Abstract
Broilers that are susceptible to pulmonary hypertension syndrome (PHS, ascites) have an elevated pulmonary arterial pressure (PAP) when compared with PHS-resistant broilers. Two distinctly different syndromes, pulmonary arterial hypertension (PAH) and pulmonary venous hypertension (PVH), both are associated with increases in PAP. Pulmonary arterial hypertension occurs when the right ventricle must elevate the PAP to overcome increased resistance to flow through restrictive pulmonary arterioles upstream from the pulmonary capillaries. In contrast, PVH is commonly caused by increased downstream (post-capillary) resistance. The sites of resistance to pulmonary blood flow are deduced by making contemporaneous measurements of the PAP and the wedge pressure (WP), and calculating the trans-pulmonary pressure gradient (TPG = PAP-WP). We obtained PAP and WP values from 8, 12, 16, 20 and 24 wk old anesthetized male and female broilers from a PHS-susceptible line. Pressures were recorded as a catheter was advanced through a wing vein to the pulmonary artery and onward until the WP was obtained. In addition to gender and age comparisons of vascular pressure gradients, the data also were pooled to obtain three cohorts for broilers having the lowest PAP values (n = 52; range: 12 to 22.9 mmHg), intermediate PAP values (n = 63; range: 23 to 32.9 mmHg), and highest PAP values (n = 62; range: 33 to 62 mmHg) independent of age or gender. Within each of the age, gender and PAP cohort comparisons, broilers with elevated PAP consistently exhibited the hemodynamic characteristics of PAH (elevated PAP and TPG combined with a normal WP) and not PVH (elevated PAP and WP combined with a normal or reduced TPG). Susceptibility to PHS can be attributed primarily to pulmonary arterial hypertension associated with increased pre-capillary (arteriole) resistance.
Keywords: Broiler, pulmonary hypertension, transpulmonary pressure gradient
INTRODUCTION
Broilers that are susceptible to pulmonary hypertension syndrome (PHS, ascites syndrome) have an elevated pulmonary arterial pressure (PAP) when compared with broilers that are resistant to PHS (Wideman et al., 2000, 2002, 2006, 2007; Chapman and Wideman, 2001, 2006; Bowen et al., 2006; Lorenzoni et al., 2008). Sustained increases in PAP typically reflect sustained increases in the cardiac output (CO) or increased resistance to blood flow through the pulmonary vasculature (increased pulmonary vascular resistance, PVR) (Peacock et al., 1989; Wideman and Bottje, 1993; Wideman, 2000, 2001; Wideman et al., 2007). Previous physiological comparisons of clinically healthy PHS-susceptible and PHS-resistant broilers failed to demonstrate consistent differences in CO, whereas both the PAP and PVR were consistently higher in susceptible than in resistant broilers. Thus for PHS-susceptible broilers to maintain a normal CO the right ventricle of the heart must increase the PAP sufficiently to overcome the excessively elevated PVR (Wideman et al., 2002, 2007; Bowen et al., 2006; Chapman and Wideman, 2006; Lorenzoni et al., 2008). Efforts to further elucidate the pathogenesis of PHS have focused on deducing the underlying sources of excessive resistance to pulmonary blood flow (Wideman et al., 2004, 2007).
Two distinctly different syndromes, pulmonary arterial hypertension (PAH) and pulmonary venous hypertension (PVH), both are associated with increases in PAP. Pulmonary arterial hypertension occurs when the right ventricle must elevate the PAP to overcome increased resistance to flow through anatomically restrictive or physiologically constricted pulmonary arterioles “upstream” from the capillaries and veins. In contrast, PVH is commonly caused by left-sided valvular or myocardial diseases that prevent the left ventricle from efficiently pumping blood returning from the lungs. The resulting accumulation of blood in the left atrium and pulmonary veins forces the right ventricle to increase the PAP to overcome this increased “downstream” (post-capillary) congestion (Hermo-Weiler et al, 1998; Simonneau et al., 2004; Benza and Tallaj, 2006).
The sites of excessive resistance to pulmonary blood flow can be deduced as being “upstream” or “downstream” to the capillaries by making contemporaneous measurements of the PAP and the wedge pressure (WP). Wedge pressures are obtained by advancing a catheter through the pulmonary arterial branches until the tip of the catheter reaches an arteriole of a similar or smaller diameter, causing the catheter’s tip to wedge in and thereby occlude blood flow and pressure transmission from the upstream arterial tree. The column of blood that remains between the catheter tip and the downstream vasculature transmits the blood pressure from the venous region, allowing the measurement of the post-capillary “downstream” blood pressure via the wedged catheter (Chapman and Wideman, 2001; Lorenzoni et al., 2008). The difference between the PAP and WP equals the trans-pulmonary pressure gradient (TPG). The typical hemodynamic characteristics of PAH include elevated values for PAP and TPG combined with a normal (low) WP and thus low left atrial or pulmonary venous pressures. In contrast, PVH is characterized by elevated values for PAP accompanied by a proportionally elevated WP in combination with a normal or reduced TPG (Zidulka and Hakim, 1985; Hermo-Weiler et al, 1998; Chemla et al., 2002; Benza and Tallaj, 2006).
Using broilers from PHS-susceptible, PHS-resistant, and relaxed lines that had been selected for multiple generations under conditions of hypobaric hypoxia (Anthony et al., 2001; Balog, 2003; Pavlidis et al., 2007), previous comparisons of CO, PVR, PAP, WP and TPG values provided direct evidence that elevated pre-capillary resistance (PAH) serves as the underlying basis for PHS susceptibility in 5 to 7 wk old males and females (Chapman and Wideman, 2001; Bowen et al., 2006; Wideman et al., 2007; Lorenzoni et al., 2008). In the present study we obtained PAP, WP and TPG values from 8 to 24 wk old males and females from the PHS-susceptible line. It was our hypothesis that if left ventricular valvular or myocardial deterioration contributed to pulmonary hypertension attributable to PVH (Olkowski et al., 1998; Olkowski, 2007), then evidence of progressively elevated post-capillary resistance might be detected more readily in 12 to 24 wk old broilers. Alternatively, if excessive arteriole resistance is wholly responsible for spontaneously initiating pulmonary hypertension, then the hemodynamic characteristics of PAH (elevated PAP and TPG combined with a normal WP) should persist regardless of age or gender in PHS-susceptible broilers grown under ideal environmental and management conditions.
MATERIALS AND METHODS
Animal procedures were approved by the University of Arkansas Institutional Animal Care and Use Committee (Protocol #08036). Male and female chicks from three separate hatches of the PAH-susceptible broiler line (Anthony et al., 2001; Balog, 2003; Pavlidis et al., 2007) were reared on wood shavings litter in environmental chambers (dimensions: 3.7 m long × 2.5 m wide × 2.5 m high) within the Poultry Environmental Research Lab at the University of Arkansas Poultry Research Farm. Single-pass ventilation was maintained at a constant rate of 6 m3 per minute per chamber. The photoperiod was set for 23 h light:1 h dark for the first 4 d, and 16 h light:8 h dark thereafter. Thermoneutral temperatures were maintained throughout: 32 °C for d 1 to 3, 31 °C for d 4 to 6, 29 °C for d 5 to10, 26 °C for d 11 to 14, and 24 °C thereafter. Chicks were placed at an initial density of 930 cm2/bird, and by 6 wk of age the bird density had been reduced to ≥2000 cm2/bird. A 23% CP corn and soybean meal-based feed formulated to meet minimum National Research Council (1994) standards for all ingredients was provided ad libitum until the birds reached 15 wk of age, after which all birds were subjected to complete feed withdrawal on 1 out of every 3 d to reduce the onset of obesity and lameness. Water was available ad libitum via nipple watering systems throughout the study.
At 8, 12, 16, 20 and 24 wk of age a minimum of 12 birds per gender (Table 1) were weighed and anesthetized to a surgical plane with intra-muscular injections of allobarbitol (5,5-diallylbarbituric acid, 3.0 mL, 25 mg/mL) and ketamine HCl (1.0 to 2.5 mL of 100 mg/mL). Sufficient numbers of birds were available from the second and third hatches (H2 and H3) to obtain duplicate groups at 24 wk of age (24 Wk H2, 24 Wk H3). These duplicate groups were obtained from the same breeder parents but were hatched 25 d apart and were raised to 24 wk of age in separate environmental chambers. The anesthetized broilers were fastened in dorsal recumbency on a surgical board. Lidocaine1 HCl (2%) was injected subcutaneously around the basilica vein, then the proximal end of a Silastic® catheter2 (0.012 in I.D., 0.025 in O.D.) filled with heparinized saline (200 IU of heparin/mL of 0.9% NaCl) was inserted into the basilica vein. The distal end of the catheter was attached to a blood pressure transducer3 interfaced through a Transbridge preamplifier3 to a Biopac MP100 data acquisition system using Acqknowledge software4. Venous pressure was recorded with the catheter inserted approximately 2 cm into the basilica vein. Characteristic pulse pressures (Guthrie et al., 1987; Owen et al., 1995; Wideman et al., 1996, 1999) were monitored to identify the catheter’s location as it was slowly advanced to the right atrium, right ventricle, main trunk of the pulmonary artery, and onward until a sudden drop in the pressure indicated the tip of the catheter had become wedged. A sudden rise in the pressure as the catheter was slightly withdrawn confirmed the recording of WP. The catheter then was gradually withdrawn to again record the pulmonary arterial, right ventricular, right atrial and venous pressures. Typical blood pressure recordings from individual broilers undergoing WP experiments have been published previously (Chapman and Wideman, 2001, Lorenzoni et al., 2008).
Table 1.
Number of broilers evaluated per gender and their body weights, heart ventricle weights, and right to total ventricular weight ratios (RV:TV ratio) at 8 to 24 wk of age1
| Variable | Gender | Wk of Age2 |
|||||
|---|---|---|---|---|---|---|---|
| 8 | 12 | 16 | 20 | 24 Wk H2 | 24 Wk H3 | ||
| Number of birds | F | 13 | 11 | 14 | 16 | 13 | 19 |
| M | 14 | 11 | 17 | 15 | 16 | 18 | |
| Body Weight, g | F | 2,409 ± 69e | 3,419 ± 112d | 4,253 ± 125c | 4,515 ± 91c | 4,993 ± 154b | 4,814 ± 166b |
| M | 2,862 ± 164e | 4,374 ± 179c | 4,631 ± 198bc | 5,285 ± 164b | 6,163 ± 163a | 6,042 ± 220a | |
| Right Ventricle, g | F | 2.03 ± 0.12b | 1.97 ± 0.11b | 2.45 ± 0.21b | 2.52 ± 0.20b | 2.63 ± 0.26b | 2.92 ± 0.23b |
| M | 2.67 ± 0.20b | 3.07 ± 0.18b | 3.11 ± 0.27b | 4.05 ± 0.37ab | 4.89 ± 0.33a | 4.61 ± 0.33a | |
| Left Ventricle, g | F | 6.02 ± 0.18d | 6.92 ± 0.28cd | 8.11 ± 0.26c | 8.90 ± 0.53c | 8.88 ± 0.43c | 8.44 ± 0.37c |
| M | 7.84 ± 0.34cd | 11.13 ± 0.58bc | 11.25 ± 0.48b | 13.18 ± 0.66ab | 14.60 ± 0.59a | 13.88 ± 0.57a | |
| Total Ventricle, g | F | 8.05 ± 0.29c | 8.89 ± 0.36c | 10.56 ± 0.44c | 11.41 ± 0.65bc | 11.51 ± 0.65bc | 11.35 ± 0.57bc |
| M | 10.51 ± 0.48c | 14.20 ± 0.65b | 14.36 ± 0.71b | 17.23 ± 0.96ab | 19.49 ± 0.82a | 18.49 ± 0.85a | |
| Right:Total Ventricle (RV:TV ratio) | F | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 |
| M | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | |
Superscripts designate differences (P ≤ 0.05) between genders and ages within a variable.
Data are presented as mean ± SEM.
Sufficient numbers of birds were available from the second and third hatches (H2 and H3) to obtain duplicate groups at 24 wk of age.
After a satisfactory recording was obtained the birds were euthanized with a 10-mL i.v. injection of 0.1 M KCl. The heart was removed and the ventricles were dissected and weighed to calculate the right-to-total ventricular weight ratio (RV:TV ratio) which is positively correlated with right ventricular work and the PAP (Burton et al., 1967, 1968; Cueva et al., 1974; Sillau and Montalvo, 1982; Hernandez, 1987; Huchzermeyer and DeRuyck, 1986; Julian et al., 1987; Julian, 1988, 1993; Peacock et al., 1989; Wideman, 2000). The Biopac MP 100 data acquisition system continuously recorded the blood pressure in mmHg. The venous, right atrial, right ventricular, pulmonary arterial and wedge pressures were averaged electronically over 10-s intervals from the Biopac recording. The TPG was calculated as PAP minus WP (Chemla et al., 2002; Benza and Tallaj, 2006). Gender and age interactions were evaluated using the SigmaStat® ANOVA package (Jandel Scientific, 1994). The individual bird was used as the experimental unit, and the numbers of birds included within each age and gender category are shown in Table 1. Means were separated by the Tukey test when the F test from the ANOVA was declared significant (P < 0.05). The SigmaStat® linear regression procedure was used to evaluate relationships between RV:TV and PAP.
RESULTS
Table 1 shows the number of birds evaluated per gender within each age category, as well as the BW, ventricle weights, and RV:TV ratios. The BW increased with age for both males and females, and males were heavier than females at 12, 20 and 24 wk of age. Right ventricle weight did not increase with age in females, but was higher in males at 24 wk than at 8, 12 and 16 wk. The right ventricle weight did not differ between males and females until wk 24. Left ventricle weights increased with age in both males and females, and were heavier in males than in females at 16, 20 and 24 wk of age. Total ventricle weights increased with age in males but not in females, and were heavier in males than in females at 12, 16, and 24 wk of age. There were no age or gender differences in RV:TV ratios. Within a gender there were no differences between birds from the 24 Wk H2 and 24 Wk H3 groups for any of the variables shown in Table 1.
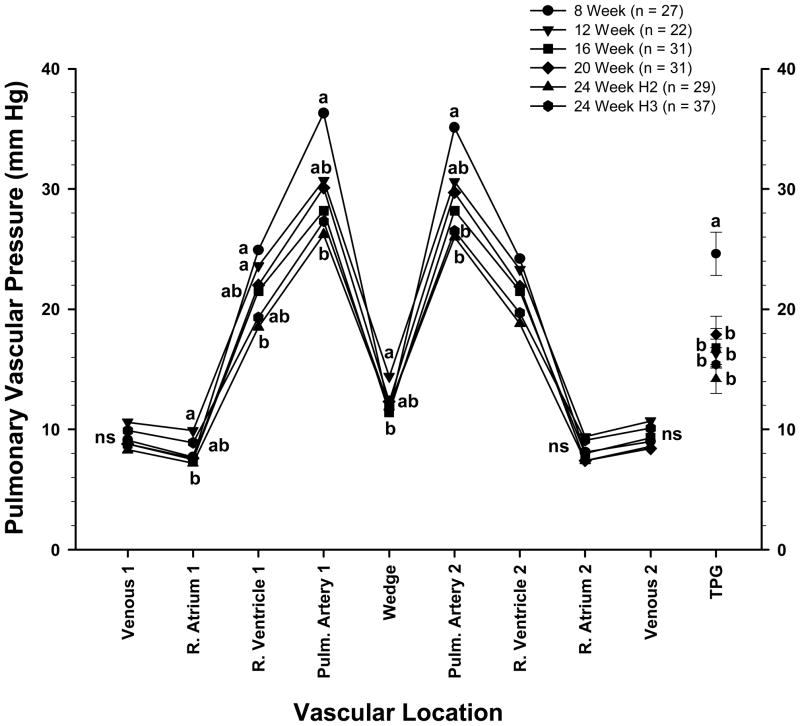
Pulmonary vascular pressures are shown for each age and gender category in Table 2. These values include the pressures recorded as the catheter was advanced through the vascular tree (Venous 1, R. Atrium 1, R. Ventricle 1, and Pulmonary Artery 1), the WP, the pressures recorded as the catheter was withdrawn from the vascular tree (Pulmonary Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2), and the calculated TPG. There were no age-related differences in the pressures recorded for Venous 1 or 2, R. Atrium 1 or 2, Pulmonary Artery 2, or R. Ventricle 2. The pressures recorded for R. Ventricle 1, Pulmonary Artery 1, WP and TPG were higher in males at 8 or 12 wk of age than in males from the 24 Wk H2 or 24 Wk H3 groups. Within each of the ages evaluated there were no gender differences in the pressures recorded at any of the vascular locations (Table 2). Pooling all birds by gender regardless of age revealed higher values for R. Atrium 1 and 2 and WP, and lower values for TPG in females than in males (Table 3). Pooling all birds by age regardless of gender (Figure 1) revealed higher average values for PAP 1 and PAP 2 in 8 wk old broilers (36.3 ± 2.1 mmHg and 35.1 ± 1.9 mmHg, respectively) than in broilers from the 24 Wk H2 group (26.2 ± 1.4 and 26.0 ± 1.3 mmHg, respectively) and the 24 Wk H3 group (27.3 ± 1.6 and 26.5 ± 1.5 mmHg, respectively). The PAP 1 and 2 values for broilers in the remaining age categories were intermediate (ns) between the 8 and 24 wk extremes. The high PAP 1 and 2 values in 8 wk old birds were associated with the second lowest average value for WP (11.6 ± 0.7 mmHg) combined with the highest TPG (24.6 ± 1.8 mmHg). The highest and lowest average WP values were observed in 12 wk old (14.4 ± 0.6) and 16 wk old (11.4 ± 0.8 mmHg) broilers, respectively (Figure 1).
Table 2.
Locations of blood pressures recorded in 8 to 24 wk old female (F) and male (M) broilers as a catheter was advanced through the vasculature (Venous 1, R. Atrium 1, R. Ventricle 1, and Pulmonary Artery 1 pressures) until the wedge pressure was obtained, and as the catheter was withdrawn from the vasculature (Pulmonary Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2 pressures)1
| Vascular Location | Gender | Wk of Age2 |
|||||
|---|---|---|---|---|---|---|---|
| 8 | 12 | 16 | 20 | 24 Wk H2 | 24 Wk H3 | ||
| Venous 1, mmHg | F | 8.5 ± 0.4 | 10.9 ± 0.7 | 8.4 ± 0.8 | 9.3 ± 0.8 | 9.7 ± 1.1 | 10.4 ± 0.7 |
| M | 9.8 ± 1.2 | 10.4 ± 0.8 | 9.2 ± 1.2 | 8.4 ± 0.9 | 7.3 ± 0.5 | 9.3 ± 0.8 | |
| R. Atrium 1, mmHg | F | 7.3 ± 0.3 | 10.2 ± 0.9 | 7.2 ± 0.9 | 8.7 ± 0.7 | 8.5 ± 1.0 | 9.6 ± 0.7 |
| M | 8.2 ± 1.0 | 9.6 ± 1.2 | 7.7 ± 1.1 | 6.4 ± 0.6 | 6.2 ± 0.4 | 8.1 ± 0.8 | |
| R. Ventricle 1, mmHg | F | 23.7 ± 1.3ab | 24.6 ± 1.2ab | 21.0 ± 1.2ab | 22.2 ± 1.3ab | 20.1 ± 1.5ab | 19.5 ± 1.5ab |
| M | 25.9 ± 2.0a | 22.6 ± 1.5ab | 21.9 ± 1.8ab | 21.8 ± 1.6ab | 17.1 ± 1.1ab | 19.0 ± 1.4b | |
| Pulmonary Artery 1, mmHg | F | 34.2 ± 2.2ab | 29.5 ± 1.4ab | 26.6 ± 2.1b | 28.3 ± 1.7ab | 25.5 ± 2.5b | 27.2 ± 2.4b |
| M | 38.2 ± 3.4a | 32.0 ± 2.0ab | 29.5 ± 2.7ab | 32.0 ± 2.9ab | 26.7 ± 1.6b | 27.4 ± 2.1ab | |
| Wedge, mmHg | F | 11.4 ± 0.7ab | 14.2 ± 0.9ab | 11.8 ± 0.9ab | 12.9 ± 0.7ab | 13.0 ± 0.9ab | 13.5 ± 0.6ab |
| M | 11.9 ± 1.2ab | 14.6 ± 0.8a | 11.0 ± 1.2ab | 11.6 ± 0.7ab | 11.0 ± 0.5ab | 10.1 ± 0.9b | |
| Pulmonary Artery 2, mmHg | F | 34.2 ± 2.2 | 29.1 ± 1.4 | 26.4 ± 2.1 | 28.4 ± 1.7 | 25.7 ± 2.3 | 26.4 ± 2.4 |
| M | 35.9 ± 3.2 | 32.2 ± 1.7 | 29.6 ± 3.0 | 31.1 ± 2.8 | 26.2 ± 1.4 | 26.6 ± 2.0 | |
| R. Ventricle 2, mmHg | F | 23.8 ± 1.4 | 23.0 ± 1.1 | 20.9 ± 1.7 | 22.1 ± 1.2 | 20.0 ± 1.6 | 20.2 ± 1.6 |
| M | 24.6 ± 2.4 | 23.7 ± 1.8 | 22.0 ± 2.0 | 21.6 ± 1.8 | 17.8 ± 1.4 | 19.2 ± 1.4 | |
| R. Atrium 2, mmHg | F | 7.8 ± 0.4 | 10.0 ± 0.8 | 7.8 ± 0.8 | 8.3 ± 0.6 | 8.8 ± 0.9 | 9.6 ± 0.6 |
| M | 8.3 ± 1.1 | 8.9 ± 0.9 | 8.2 ± 1.2 | 6.4 ± 0.6 | 6.3 ± 0.4 | 8.6 ± 0.8 | |
| Venous 2, mmHg | F | 8.5 ± 0.4 | 10.8 ± 0.8 | 8.6 ± 0.8 | 9.0 ± 0.7 | 9.8 ± 1.0 | 10.3 ± 0.7 |
| M | 9.5 ± 1.0 | 10.6 ± 0.8 | 9.8 ± 1.2 | 7.7 ± 0.6 | 7.5 ± 0.4 | 9.8 ± 0.8 | |
| Trans-Pulmonary Gradient, mmHg | F | 22.8 ± 2.1ab | 15.4 ± 1.4b | 14.8 ± 1.8b | 15.5 ± 1.4b | 12.5 ± 2.1b | 13.7 ± 2.2b |
| M | 26.3 ± 2.9a | 17.3 ± 2.0ab | 18.5 ± 2.5ab | 20.4 ± 2.6ab | 15.7 ± 1.4b | 17.3 ± 1.7ab | |
Superscripts designate differences (P ≤ 0.05) between genders and ages within a variable.
Data are presented as mean ± SEM. The trans-pulmonary gradient equals the pulmonary artery pressure minus the wedge pressure.
Sufficient numbers of birds were available from the second and third hatches (H2 and H3) to obtain duplicate groups at 24 wk of age.
Table 3.
Values for broilers pooled by gender regardless of age, including their body weights, heart ventricle weights, right to total ventricular weight ratios (RV:TV ratio), and blood pressures recorded as a catheter was advanced through the vasculature (Venous 1, R. Atrium 1, R. Ventricle 1, and Pulmonary Artery 1 pressures) until the wedge pressure was obtained, and as the catheter was withdrawn from the vasculature (Pulmonary Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2 pressures)1
| Variables | Gender |
|
|---|---|---|
| Females (n = 86) | Males (n = 91) | |
| Body Weight, g | 4,152 ± 108b | 4,984 ± 139a |
| Right:Total Ventricle wt. ratio | 0.234 ± 0.004 | 0.235 ± 0.004 |
| Venous 1, mmHg | 9.5 ± 0.3z | 9.0 ± 0.4z |
| R. Atrium 1, mmHg | 8.6 ± 0.3a,z | 7.6 ± 0.4b,z |
| R. Ventricle 1, mmHg | 21.6 ± 0.6x | 21.2 ± 0.7x |
| Pulmonary Artery 1, mmHg | 28.4 ± 0.9w | 30.6 ± 1.1w |
| Wedge, mmHg | 12.8 ± 0.3a,y | 11.5 ± 0.4b,y |
| Pulmonary Artery 2, mmHg | 28.2 ± 0.9w | 29.9 ± 1.0w |
| R. Ventricle 2, mmHg | 21.5 ± 0.6x | 21.2 ± 0.8x |
| R. Atrium 2, mmHg | 8.7 ± 0.3a,z | 7.7 ± 0.4b,z |
| Venous 2, mmHg | 9.5 ± 0.3z | 9.1 ± 0.4yz |
| Trans-Pulmonary Gradient, mmHg | 15.6 ± 0.8b | 19.1 ± 1.0a |
Superscripts designate differences (P ≤ 0.05) between genders within a variable.
Superscripts designate differences (P ≤ 0.05) between blood pressures regardless of gender.
Data are presented as mean ± SEM. The trans-pulmonary gradient equals the pulmonary artery pressure minus the wedge pressure.
Figure 1.
Symbols represent the mean blood pressures (in millimeters of mercury) of broilers (males and females pooled) from a PHS-susceptible line at 8 through 24 wk of age, including duplicate groups from hatches 2 and 3 at wk 24 (24 Wk H2, 24 WK H3). The pressures were recorded as a catheter was advanced through the wing vein (Venous 1), right atrium (R. Atrium 1), right ventricle (R. Ventricle 1), and pulmonary artery (Pulm. Artery 1) until the wedge pressure was obtained (Wedge). Pressures also were recorded as the catheter was withdrawn from the vasculature (Pulm. Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2). The transpulmonary gradient (TPG; mean values ± SEM) represents the pulmonary artery pressure minus the WP. a,b Letters represent differences (P ≤ 0.05) between the age groups within a single anatomical location.
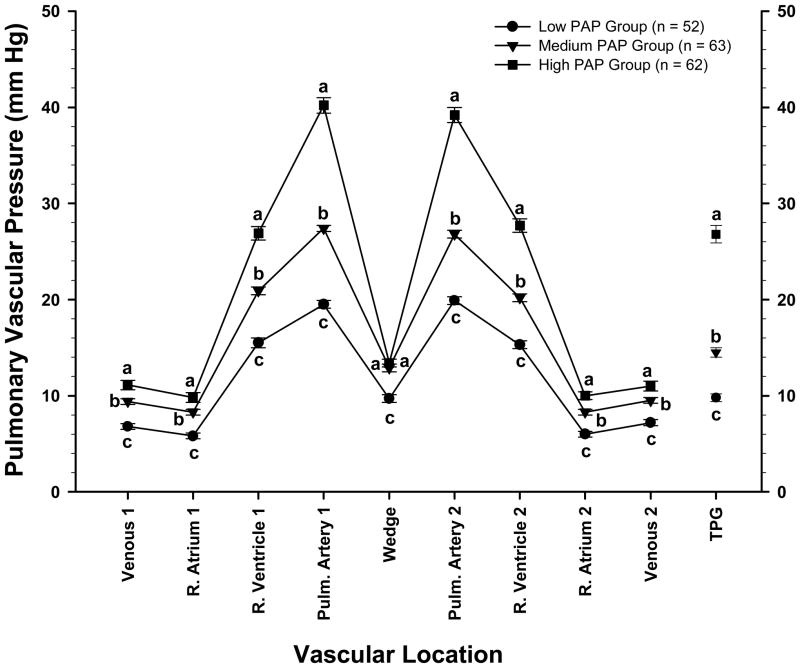
To further characterize the relationship between PAP, WP and TPG the vascular pressure data were pooled regardless of age or gender. These pooled data were grouped into three cohorts of approximately equal size to compare vascular pressures in birds having the lowest average PAP values (n = 52; range: 12 to 22.9 mmHg), intermediate PAP values (n = 63; range: 23 to 32.9 mmHg), and highest PAP values (n = 62; range: 33 to 62 mmHg). As shown in Figure 2, these High, Medium, and Low cohort groupings proportionally differentiated pressures within each of the vascular locations with the exception of WP. Thus the differences in PAP between the High and Medium cohorts (40.2 ± 0.8 vs. 27.4 ± 0.3 mmHg, respectively; P < 0.001; Δ 12.8 mmHg) were not matched by differences in WP (13.4 ± 0.4 vs. 12.9 ± 0.4 mmHg, respectively; ns; Δ 0.5 mmHg) but instead were attributable to large differences in TPG (26.8 ± 0.9 vs. 14.5 ± 0.5 mmHg, respectively; P < 0.001; Δ 12.3 mmHg). Similarly the differences in PAP between the High and Low cohorts (40.2 ± 0.8 vs. 19.5 ± 0.4 mmHg, respectively; P < 0.001; Δ 20.7 mmHg) were minimally associated with differences in WP (13.4 ± 0.4 vs. 9.7 ± 0.4 mmHg, respectively; P < 0.001; Δ 4.0 mmHg) but instead were primarily attributable to large differences in TPG (26.8 ± 0.9 vs. 9.8 ± 0.4 mmHg, respectively; P < 0.001; Δ 17.0 mmHg). The RV:TV ratio for the High PAP group (0.26 ± 0.005) was higher (P<0.001) than the RV:TV ratios for the Medium and Low PAP groups (0.23 ± 0.005 and 0.21 ± 0.004, respectively; ns).
Figure 2.
Symbols represent the mean ± SEM of blood pressures (in millimeters of mercury) of broilers (genders and ages pooled) from a PHS-susceptible line grouped into three cohorts of approximately equal size to compare vascular pressures in birds having the lowest average PAP values (range: 12 to 22.9 mmHg), intermediate PAP values (range: 23 to 32.9 mmHg), and the highest PAP values (range: 33 to 62 mmHg). The pressures were recorded as a catheter was advanced through the wing vein (Venous 1), right atrium (R. Atrium 1), right ventricle (R. Ventricle 1), and pulmonary artery (Pulm. Artery 1) until the wedge pressure was obtained (Wedge). Pressures also were recorded as the catheter was withdrawn from the vasculature (Pulm. Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2). The transpulmonary gradient (TPG; mean values ± SEM) represents the pulmonary artery pressure minus the WP. a,b,c Letters represent differences (P ≤ 0.05) between the age groups within a single anatomical location.
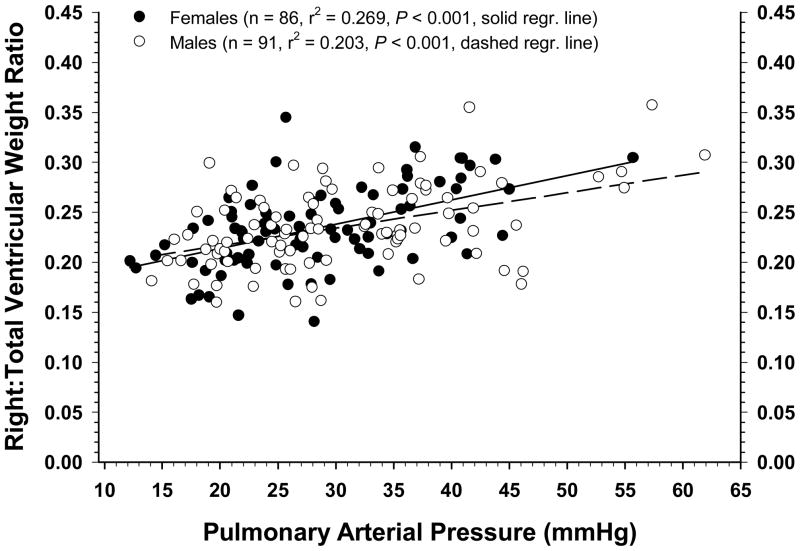
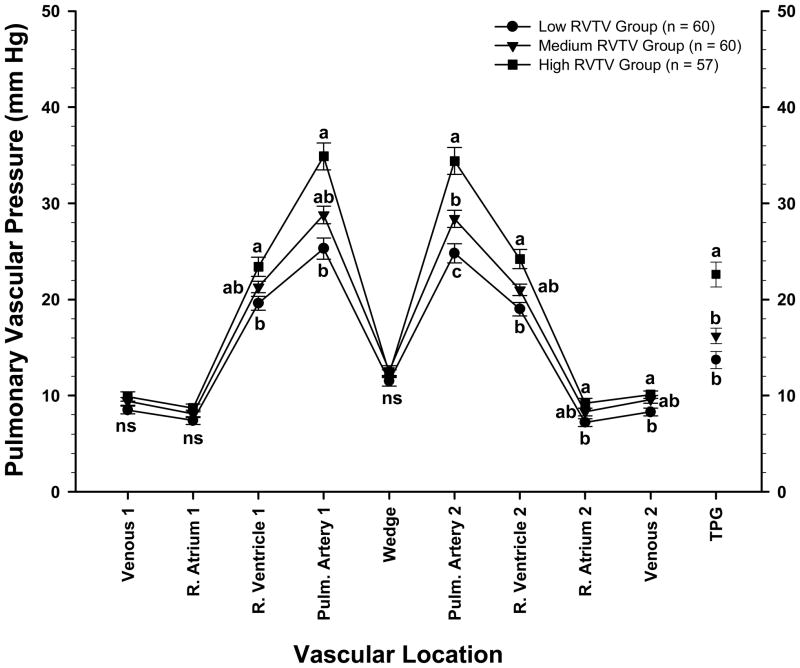
The relationship between average PAP values for individual males and females and their corresponding RV:TV ratios are shown with all ages pooled in Figure 3. The slope of the regression line was significant (P <0 .001) for both genders, and the coefficients of determination indicated that approximately 20% of the variation in PAP for males and 27% of the variation for females could be predicted by differences in the RV:TV ratios. To further characterize the relationship between RV:TV ratios and vascular pressure profiles, data pooled regardless of age or gender were subdivided into three cohorts of approximately equal size to compare birds having the lowest RV:TV ratios (n = 60; range: 0.14 to 0.217), intermediate RV:TV ratios (n = 60; range: 0.22 to 0.25), and highest RV:TV ratios (n = 57; range: 0.251 to 0.36) (Figure 4). The RV:TV ratio for the High group (0.28 ± 0.003) was higher (P < 0.001) than the RV:TV ratio for the Medium group (0.23 ± 0.012) which in turn was higher than the RV:TV ratio of the Low group (0.19 ± 0.002). Broilers in the High RV:TV group had higher PAP and TPG values combined with essentially normal WP values when compared with broilers in the Low RV:TV group (Figure 4).
Figure 3.
The relationship between pulmonary arterial pressure values and the corresponding right to total ventricular weight ratios for individual males and females are shown with all ages combined. The slope of the regression line was significant (P < 0.001) for both genders, and the coefficients of determination indicated that approximately 20% of the variation in PAP for males and 27% of the variation for females could be predicted by differences in the RV:TV ratios.
Figure 4.
Symbols represent the mean ± SEM of blood pressures (in millimeters of mercury) of broilers (genders and ages pooled) from a PHS-susceptible line grouped into three cohorts of approximately equal size to compare vascular pressures in birds having the lowest RV:TV ratios (range: 0.14 to 0.217), intermediate RV:TV ratios (range: 0.22 to 0.25), and the highest RV:TV ratios (range: 0.251 to 0.36). The pressures were recorded as a catheter was advanced through the wing vein (Venous 1), right atrium (R. Atrium 1), right ventricle (R. Ventricle 1), and pulmonary artery (Pulm. Artery 1) until the wedge pressure was obtained (Wedge). Pressures also were recorded as the catheter was withdrawn from the vasculature (Pulm. Artery 2, R. Ventricle 2, R. Atrium 2, and Venous 2). The transpulmonary gradient (TPG; mean values ± SEM) represents the pulmonary artery pressure minus the WP. a,b,c Letters represent differences (P ≤ 0.05) between the age groups within a single anatomical location.
DISCUSSION
Previous comparisons of 5 to 7 wk old PHS-susceptible and PHS-resistant broilers did not reveal differences in cardiac output but did provide direct evidence supporting elevated pre-capillary resistance (PAH) rather than elevated post-capillary resistance (PVH) as the primary cause of pulmonary hypertension (Chapman and Wideman, 2001; Lorenzoni et al., 2008). If a component of PHS susceptibility involves progressive deterioration of left ventricular valvular or myocardial function, then evidence of PVH theoretically should be detected more readily in older broilers. In the present study pulmonary vascular pressure profiles were measured in 8 to 24 wk old males and females from a PHS-susceptible line. All birds were reared under nominal conditions to emphasize the innate basis of susceptibility during the spontaneous onset of pulmonary hypertension. No differences in vascular pressures were detected between genders within any age group (Table 2). Thereafter the vascular pressure profiles were compared based on: gender regardless of age (Table 3); age regardless of gender (Figure 1); PAP cohorts regardless of age or gender (Figure 2); and RV:TV cohorts regardless of age or gender (Figure 4). Within each of these comparisons the group or cohort having the highest PAP consistently exhibited a directly proportional increase in TPG with little or no increase in WP. For example, 8 wk old broilers had the highest PAP and TPG values in combination with the second lowest WP value of all age groups. Similarly the broilers in the cohort with the highest RV:TV ratios had higher PAP and TPG values but no difference in WP compared with broilers in the cohort with the lowest RV:TV ratios. For the PAP cohort comparisons, differences in PAP between the high and medium cohorts were directly proportional to differences in TPG, and no difference in WP was detected.
None of the pulmonary vascular pressure profiles for the age, gender, PAP and RV:TV groups or cohorts evaluated in the present study matched the essential criteria necessary for a diagnosis of PVH caused by left-sided valvular or myocardial diseases (elevated PAP and WP accompanied by a low TPG). Indeed, a diagnosis of PVH requires a direct 1:1 proportionality between increments in PAP and WP, accompanied by TPG values that remain within the “normal” (low) range. A proportional elevation in WP reflects increased post-capillary resistance to blood flow or congestion within the pulmonary veins and left atrium (Zidulka and Hakim, 1985; Fawzy et al., 1996; Hermo-Weiler et al., 1998; Chemla et al., 2002; Benza and Tallaj, 2006). Instead, in the present study elevations in PAP consistently were associated with directly proportional increases in the TPG accompanied by virtually no increase in the WP. The largest difference in WP was detected when the pressure profiles for the high and low PAP cohorts were compared (Figure 2). In that comparison the very large (Δ 20.7 mmHg) difference in PAP was minimally associated with the WP (Δ 4 mmHg) and instead was paralleled by large differences in the TPG (Δ 17 mmHg). The slight elevation in WP for broilers with very high PAP values likely reflects an elevated pressure dissipation profile transmitted along the length of the pulmonary vasculature. It also is possible that slight elevations in the WP reflect very modest increases in post-capillary resistance to blood flow through the pulmonary venous drainage, likely caused by minor left ventricular diastolic dysfunction attributable to right ventricular dilation or hydropericardium (Zidulka and Hakim, 1985; Lorenzoni et al., 2008). However, any effect of right ventricular dilation or hydropericardium on cardiopulmonary hemodynamics would only be anticipated after the evolution of severe pathological changes in the heart associated with the cardiac decompensation that typically can follow sustained “work hypertrophy” (Diwan and Dorn, 2007). In the present study age-related increases in left and total ventricle weights reflect the anticipated increments in the cardiac output needed to support growth (Wideman, 1999). Based on the pulmonary vascular pressure profiles obtained in the present and previous studies, we conclude that the initial pathogenesis of PHS in susceptible broilers cannot be attributed to increases in downstream resistance (e.g., pulmonary venoconstriction), left myocardial failure, cardiac decompensation, or mitral valve degeneration (Chapman and Wideman, 2001; Lorenzoni et al., 2008). Instead, all modes of data comparison were wholly consistent with PAH rather than PVH as the primary cause of increased PAP in broilers developing pulmonary hypertension.
The significant but relatively low correlations between PAP and RV:TV ratios for males and females of all age groups (Figure 3) are consistent with previous observations in which the earliest changes associated with pulmonary hypertension were detected through direct measurements of PAP rather than by evaluating RV:TV ratios. Repeated observations consistently indicate that differences in RV weights in birds undergoing different degrees of pulmonary hypertension presumably develop after differences in PAP have been sustained chronically (Wideman et al., 2000; Liu, 2001; Lorenzoni, 2006; Lorenzoni et al., 2008). Parallel increases in pulmonary vascular pressure profiles associated with increasingly severe PAH (Figure 2) further support the hypothesis that pooling in large systemic veins may be one of the earliest hemodynamic changes associated with the onset of right-sided congestive heart failure (Wideman et al., 1999; Lorenzoni et al., 2008). The hemodynamic changes associated with PAH occurred most dramatically in fast-growing 8 wk old broilers and were progressively less evident as the broilers aged (Figure 1), whereas evidence of more chronic RV “work hypertrophy” persisted throughout all age categories (Table 1). An early, rapid onset of a mild right-sided congestion would explain the consistent increase in VP and PAP but not necessarily in RV mass or RV:TV ratios as broilers develop PAH (Figure 3). Evidently increases in systemic venous and pulmonary vascular pressures develop rapidly and precede significant work hypertrophy by the RV as it propels the requisite cardiac output through the elevated pre-capillary pulmonary vascular resistance of PAH-susceptible broilers.
Acknowledgments
Supported by NIH/National Heart Lung Blood Institute Grant 1R15HL092517 01
Abbreviation Key
- CO
cardiac output
- PAH
pulmonary arterial hypertension
- PAP
pulmonary arterial pressure
- PHS
pulmonary hypertension syndrome
- PVH
pulmonary venous hypertension
- PVR
pulmonary vascular resistance
- RV
TV, right: total ventricular weight ratio
- TPG
transpulmonary gradient
- WP
wedge pressure
Footnotes
Interstate Drug Exchange, Inc., Amityville, NY 11701.
Dow Corning Corp., Midland, MI 48686-0994
World Precision Instruments, Sarasota, FL 34230.
Biopac Systems, Inc., Goleta, CA 93117
References
- Anthony NB, Balog JM, Hughes JD, Stamps L, Cooper MA, Kidd BD, Liu X, Huff GR, Huff WE, Rath NC. Genetic selection of broiler lines that differ in their ascites susceptibility 1. Selection under hypobaric conditions; Proc. 13th Eur. Symp. Poult. Nutr; Belgium: Blankenberge; 2001. pp. 327–328 . [Google Scholar]
- Balog JM. Ascites syndrome (pulmonary hypertension syndrome) in broiler chickens: are we seeing the light at the end of the tunnel? Avian Poult Biol Rev. 2003;14:99–126. [Google Scholar]
- Benza RL, Tallaj JA. Pulmonary hypertension out of proportion to left heart disease. Adv Pulm Hypertension. 2006;5:21–29. [Google Scholar]
- Bowen OT, Erf GF, Anthony NB, Wideman RF. Pulmonary hypertension triggered by lipopolysaccharide in ascites-susceptible and -resistant broilers is not amplified by aminoguanidine, a specific inhibitor of inducible nitric oxide synthase. Poult Sci. 2006;85:528–536. doi: 10.1093/ps/85.3.528. [DOI] [PubMed] [Google Scholar]
- Burton RR, Smith AH. The effect of polycythemia and chronic hypoxia on heart mass in the chicken. J Appl Physiol. 1967;22:782–785. doi: 10.1152/jappl.1967.22.4.782. [DOI] [PubMed] [Google Scholar]
- Burton RR, Besh EL, Smith AH. Effect of chronic hypoxia on the pulmonary arterial blood pressure of the chicken. Am J Physiol. 1968;214:1438–1442. doi: 10.1152/ajplegacy.1968.214.6.1438. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Pulmonary wedge pressures confirm pulmonary hypertension in broilers is initiated by an excessive pulmonary arterial (precapillary) resistance. Poult Sci. 2001;80:468–473. doi: 10.1093/ps/80.4.468. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Evaluation of the serotonin blocker methiothepin in broilers injected intravenously with lipopolysaccharide and microparticles. Poult Sci. 2006;85:2222–2230. doi: 10.1093/ps/85.12.2222. [DOI] [PubMed] [Google Scholar]
- Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Resp J. 2002;20:1314–1331. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- Cueva S, Sillau H, Valenzuela A, Ploog H. High altitude induced pulmonary hypertension and right ventricular failure in broiler chickens. Res Vet Sci. 1974;16:370–374. [PubMed] [Google Scholar]
- Diwan A, Dorn GW. Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology. 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- Fawzy ME, Mimish LM, Sivanandam V, Lingamanaicker J, Patel A, Khan B, Duran CM. Immediate and long-term effect of mitral ballon valvotomy on severe pulmonary hypertension in patients with mitral stenosis. Am Heart J. 1996;131:89–93. doi: 10.1016/s0002-8703(96)90055-1. [DOI] [PubMed] [Google Scholar]
- Guthrie AJ, Cilliers JA, Huchzermeyer FW, Killeen VM. Broiler pulmonary hypertension syndrome. II. The direct measurement of right ventricular and pulmonary artery pressures in the closed chest domestic fowl. J Vet Res. 1987;54:599–602. [PubMed] [Google Scholar]
- Hermo-Weiler CI, Koizumi T, Parker R, Newman JH. Pulmonary vasoconstriction induced by mitral valve obstruction in sheep. J Appl Physiol. 1998;85:1655–1660. doi: 10.1152/jappl.1998.85.5.1655. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Hypoxic ascites in broilers: A review of several studies done in Colombia. Avian Dis. 1987;31:171–183. [PubMed] [Google Scholar]
- Huchzermeyer FW, DeRuyck AMC. Pulmonary hypertension syndrome associated with ascites in broilers. Vet Rec. 1986;119:94. doi: 10.1136/vr.119.4.94. [DOI] [PubMed] [Google Scholar]
- Jandel Scientific. SigmaStat® Statistical Software User’s Manual. Jandel Scientific Software; San Rafael, CA: 1994. [Google Scholar]
- Julian RJ. Pulmonary hypertension as a cause of right ventricular failure and ascites in broilers. Zootecnica International (November, #11) 1988:58–62. [Google Scholar]
- Julian RJ. Ascites in poultry. Avian Pathol. 1993;22:419–454. doi: 10.1080/03079459308418934. [DOI] [PubMed] [Google Scholar]
- Julian RJ, Friars GW, French H, Quinton M. The relationship of right ventricular hypertrophy, right ventricular failure, and ascites to weight gain in broiler and roaster chickens. Avian Dis. 1987;31:130–135. [PubMed] [Google Scholar]
- Liu X. MSc Diss. Univ. Arkansas; Fayetteville: 2001. Effect of cold stress or bronchus challenge on ascites resistant or susceptible lines of chickens. [Google Scholar]
- Lorenzoni AG. MSc Diss. McGill Univ; Montreal: 2006. Effects of alpha-tocopherol and L-arginine on cardiopulmonary function in broilers. [Google Scholar]
- Lorenzoni AG, Anthony NB, Wideman RF. Transpulmonary pressure gradient verifies pulmonary hypertension is initiated by increased arterial resistance in broilers. Poult Sci. 2008;87:146–644. doi: 10.3382/ps.2007-00178. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry. 9. National Academy Press; Washington, DC: 1994. rev. [Google Scholar]
- Olkowski AA. Pathophysiology of heart failure in broiler chickens: structural, biochemical, and molecular characteristics. Poult Sci. 2007;86:999–1005. doi: 10.1093/ps/86.5.999. [DOI] [PubMed] [Google Scholar]
- Olkowski AA, Classen HL, Kumor L. Left atrio-ventricular valve degeneration, left ventricular dilation and right ventricular failure: a possible association with pulmonary hypertension in the etiology of ascites in broiler chickens. Avian Pathol. 1998;27:51–59. doi: 10.1080/03079459808419274. [DOI] [PubMed] [Google Scholar]
- Owen RL, Wideman RF, Cowen BS. Changes in pulmonary arterial and femoral arterial blood pressure upon acute exposure to hypobaric hypoxia in broiler chickens. Poult Sci. 1995;74:708–715. doi: 10.3382/ps.0740708. [DOI] [PubMed] [Google Scholar]
- Pavlidis HO, Balog JM, Stamps LK, Hughes JD, Jr, Huff WE, Anthony NB. Divergent selection for ascites incidence in chickens. Poult Sci. 2007;86:2517–2529. doi: 10.3382/ps.2007-00134. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Pickett C, Morris K, Reeves JT. The relationship between rapid growth and pulmonary hemodynamics in the fast-growing broiler chicken. Am Rev Respir Dis. 1989;139:1524–1530. doi: 10.1164/ajrccm/139.6.1524. [DOI] [PubMed] [Google Scholar]
- Sillau AH, Montalvo C. Pulmonary hypertension and the smooth muscle of pulmonary arterioles in chickens at high altitude. Comp Biochem Physiol. 1982;71A:125–130. [Google Scholar]
- Simonneau G, Galié N, Rubin LJ, Langleben D, Segger W, Dominighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am College Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardiac output in four-, five-, and six-week-old broilers, and hemodynamic responses to intravenous injections of epinephrine. Poult Sci. 1999;78:392–403. doi: 10.1093/ps/78.3.392. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardio-pulmonary hemodynamics and ascites in broiler chickens. In: Dietert RR, Ottinger MA, editors. Poult and Av Biol Rev. Vol. 11. 2000. pp. 21–43. [Google Scholar]
- Wideman RF. Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. World’s Poult Sci J. 2001;57:289–307. [Google Scholar]
- Wideman RF, Bottje WG. Current understanding of the ascites syndrome and future research directions. Nutrition and Technical Symposium Proceedings; St. Louis, MO: Novus International Inc; 1993. pp. 1–20. [Google Scholar]
- Wideman RF, Bowen OT, Erf GF, Chapman ME. Influence of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on the pulmonary hypertensive response to microparticle injection in broilers. Poult Sci. 2006;85:511–527. doi: 10.1093/ps/85.3.511. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Hamal KR, Bowen OT, Lorenzoni AG, Erf GF, Anthony NB. An inadequate pulmonary vascular capacity and susceptibility to pulmonary arterial hypertension in broilers. Poult Sci. 2007;86:984–998. doi: 10.1093/ps/86.5.984. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Wang W, Erf GF. Immune modulation of the pulmonary hypertensive response to bacterial Lipopolysaccharide (LPS, endotoxin) in broilers. Poult Sci. 2004;83:432–441. doi: 10.1093/ps/83.4.624. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Erf GF, Chapman ME, Wang W, Anthony NB, Xiaofang L. Intravenous micro-particle injections and pulmonary hypertension in broiler chickens: acute post-injection mortality and ascites susceptibility. Poult Sci. 2002;81:1203–1217. doi: 10.1093/ps/81.8.1203. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Fedde MR, Tackett CD, Weigle GE. Cardio-pulmonary function in preascitic (hypoxemic) or normal broilers inhaling ambient air or 100% oxygen. Poult Sci. 2000;79:415–425. doi: 10.1093/ps/79.3.415. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Kirby YK, Tackett CD, Marson NE, McNew RW. Cardio-pulmonary function during acute unilateral occlusion of the pulmonary artery in broilers fed diets containing normal or high levels of arginine-HCL. Poult Sci. 1996;75:1587–1602. doi: 10.3382/ps.0751587. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Maynard P, Bottje W. Venous blood pressure in broilers during acute inhalation of five percent carbon dioxide or unilateral pulmonary artery occlusion. Poult Sci. 1999;78:1443–1451. doi: 10.1093/ps/78.10.1443. [DOI] [PubMed] [Google Scholar]
- Zidulka A, Hakim TS. Wedge pressure in large vs. small pulmonary arteries to detect pulmonary venoconstriction. J Appl Physiol. 1985;59:1329–1332. doi: 10.1152/jappl.1985.59.4.1329. [DOI] [PubMed] [Google Scholar]