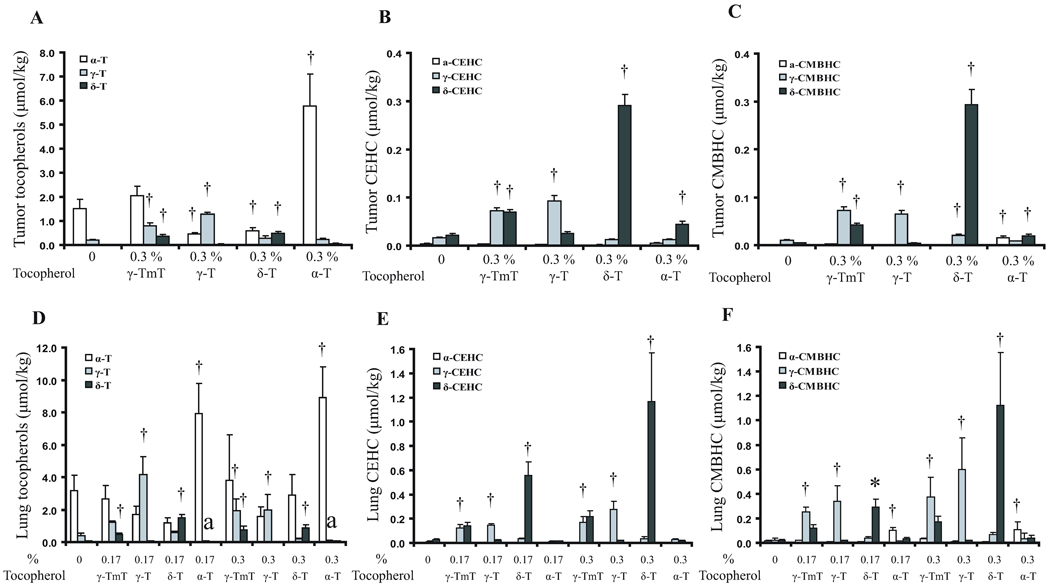
In analyzing the levels of tocopherols and their metabolites in the tumors, only the control and the 0.3% tocopherol supplemented groups were used. As shown in Figure 5A, the α-T levels in the tumors were significantly increased (from 1.5 µmol/kg to 5.5 µmol/kg) by supplementation with α-T, but were significantly decreased by γ-T and δ-T. As expected, supplementation with γ-TmT, γ-T or δ-T increased the levels of γ-T or δ-T, but their levels were still lower than those of α-T in the α-T supplement group. δ-CEHC was the most abundant side-chain degradation metabolite, reaching 0.28 µmol/kg in the δ-T supplemented group (Fig. 5B). The δ-CEHC levels were also increased by supplementation with γ-TmT (containing 24% δ-T). The γ-CEHC levels were generally lower than the corresponding δ-CEHC levels, and those for α-CEHC were very low. The tumor levels of CMBHCs were similar to those of CEHCs and were increased by supplementation with the respective forms of tocopherols (Fig. 5C).
Fig 5.
Effects of tocopherol supplementation on the levels of tocopherols and their metabolites in tumors and lung tissues. (A), (B) and (C): levels of tocopherols, CEHCs and CMBHCs in tumors; (D), (E) and (F): levels of tocopherols, CEHCs and CMBHCs in lung tissues, respectively. Designations for statistical analysis are the same as Fig 3.
Similar to serum levels, the lung tissue levels of α-T were significantly increased, but the levels of γ-T were decreased, by the supplementation with α-T (Fig. 5D). Supplementation with γ-T or δ-T increased γ-T or δ-T levels, respectively. The most abundant metabolite in the lung was δ-CEHC, showing mean levels of 0.55 and 1.17 µM in mice supplemented with 0.17% and 0.3% δ-T, respectively (Fig. 5E). γ-CEHC was found at 0.30 µM in mice supplemented with 0.3% γ-T. The levels of α-CEHC were very low. The levels of CMBHCs (Fig. 5F) were similar to those of CEHCs in the lung.
Effects of tocopherol supplementation on colon and urinary levels of tocopherols and their metabolites
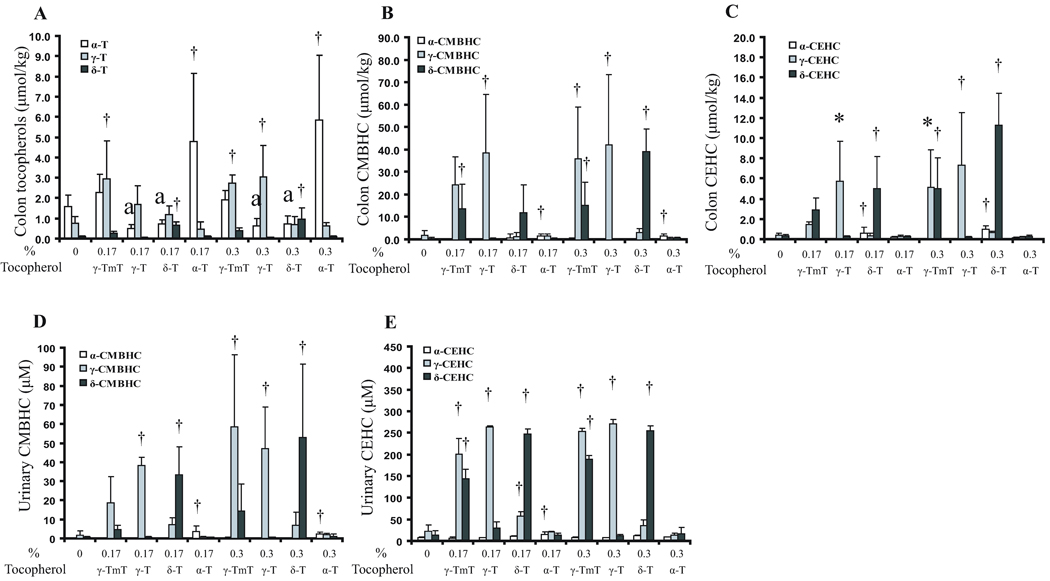
Substantial amounts of tocopherols were found in the colon tissues, especially in mice that received supplementation (0.3% in the diet), showing highest levels of 5.7, 3.0 and 1.0 µM for α-, γ- and δ-tocopherols, respectively (Fig. 6A). However, supplementation with γ-T and δ-T decreased the levels of α-T. γ-CMBHC was the most abundant colonic metabolite, reaching levels of 39–45 µM; δ-CMBHC reached a level of 40 µM; and the levels of α-CMBHC were much lower (Fig. 6B). The colonic levels of γ- and δ-CEHCs were also increased in response to supplementations, following the pattern of CMBHCs, but the levels were 3 to 4-fold lower (Fig. 6C). Interestingly, long chain metabolites (those with 2, 4, and 6 carbons cleaved from the main side-chain) were also observed at levels of 1–4 µM (data not shown in the figure).
Fig 6.
Effects of tocopherol supplementation on colonic and urinary levels of tocopherols and their metabolites. Levels of tocopherols (A), CMBHCs (B) and CEHCs (C) in colon tissues; levels of CMBHCs (D) and CEHCs (E) in urine samples. Designations for statistical analysis are the same as Fig 3.
In the urine samples, tocopherols were not detected, but high levels of metabolites were observed. Supplementation with tocopherols markedly increased the urinary levels of γ- and δ-CMBHCs to 53–59 µM, but the levels of α-CMBHC were low (~ 3 µM) (Fig. 6D). The urinary levels of γ-CEHC and δ-CEHC were higher than CMBHC levels, and even higher after supplementation showing highest levels of 270 µM and 251 µM, respectively (Fig. 6E).
Discussion
In planning this study to compare the inhibitory activities of different tocopherols, we hypothesized that γ-T would be more active than other tocopherols based on published results on the anti-inflammatory and anticancer activities of γ-T (13–17, 26). We found, however, that the activity of δ-T was superior to that of α- or γ-T. To our knowledge, this is the first in-vivo study of δ-T in tumorigenesis and the first systematic study of tissue levels of different tocopherols and their metabolites after dietary supplementation with different doses of pure and mixed tocopherols in mice.
The higher activity of δ-T in the inhibition of tumor growth corresponded well with its ability to inhibit the formation of 8-OHdG, γ-H2AX and nitrotyrosine as well as to induce cell apoptosis (Fig. 3). 8-OHdG is a commonly used marker for oxidative stress (27), whereas γ-H2AX is a sensitive marker for the presence of double-strand breaks in cells and tissues (28). Nitrotyrosine is a product formed between reactive nitrogen species and tyrosines in proteins (24). The ability in affecting these four parameters by tocopherols follows the order: δ-T > γ-TmT > γ-T, whereas α-T has no or little activity. This ranking order correlates well with the relative activity of different tocopherols in inhibiting tumor growth (Fig. 2). These results suggest that δ-T inhibits xenograft tumor growth by trapping RONS and inducing apoptosis. The oxidative stress levels in tumor cells were generally higher than normal tissues, and RONS can function as secondary messenger molecules for transducer receptor-mediated signal cascades and promote cell growth (29–33). Therefore, trapping RONS could be an important mechanism for the inhibition of tumor growth. The mechanisms by which δ-T induces apoptosis remains to be further investigated.
Information on blood and tissue levels of tocopherols and their metabolites is important for understanding the biological activities observed in the present study and in planning future studies. With our sensitive HPLC method, the levels of different tocopherols and their metabolites were systematically analyzed. Upon supplementation, the serum α-T level was elevated from 20 µM to 40 µM, γ-T level was elevated 36-fold to a level of 9.5 µM, and δ-T was lower at 1.1 µM even with 0.3% δ-T supplementation. The α-, γ- and δ-tocopherol levels in the tumor, lung and colon tissues followed the same pattern as those of the serum, but the levels were several-fold lower. This comparison is made based on the approximation that 1 µmol/kg = 1 µM. A possible competition among different tocopherols in entering the blood is suggested by the lowering of serum, tumor and colon α-T levels by supplementation with γ-T or δ-T as well as the lowering of serum and lung γ-T by supplementation with α-T. This result is consistent with the reports that intake of high levels of α-T lowered the plasma levels of γ-T in rodents and humans (9, 34–37) and that γ-T administration decreased plasma α-T concentrations in mice (38). It is known that α-T is preferentially transferred from liver to blood by α-T-transfer protein, and then distributed to different nonhepatic tissues (39). It has been suggested that the scavenger receptor class B type 1 (SR-B1) serves as a transporter of α-T from the apical to the basal side of the enterocyte in mice (31). Our present work further demonstrates that γ-T is more efficient than δ-T in getting into the blood. When given in a mixture, the serum and tissue concentrations of α-T, γ-T and δ-T are likely a reflection of the relative affinities of different tocopherols to the α-T-transfer protein and SR-B1. These two proteins are also possible sites for the competition among tocopherols as discussed previously.
A major pathway for tocopherol metabolism is through side-chain degradation, which is initiated by cytochrome P450 4F (or 3A)-catalyzed ω-hydroxylation followed by β-oxidation, in which two carbons are removed from the chain in each cycle (1, 40, 41). δ- and γ-Tocopherols are much more extensively degraded than α-T because they are not transferred to the blood and remain in the liver to be metabolized by liver enzymes. It has also been suggested that γ- and δ-tocopherols are preferentially metabolized at the enzymic level (40). The end products of this metabolic pathway, CEHCs, have been widely reported in the literature (42–45). In the present work, CMBHCs were also found in the serum, tumor, and lung tissues in similar concentration to CEHCs. In colon tissues, CMBHCs were detected at higher levels than CEHCs, and long chain metabolites were also observed. δ-CMBHC and CEHC were found at higher concentrations than the corresponding γ-forms of these metabolites. However, the higher concentration of γ-CEHC (than δ-CEHC) found in the urine suggests that this metabolite is also formed in large quantities. Therefore, we suggest that the tissue uptake and the route of excretion determine the ratio of γ- and δ-forms of CEHCs and CMBHCs that are distributed in different tissues. The tocopherol level of 0.17% or 1.7mg/g in the diet, according to the concept of allometric scaling (46, 47) is equivalent to 0.425 mg/kcal. This value is close to the intake of 800 mg of tocopherol (usually in one or two capsules of vitamin E dietary supplement) for a person requiring 2,000 kcal daily (i.e., 0.4 mg/kcal).
The present work found δ-T to be more effective than α- or γ-T in inhibiting tumor growth, but the serum and tumor levels of δ-T were much lower than levels of the other tocopherols. On the other hand, the δ-T metabolites CMBHC and CEHC were much higher than the corresponding forms of γ- and α-T metabolites. These interesting results provide strong support for further preclinical studies of δ-T and its metabolites in tumorigenesis.
Acknowledgments
We thank Yu-Hai Sun for her technical assistance with the manuscript.
Grant Support
This work was supported by grants from the U.S. National Institutes of Health (RO1 CA120915, RO1 CA122474 and RO1 CA133021) and the John L. Colaizzi Chair endowment Fund to C.S. Yang as well as by shared Facilities funded by National Cancer Institute Cancer Center Support Grant (CA72720) and National Institute of Environmental Health Center Grant (ES05022).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations
- γ-T
γ-tocopherol
- RONS
reactive oxygen and nitrogen species
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- HPLC
high-performance liquid chromatography
- γ-H2AX
histone 2AX phospho at Ser139
- CEAS
Coulochem electrode array system
- CEHC
carboxyethyl hydroxychromans
- CMBHC
carboxymethylbutyl hydroxychromans
- ANOVA
Analyses of Variance
- SR-B1
scavenger receptor class B type 1
Footnotes
Disclosure of Potential Conflicts of Interest
No Potential conflicts of interest were disclosed.
REFERENCES
- 1.Traber MG. Vitamin E. In: Bowman BA, M RR, editors. Present Knowledge in Nutrition. Washington DC: ILSI Press; 2006. pp. 211–219. [Google Scholar]
- 2.Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor PR, Qiao YL, Abnet CC, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1414–1416. doi: 10.1093/jnci/djg044. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 5.Knekt P, Aromaa A, Maatela J, et al. Vitamin E and cancer prevention. Am J Clin Nutr. 1991;53:283S–286S. doi: 10.1093/ajcn/53.1.283S. [DOI] [PubMed] [Google Scholar]
- 6.Mahabir S, Schendel K, Dong YQ, Barrers SL, Spitz MR, Forman MR. Dietary alpha-, beta-, gamma- and delta-tocopherols in lung cancer risk. Int J Cancer. 2008;123:1173–1180. doi: 10.1002/ijc.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 8.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf G. gamma-Tocopherol: an efficient protector of lipids against nitric oxide-initiated peroxidative damage. Nutr Rev. 1997;55:376–378. doi: 10.1111/j.1753-4887.1997.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 12.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 14.Grammas P, Hamdheydari L, Benaksas EJ, et al. Anti-inflammatory effects of tocopherol metabolites. Biochem Biophys Res Commun. 2004;319:1047–1052. doi: 10.1016/j.bbrc.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 15.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu W, Park SK, Jia L, et al. RRR-gamma-tocopherol induces human breast cancer cells to undergo apoptosis via death receptor 5 (DR5)-mediated apoptotic signaling. Cancer Lett. 2008;259:165–176. doi: 10.1016/j.canlet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu W, Jia L, Park SK, et al. Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol. Mol Nutr Food Res. 2009;53:1573–1581. doi: 10.1002/mnfr.200900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju J, Hao X, Lee MJ, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila Pa) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek AK, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-g. Clin Can Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh N, Paul S, Lee HJ, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 22.Barve A, Khor TO, Nair S, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert JD, Lu G, Lee MJ, Hu J, Yang CS. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol Nutr Food Res. 2009;53:1030–1035. doi: 10.1002/mnfr.200800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu G, Xiao H, Li GX, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 31:687–694. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Lee M-J, Cheng C, et al. Analysis of multiple metabolites oftocopherols and tocotrienols in mice and humans. J Agri Food Chem. 2010;58:4844–4852. doi: 10.1021/jf904464u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 27.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2'-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 28.Albino AP, Huang X, Jorgensen E, et al. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle. 2004;3:1062–1068. [PubMed] [Google Scholar]
- 29.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 30.Choi MH, Lee IK, Kim GW, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 31.Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 32.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 34.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 35.Handelman GJ, Epstein WL, Peerson J, Spiegelman D, Machlin LJ, Dratz EA. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr. 1994;59:1025–1032. doi: 10.1093/ajcn/59.5.1025. [DOI] [PubMed] [Google Scholar]
- 36.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49:517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 37.Huang HY, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr. 2003;133:3137–3140. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa S, Morinobu T, Hamamura K, Hirahara F, Iwamoto T, Tamai H. The effect of gamma-tocopherol administration on alpha-tocopherol levels and metabolism in humans. Eur J Clin Nutr. 2005;59:900–905. doi: 10.1038/sj.ejcn.1602154. [DOI] [PubMed] [Google Scholar]
- 39.Traber MG, Burton GW, Hughes L, et al. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J Lipid Res. 1992;33:1171–1182. [PubMed] [Google Scholar]
- 40.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 41.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 42.Hensley K, Benaksas EJ, Bolli R, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40:665–671. [PubMed] [Google Scholar]
- 44.Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. J Lipid Res. 1984;25:40–48. [PubMed] [Google Scholar]
- 45.Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev. 2006;64:295–299. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 46.Newmark HL. Nutrient density: an important and useful tool for laboratory animal studies. Carcinogenesis. 1987;8:871–873. doi: 10.1093/carcin/8.7.871. [DOI] [PubMed] [Google Scholar]
- 47.Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment--empirical investigations. Regul Toxicol Pharmacol. 2004;39:334–347. doi: 10.1016/j.yrtph.2004.03.001. [DOI] [PubMed] [Google Scholar]