Abstract
Secondary palate fusion requires adhesion and epithelial to mesenchymal transition (EMT) of the epithelial layers on opposing palatal shelves. This EMT requires transforming growth factor β3 (TGFβ3), and its failure results in cleft palate. Ephrins, and their receptors, the Ephs, are responsible for migration, adhesion, and midline closure events throughout development. Ephrins can also act as signal transducing receptors in these processes, with the Ephs serving as ligands (termed "reverse" signaling). We found that activation of ephrin reverse signaling in chicken palates induced fusion in the absence of TGFβ3, and that PI3K inhibition abrogated this effect. Further, blockage of reverse signaling inhibited TGFβ3-induced fusion in the chicken and natural fusion in the mouse. Thus, ephrin reverse signaling is necessary and sufficient to induce palate fusion independent of TGFβ3. These data describe both a novel role for ephrins in palate morphogenesis, and a previously unknown mechanism of ephrin signaling.
Keywords: palate, ephrin, Eph, PI3 kinase, epithelium, mouse, chicken
Introduction
Cleft palate is a common birth defect, occurring on its own in an estimated one in 1,500 live births and even more often in conjunction with cleft lip (Croen et al., 1998). Yet, the molecular mechanisms that control mammalian palate development are poorly understood. In the mouse, the mesenchymal extrusions of tissue that will ultimately form the hard palate elevate over the tongue starting at e11.5. By e14.5, they have grown to meet at the midline, at which time the layers of epithelium on the opposing shelves adhere (Murray and Schutte, 2004). Current evidence indicates that these cells then undergo epithelial to mesenchymal transition (EMT) and apoptosis to achieve a fused palate of confluent mesenchyme, and both these aspects of the fusion process require the action of transforming growth factor beta 3 (TGFβ3) (Ahmed et al., 2007; Nawshad, 2008). Kang and Svoboda (2002) showed that PI3 kinase signaling is required for fusion (Kang and Svoboda, 2002). And recently, Xu et al. (2008) demonstrated that both Smad4 and p38 act downstream of TGFβ to cause palate fusion, although either alone appeared dispensable (Xu et al., 2008).
The palate develops from neural crest cells (NCC), migration and segregation of which depend on the action of ephrins and their Eph receptors. The Ephs are the largest family of receptor tyrosine kinases (RTKs), and are subdivided into A and B groups based on their preferential binding to the glycosylphosphatidyl inositol-linked A ephrin or the transmembrane B ephrin ligands, although binding can be promiscuous across classes (Orioli and Klein, 1997). Ephrins are unique among RTK ligands in that they can also act as receptors, with the Eph acting as ligand, a process called “reverse signaling” (Murai and Pasquale, 2003). Early migration of embryonic NCCs in the mouse requires both forward and reverse signaling of ephrin-B1 and ephrin-B2, and ablation of either ephrin causes defects in NCC-derived tissues including the palate (Davy et al., 2004; Davy and Soriano, 2007). In humans, ephrin-B1 mutations are associated with syndromes that include cleft palate (Twigg et al., 2004; Wieland et al., 2005; Torii et al., 2007), highlighting a likely conserved role for ephrin signaling in craniofacial development.
Recent work by Risley, et al. (2009) reported a role for ephrin-B/EphB forward signaling in a later stage of palate development, namely the proliferation of palate shelf mesenchyme prior to midline apposition (Risley et al., 2009). This study was closely followed by a report from Bush, et al. (2010) showing that ephrin-B1 forward signaling controls NCC-derived mesenchyme proliferation through the mitogen activated protein kinase (MAPK) pathway (Bush and Soriano, 2010). These findings are consistent with known roles for EphB forward signaling in progenitor proliferation in other systems, such as the hippocampus (Chumley et al., 2007) and the intestine (Holmberg et al., 2006; Genander et al., 2009).
Both the Risley and Bush studies showed evidence of Eph and ephrin in palate at a stage when the shelves begin to undergo EMT and fuse. Risley et al. (2009) reported Ephs B2 and B3 and all three B ephrins in mouse palate epithelium and mesenchyme at e14.5, and Bush et al. (2010) showed ephrin-B1 at the same stage, albeit only in the mesenchyme. Because B Ephs and ephrins direct midline adhesion and fusion events in other developmental processes, such as urethral closure and urorectal septation (Dravis et al., 2004), we asked whether they play a similar role in palate fusion.
Here we report our analysis of B Eph and ephrin expression in fusing palate epithelium and document a requirement for ephrin reverse signaling in palate fusion. Our findings describe a novel role for ephrins in craniofacial development and point to a heretofore unknown mechanism of ephrin reverse signaling.
Results
Eph and ephrin expression in fusing palate epithelium
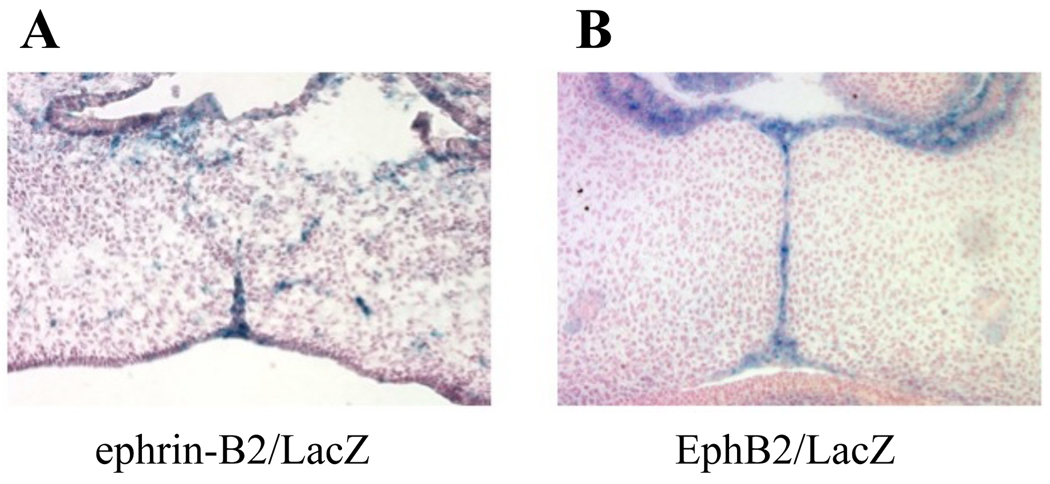
We examined beta-galactosidase (βgal) expression in mouse embryos that were genetically engineered to express a chimeric ephrin-B2 (EB2) allele in which the intracellular domain was replaced by a βgal moiety (Dravis et al., 2004). At e14.5, we found EB2/βgal specifically in the palate epithelial cells, many of which had begun to migrate inward to mix with mesenchymal cells in the interior of the shelf (Figure 1A). This migration parallels the previously documented movement of fluorescently labeled epithelial cells during the EMT process that leads to fusion (Kang and Svoboda, 2005) and validates EB2 as a marker for palate epithelium. We also noted βgal expression in the same layer in the EphB2/LacZ mouse, in which the lacZ gene replaces the EphB2 allele (Henkemeyer et al., 1996) (Figure 2B). These data suggested that Eph/ephrin forward and/or reverse signaling in the epithelial layers plays a role in palate adhesion and fusion.
Figure 1. EphB2 and ephrin-B2 expression in fusing palate epithelium.
Day 14.5 embryos from mice harboring: (A) the EB2/LacZ chimeric allele or (B) the LacZ knock-in to the EphB2 locus were sectioned coronally and stained with X-gal. Counter stain is Nuclear Fast Red. βgal expression was found in the palate epithelium, suggesting a role in adhesion and/or fusion. Note the breakup and dispersion of EB2/βgal during EMT.
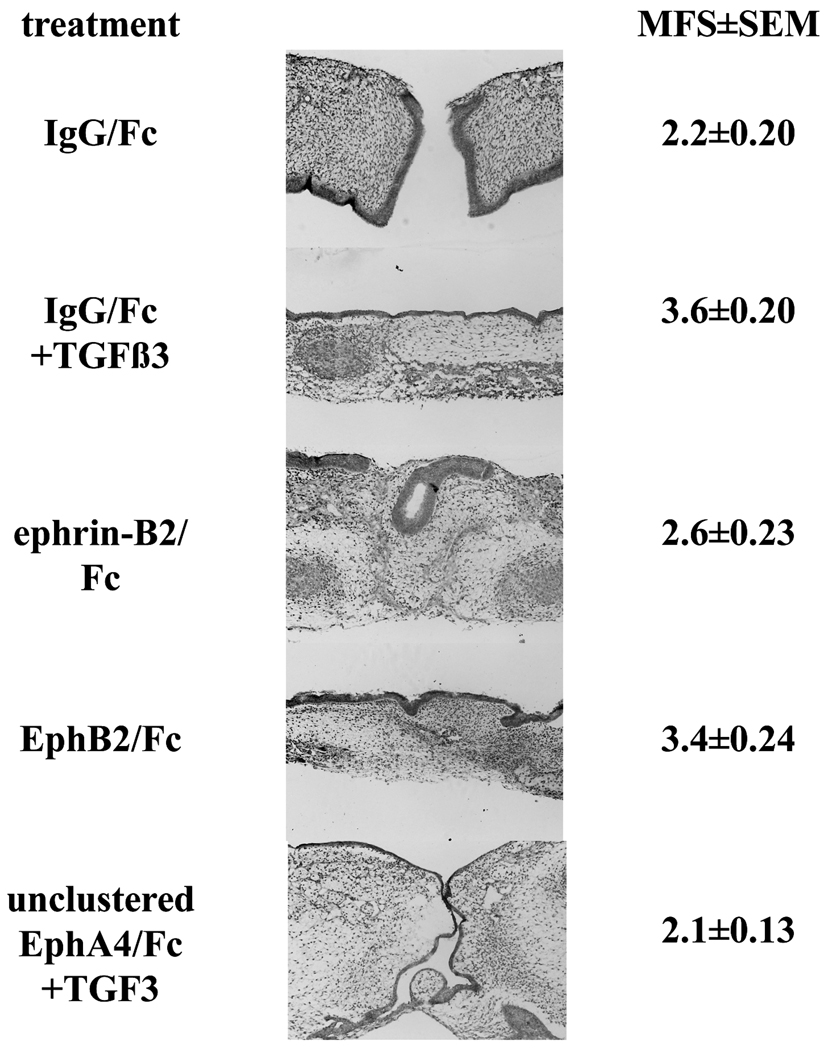
Figure 2. Eph and ephrin effects on palate fusion.
Palatal shelves were dissected from eight day old chicken embryos and cultured in contact on a support for 72 h in the presence of specific treatments, as indicated. Tissues were fixed, paraffin processed, and sectioned. Shown are representative H&E stained sections from each treatment. Note the darkened epithelial layer that disappears as fusion proceeds. H&E stained sections from anterior to posterior were scored on for fusion on a scale of 1 to 5 at anterior, middle, and posterior points and these scores averaged to yield the mean fusion score (MFS) shown. Values are ±SEM, with n=7 to 9 for each group across three separate experiments. Magnification is 100×.
Activation of ephrin reverse signaling causes palate fusion
To examine the role of ephrins in palate fusion, we employed a well established ex vivo chicken palate culture system. The chicken palate does not naturally fuse, unlike the mouse palate. However, the palatal shelves will fuse if placed in contact and given exogenous TGFβ3 (Sun et al., 1998). In this respect, the functional difference between mouse and chicken is that the mouse palate makes its own TGFβ3 (Taya et al., 1999); anti-TGF antibodies will block mouse palate fusion (Brunet et al., 1995). Thus, the chicken system allows us to examine ephrin signaling apart from TGFβ3 simply by adding or withholding this factor. Furthermore, principles of ephrin signaling governing axon guidance in the chicken retino-tectal system have proved to be directly applicable to the analogous mouse visual system, indicating conservation of Eph/ephrin signaling across these species (Scicolone et al., 2009). Since the palatal shelves in this culture system are placed in contact throughout the experiment, it allows examination of the fusion event independent of tissue proliferation. To stimulate Eph forward signaling, we used purified recombinant EB2 extracellular domain fused to human Fc. In order for this protein to be biologically active, it must be artificially clustered by the addition of anti-Fc antibody to stimulate the receptor aggregation that is required to initiate intracellular signaling (Knöll et al., 2007). We applied this protein, with or without TGFβ3, to palatal shelves placed in contact for 72 h, after which we fixed the tissues and evaluated them histologically for fusion using our previously published one to five scale for mean fusion score (MFS) (Kang and Svoboda, 2002). Palates treated with IgG Fc as a negative control did not fuse (MFS=2.2±0.20), while those treated with TGFβ3+IgG Fc fused as expected (MFS=3.6±0.20; Figure 2). In comparison, palates treated with clustered EB2/Fc exhibited partial fusion that was not statistically different from IgG Fc controls across all experimental replicates (MFS=2.6±0.23). Because the ephrin treatment experiments gave negative results, we performed three tests to confirm that the lot of recombinant EB2/Fc we used was both biologically active and stable in culture over the time course of our experiments. First, administration of clustered EB2/Fc to COS1 cells caused clustering and tyrosine phosphorylation of endogenous EphB2, a hallmark of Eph activation (Fig. S1A) (Himanen et al., 2004). Second, incubation of clustered EB2/Fc with cultured spinal cord neurons positive for EphB2 caused both clustering of EphB2 and co-localization of those clusters with N-methyl-D-aspartate receptors (NMDARs), as has been previously been documented in neurons (Fig. S1B) (Dalva et al., 2000). Finally, we produced collagen gels infused with clustered EB2/Fc and incubated them in culture conditions, replacing the liquid every 12 hours. Western analysis of these gel eluates demonstrated that the protein remained undegraded in culture over at least five days (Fig. S1C). The protein used in our experiments was replaced every 24 hours over a period of three days. Therefore the lack of ephrin effect on fusion was not due to inactive or degraded reagent.
We observed far more dramatic results when we treated palates with clustered EphB2/Fc recombinant protein to activate reverse signaling through endogenous ephrin-Bs. Clustered EphB2/Fc gave an MFS of 3.4±0.24, essentially equivalent to TGFβ3 treatment. When applied without clustering, Eph and ephrin Fc proteins serve as effective blocking reagents because they bind to their target ligands and receptors without activating biological signaling, thus acting as competitive inhibitors (Benson et al., 2005). We employed this strategy by adding unclustered EphA4/Fc to palate cultures. EphA4 binds to all A and B ephrins and so serves as a good pan-ephrin blocker (Pasquale, 2004). This treatment effectively blocked palate shelf fusion, even in the presence of TGFβ3 (MFS=2.1±0.13; Figure 2). Thus, these data indicate that ephrin reverse signaling is required for palate fusion and is downstream of TGFβ3.
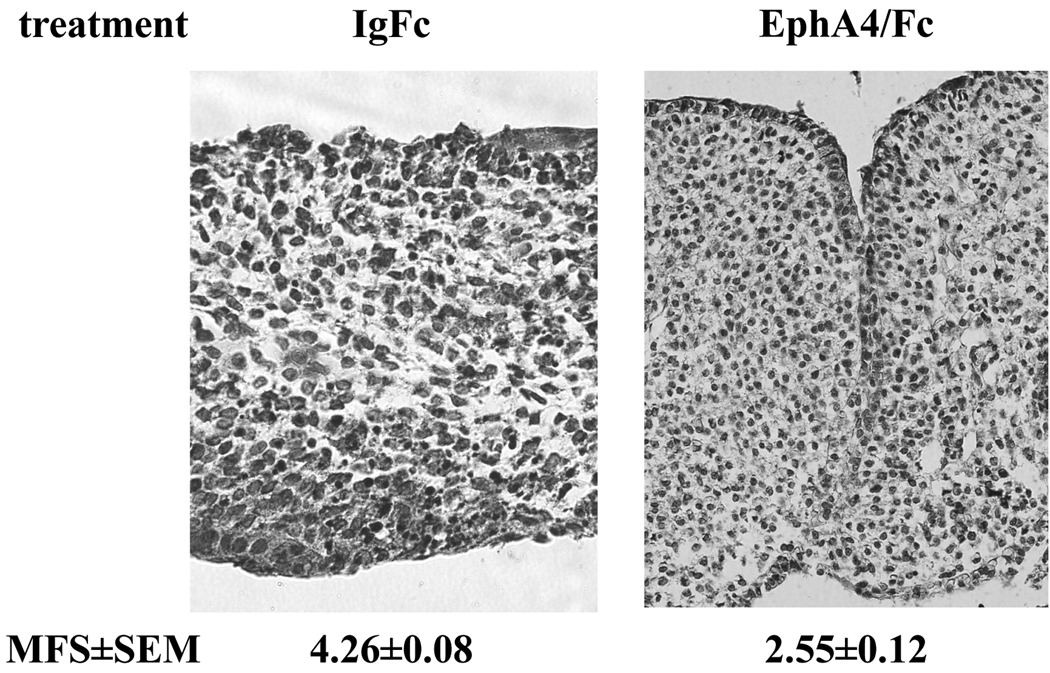
If this role of ephrins in fusion is conserved in mammals, then we would expect that inhibiting ephrin action in mouse palate would prevent the naturally occurring fusion normally observed in culture. To test this prediction, we applied unclustered EphA4/Fc to e14.5 mouse palate culture, and observed the same effect as in the chicken: an inhibition of palate fusion (MFS=2.55±0.12 vs. 4.26±0.08 in the control group) (Figure 3). Interestingly, we observed in the mouse that fused areas were interspersed with non-fused areas along the length of touching epithelium. Nevertheless, the data clearly demonstrate that the requirement for ephrin signaling is conserved between chicken and mouse.
Figure 3. Ephrin dependence of mouse palate fusion.
Embryonic day 14.5 mouse palates were cultured in the presence of unclustered EphA4/Fc soluble recombinant protein or IgG Fc control protein as described in the text. Tissues were fixed, paraffin processed, and sectioned. Shown are representative H&E stained sections from each treatment. H&E stained sections from anterior to posterior were scored on for fusion on a scale of 1 to 5 and these scores averaged to yield the mean fusion score (MFS) shown. Values are ±SEM for n=14 palates over four independent experiments. Magnification is 200×.
Ephrin-dependent fusion requires PI3 kinase
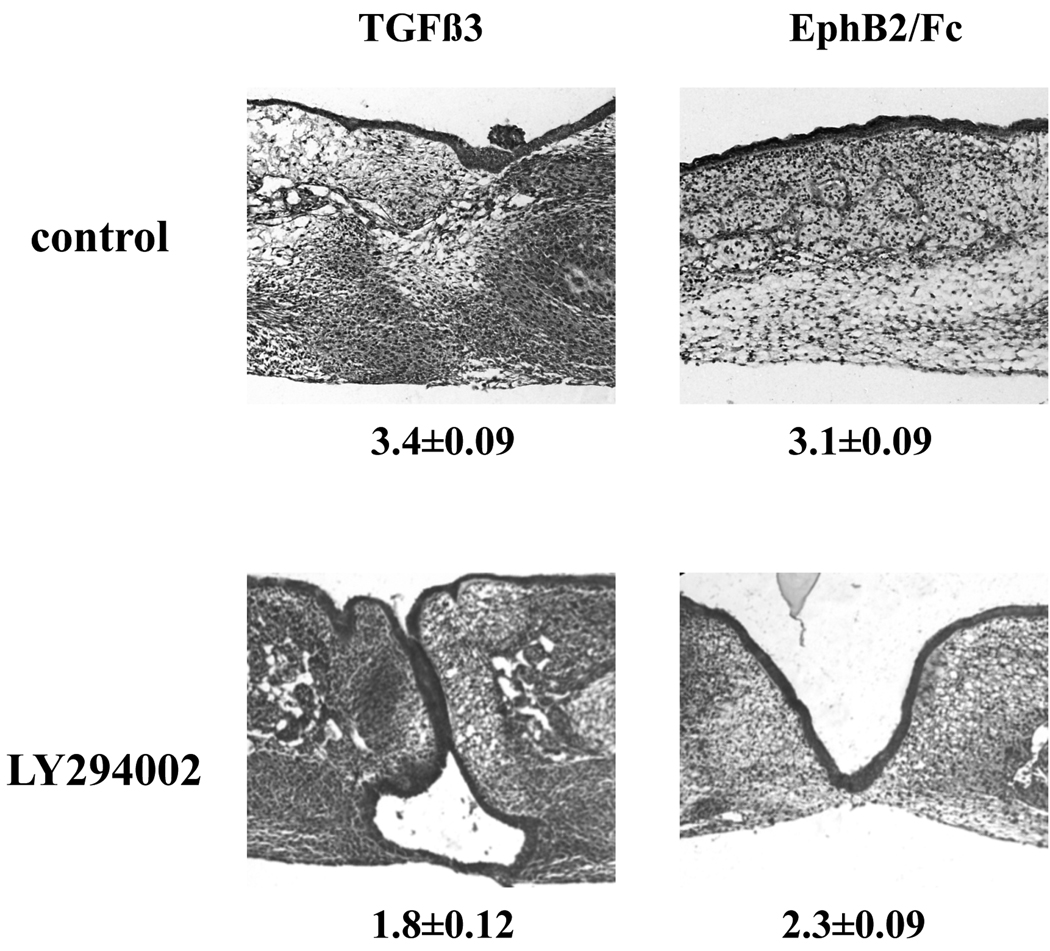
Our culture evidence indicated that ephrin signaling in palate fusion acts downstream of the receptor for TGFβ3. As noted above, previous work showed that TGFβ3-induction of fusion requires active PI3K, as PI3K inhibition abrogates or delays fusion. This would appear to place a required TGFβ3 effector downstream of ephrin signaling. We therefore tested whether ephrin reverse signaling also acts through PI3K. Chicken palates cultured in the presence of the PI3K inhibitor LY and either TGFβ3 or clustered Eph-B2 failed to fuse over the test period (Figure 4). Thus, we conclude that ephrin reverse signaling acts through the PI3K pathway to cause fusion.
Figure 4. Effect of PI3K inhibition on Eph-induced palate fusion.
Chicken palates were grown with the treatments indicated under the conditions described in the text. Samples grown in TGFβ3 or EphB2 alone fused almost completely. Addition of the PI3K inhibitor LY294002 abrogated fusion with either TGFβ3 or clustered EphB2/Fc. Shown are H&E stained examples of each group with n=16 to 19 for each group from 3 independent experiments. Mean fusion score (MFS) for each is shown ± SEM. Magnification is 100×.
Discussion
Collectively, our study shows that ephrin reverse signaling is both required and sufficient for chicken palate fusion, and that PI3K is part of this signaling mechanism. As the stimulating and blocking reagents used here are not specific to a single Eph or ephrin member, we are not able to identify the individual ephrin that mediates fusion, or even whether it is a single or multiple ephrins. We do know, from our examination of ephrin-B3/LacZ mice, that ephrin-B3 is not present in the palate at this stage, and so is not a candidate participant (data not shown). We do not know from our own experiments the expression of the remaining B ephrin, ephrin-B1, in the fusing palate. Our expression data for EB2 and EphB2 conflict with those of Risley et al. which reported all three B ephrins and EphB2 in both epithelium and mesenchyme at e14.5 (Risley et al., 2009). We are unable to explain this discrepancy except to say that we examined protein expression (through X-gal stain) while Risley et al. examined mRNA levels, and perhaps the low levels of mRNAs reported in their study do not translate to enough protein production to display detectable β-gal activity in all cases. We cannot formerly rule out A class ephrins from involvement in the reverse signaling events we observed. Although ephrin-Bs are more likely candidates for receptors of the EphB2 used in our experiments (Kullander and Klein, 2002; Murai and Pasquale, 2003), EphB2 is activated by at least one ephrin-A ligand (Himanen et al., 2004). Our initial expression evidence does suggest EB2 as the most likely candidate due to its presence in the midline epithelium at the time of palate contact. Indeed, as mentioned above, the observed migration of EB2/LacZ expressing cells into the mesenchyme during fusion parallels that seen in MEE cells labeled with vital dyes (Kang and Svoboda, 2005), and defines EB2 as a marker of these cells.
Another important question is how the signaling pathways of TGFβRs and ephrins intersect within cells of the fusing palate. Up to now, study of signal transduction in palate fusion has focused on events downstream of TGFβ3, as this was thought the chief requisite growth factor for EMT and fusion (Nawshad, 2008; Yu et al., 2009). Our chicken palate findings show that ephrin signaling is downstream, or at least independent of, TGFβR signaling in that we were able to dispense with the TGFβ3 treatment. We now know that the PI3K mechanism is a point of intersection, but the up- and downstream details of the interaction are unknown (Fig. 4). The action of the transcription factors Twist1 and Snail were shown by Yu et al. to mediate at least a substantial part of TGFβ3’s fusion-promoting activity, as RNAi against these genes’ mRNAs partially inhibited fusion (Yu et al., 2008). It will remain to be seen whether ephrin reverse signals impact these factors as well.
Our EphA4/Fc blocking experiments demonstrated that the ephrin requirement is conserved between mouse and chick. However, previous data suggests that ephrins may not be sufficient in the mouse. Blocking antibodies against TGFβ3 inhibit mouse palate fusion, and genetic ablation of TGFβ3 in mice yields cleft secondary palate (Brunet et al., 1995; Proetzel et al., 1995; Taya et al., 1999; Ahmed et al., 2007). This suggests that endogenous ephrin signals are not sufficient to compensate for loss of TGFβ3. Thus, there may be a difference in the hierarchical roles of TGFβ3 versus ephrins between the two species. Alternatively, ephrin expression in the palate epithelium may be under the control of TGFβ3 such that loss of the TGFβ3 signal eliminates the endogenous ephrin signal as well.
The findings in this study have broader implications for ephrin biology beyond the example of palate fusion. Ephs are known to mediate EMT in other processes, such as cancer metastasis, and although PI3K signaling was implicated in Eph/ephrin systems, it was in the forward direction, downstream of the Eph RTKs (Genander et al., 2009). There is a report of PI3K being required for in vitro ephrin induced proliferation in an endothelial cell line via reverse signaling (Steinle et al., 2003), but ours is the first evidence of reverse signaling mediating a biologically critical developmental EMT event via PI3K. Although PI3K is associated with cell motility, and ephrin reverse signaling is associated with cell migration, this pathway has not been connected with the known signaling elements of the ephrin-B cytoplasmic domain.
The cytoplasmic domains of B class ephrins contain conserved tyrosines that are phosphorylated upon stimulation to form SH2-binding domains that bind the adaptor Grb4 (Cowan and Henkemeyer, 2001), although it is possible they may bind other SH2 domain proteins in different contexts. Grb4 complexes with other factors such as the GTPase activating protein 1 (GIT1) and Glutamate receptor interacting protein 1 (GRIP1) (Aoto et al., 2007; Segura et al., 2007). B ephrins also have a C-terminal PDZ-binding domain, which is required for a number of biological functions (Mäkinen et al., 2005; Bush and Soriano, 2009). Binding of SH2 proteins in the PI3K pathway to EB2 is certainly plausible, but not yet demonstrated. A-class ephrins, on the other hand, do not have cytoplasmic domains. Still, they can participate in reverse signaling via a co-receptor (Lim et al., 2008; Naska et al., 2010). It is also possible that the reverse signal does not directly activate PI3K, but promotes is action indirectly. Our finding thus encourages increased investigation along a new direction of ephrin signaling.
Our ongoing studies will focus on identification and regulation of the specific Eph and ephrin molecules involved in palate fusion, the signal transduction pathways downstream of those ephrins, and their intersection with TGFβR-mediated pathways.
Experimental Procedures
Chemicals and reagents
TGFβ3 was obtained from Invitrogen (Carlsbad, CA). LY294002 PI3K inhibitor was from Cell Signaling Technology (Danvers, MA). EphB2 ectodomain Fc fusion protein and goat anti-EphB2 antibody were from R&D Systems (Minneapolis, MN). IgG Fc protein was from Calbiochem (EMD Chemicals, Gibbstown, NJ). Mouse anti-phosphotyrosine was from Cell Signaling Technology (Danvers, MA). Mouse anti-NMDAR1 was from BD Pharmingen. Cy2 and Cy3 conjugated secondary antibodies for immunofluorescence were from Jackson Immunoresearch (West Grove, PA). EB2 ectodomain fused to human Fc was produced by cloning a PCR product encompassing the extracellular portion of the coding sequence for murine EB2 into pFUSE-hIgG1e3-Fc2 (InvivoGen, San Diego, CA). The resulting plasmid was transfected into CHO cells (ATCC). Fusion protein was collected from conditioned supernatant by protein A chromatography and analyzed by Western blot with anti-Fc antibody (Jackson Immunoresearch). Detailed cloning and purification protocols available upon request. Fc clustering was accomplished with the same anti-Fc antibody.
All other chemicals were from Sigma-Aldrich (St. Louis, MO).
Embryonic palate culture
Chicken palate culture was performed as previously described (Kang and Svoboda, 2002). Briefly, palatal shelves were dissected from eight day old chicken embryos and placed nasal side down on nucleopore polycarbonate membranes and cultured with in BGJb medium (Invitrogen, Carlsbad, CA) for 72 h in 37°C with 5% CO2. Medium was replaced every 24 h with fresh treatments. TGFβ3 was used at 50 ng/ml. EphB2, EB2, and control IgG Fc proteins were used at 5 ng/ml. EphA4/Fc was used at 20 ng/ml. IgG Fc was added at 20 ng/ml when used as control for EphA4/Fc. LY294002 was used at 10 µM. To cluster Fc proteins, protein was mixed with anti-Fc in a 4 to 1 w/w ratio as a 50x or 100x stock and incubated at 22C for 1 h or overnight at 4C (Knöll et al., 2007). Mouse palates were dissected from e13.5 day old CD-1 mice and cultured as for the chicken palates, except that TGFβ3 was not added (Yu et al., 2008).
COS-1 and spinal cord neuron culture
COS-1 monkey kidney cells (American Type Culture Collection) were propagated in DMEM/10% fetal bovine serum with penicillin/streptomycin. Adult mouse spinal cord neurons were purified in Hibernate-A medium (Brain Bits, LLC, Springfield, IL) and cultured in Neurobasal-A medium supplemented with B-27 (Invitrogen) as previously described (Benson et al., 2005). Both types of cells were plated on poly-DL-ornithine-coated glass coverslips and treated with clustered EB2/Fc for from six to 24 hours with qualitatively identical results. Cells were fixed in 4% formaldehyde and processed for immunofluorescence.
Histological analysis
Cultured palates were fixed in 4% formaldehyde/phosphate buffered saline for 2 days. They were then stabilized in low melting point agarose and processed for paraffin embedding. Serial 6 µm sections were collected in the coronal orientation from anterior to posterior. Sections were stained with hematoxylin and eosin (H&E) and photographed on a light microscope with a digital camera. Every twentieth section was scored for fusion by at least two independent, blinded observers using the previously described scale (Kang and Svoboda, 2002): 1=non-fusion with no adhesion, 2=non-fusion with some apparent adhesion, 3=adhesion with some disintegration of MEE layers and clear partial mesenchymal confluence, 4=complete fusion with some traces of MEE cells or seam remaining, 5=complete fusion with no evidence of MEE cells or seam visible. All experiments were repeated at least three times, with qualitatively identical results. Scores from identical experiments were pooled to give the MFS reported ± standard error of the mean (SEM).
EphB2/LZ (Henkemeyer et al., 1996) and EB2/LZ (Dravis et al., 2004) mouse embryos were sectioned in coronal orientation at 12 µm on a cryostat. Sections were incubated in X-gal overnight (2 mM MgCl2, 0.02% NP-40, 1 mg/ml X-gal in PBS) at 37C, then rinsed in PBS, counterstained with Nuclear Fast Red, dehydrated through alcohol and xylenes and coverslipped.
Supplementary Material
(A) COS-1 cells were treated in culture for 6h with 5ng/ml clustered EB2/Fc or control IgG Fc, then fixed and incubated with goat anti-EphB2 and mouse anti-phosphotyrosine (anti-PY), followed by fluorescent secondary antibodies. Images were collected by confocal microscopy. EphB2 is shown in red, PY in green. Note the co-localization of EphB2 and PY clusters in the EB2-treated cells (white arrows, color-merged image) that does not appear in the IgG Fc-treated cells denoting activated EphB2 kinase. (B) Spinal cord neurons from adult mouse were cultured and treated with EB2/Fc or IgG Fc for 12h, fixed, and incubated with goat anti-EphB2 and mouse anti-NMDAR1, followed by fluorescent secondary antibodies. Red is EphB2, green is NMDAR1 (NR1). Note the co-localization of EphB2 and NR1 clusters in the EB2-treated cells (white arrows, color-merged image) that does not appear in the IgG Fc-treated cells indicating that activated EphB2 is associating with glutamate receptors. (C) Collagen gels were prepared in 96-well plates, infused with EB2/Fc. The gels were maintained under liquid in culture conditions for up to 144h. The liquid was collected and replaced every 12h. Equal volumes of each fraction were evaluated by SDS-PAGE, followed by Western blot and immunodetection with anti-Fc. The +C lane is fresh, unincubated protein from the same lot. Note the lack of degradation of protein even after 5 days in culture.
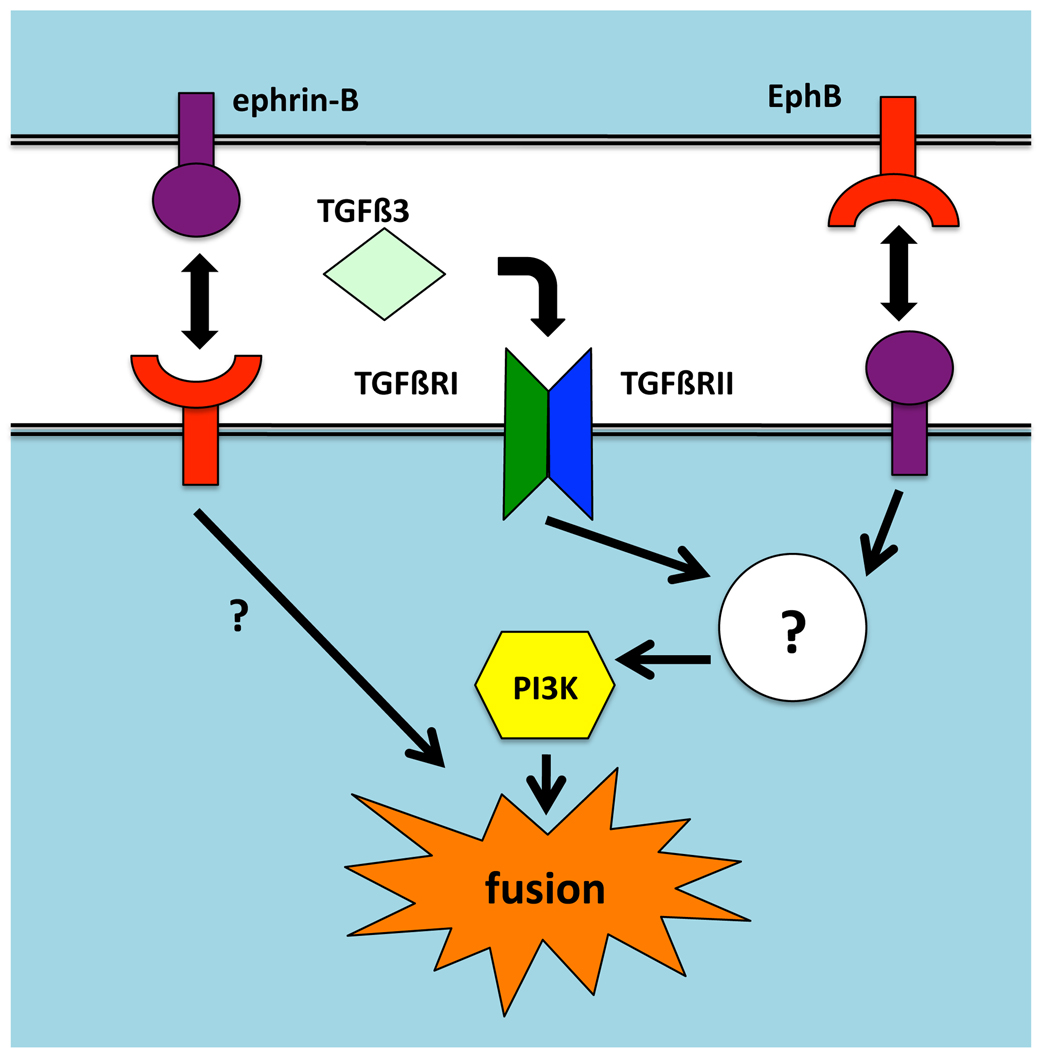
Figure 5. Model of ephrin and TGFβ3 signal transduction in palate fusion.
Ephrin and TGFβR signals intersect at a point upstream of PI3K, which is required for fusion. Other possible pathways from eprhin-Bs that do not go through PI3K are not diagrammed. Known possible effectors or ephrin-Bs in reverse signaling are described in the text. Signals from Eph RTKs that induce partial fusion are unknown.
Acknowledgements
The authors thank Dr. Dan Dimitrijevich at the University of North Texas Health Science Center for production of collagen gels, and Ms. Jan Westerlund for technical assistance. This work was funded by a grant from the March of Dimes (FY06-321) to K.K.H.S, a fellowship from T32 DE-018380 to M.S., and support from U24 DE16472 to M.D.B.
References
- Ahmed S, Liu C-C, Nawshad A. Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3. Dev Biol. 2007;309:193–207. doi: 10.1016/j.ydbio.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L. Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J Neurosci. 2007;27:7508–7519. doi: 10.1523/JNEUROSCI.0705-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet CL, Sharpe PM, Ferguson MW. Inhibition of TGF-beta 3 (but not TGF-beta 1 or TGF-beta 2) activity prevents normal mouse embryonic palate fusion. Int J Dev Biol. 1995;39:345–355. [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 regulates axon guidance by reverse signaling through a PDZ-dependent mechanism. Genes Dev. 2009;23:1586–1599. doi: 10.1101/gad.1807209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes Dev. 2010;24:2068–2080. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Wasserman CR, Tolarová MM. Racial and ethnic variations in the prevalence of orofacial clefts in California, 1983–1992. Am J Med Genet. 1998;79:42–47. doi: 10.1002/(sici)1096-8628(19980827)79:1<42::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu N-J, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, Chumley MJ, Zdunek S, Wang C, Holm T, Goff SP, Pettersson S, Pestell RG, Henkemeyer M, Frisén J. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Himanen J-P, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Genander M, Halford MM, Annerén C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisén J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Kang P, Svoboda KKH. PI-3 kinase activity is required for epithelial-mesenchymal transformation during palate fusion. Dev Dyn. 2002;225:316–321. doi: 10.1002/dvdy.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Svoboda KKH. Epithelial-mesenchymal transformation during craniofacial development. J Dent Res. 2005;84:678–690. doi: 10.1177/154405910508400801. [DOI] [PubMed] [Google Scholar]
- Knöll B, Weinl C, Nordheim A, Bonhoeffer F. Stripe assay to examine axonal guidance and cell migration. Nat Protoc. 2007;2:1216–1224. doi: 10.1038/nprot.2007.157. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lim Y-S, McLaughlin T, Sung T-C, Santiago A, Lee K-F, O'Leary DDM. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Murray JC, Schutte BC. Cleft palate: players, pathways, and pursuits. J Clin Invest. 2004;113:1676–1678. doi: 10.1172/JCI22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naska S, Lin DC, Miller FD, Kaplan DR. p75NTR is an obligate signaling receptor required for cues that cause sympathetic neuron growth cone collapse. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Nawshad A. Palatal seam disintegration: to die or not to die? that is no longer the question. Dev Dyn. 2008;237:2643–2656. doi: 10.1002/dvdy.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli D, Klein R. The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 1997;13:354–359. doi: 10.1016/s0168-9525(97)01220-1. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin promiscuity is now crystal clear. Nat Neurosci. 2004;7:417–418. doi: 10.1038/nn0504-417. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley M, Garrod D, Henkemeyer M, McLean W. EphB2 and EphB3 forward signalling are required for palate development. Mech Dev. 2009;126:230–239. doi: 10.1016/j.mod.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Meininger CJ, Chowdhury U, Wu G, Granger HJ. Role of ephrin B2 in human retinal endothelial cell proliferation and migration. Cell. Signal. 2003;15:1011–1017. doi: 10.1016/s0898-6568(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Sun D, Vanderburg CR, Odierna GS, Hay ED. TGFbeta3 promotes transformation of chicken palate medial edge epithelium to mesenchyme in vitro. Development. 1998;125:95–105. doi: 10.1242/dev.125.1.95. [DOI] [PubMed] [Google Scholar]
- Taya Y, O'Kane S, Ferguson MW. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Torii C, Izumi K, Nakajima H, Takahashi T, Kosaki K. EFNB1 mutation at the ephrin ligand-receptor dimerization interface in a patient with craniofrontonasal syndrome. Congenit Anom (Kyoto) 2007;47:49–52. doi: 10.1111/j.1741-4520.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Twigg SRF, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, Morriss-Kay GM, Wilkie AOM. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci USA. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I, Reardon W, Jakubiczka S, Franco B, Kress W, Vincent-Delorme C, Thierry P, Edwards M, König R, Rusu C, Schweiger S, Thompson E, Tinschert S, Stewart F, Wieacker P. Twenty-six novel EFNB1 mutations in familial and sporadic craniofrontonasal syndrome (CFNS) Hum Mutat. 2005;26:113–118. doi: 10.1002/humu.20193. [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Deng C, Chai Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell. 2008;15:322–329. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Kamara H, Svoboda KKH. The role of twist during palate development. Dev Dyn. 2008;237:2716–2725. doi: 10.1002/dvdy.21627. [DOI] [PubMed] [Google Scholar]
- Yu W, Ruest L-B, Svoboda KKH. Regulation of epithelial-mesenchymal transition in palatal fusion. Exp Biol Med (Maywood) 2009;234:483–491. doi: 10.3181/0812-MR-365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) COS-1 cells were treated in culture for 6h with 5ng/ml clustered EB2/Fc or control IgG Fc, then fixed and incubated with goat anti-EphB2 and mouse anti-phosphotyrosine (anti-PY), followed by fluorescent secondary antibodies. Images were collected by confocal microscopy. EphB2 is shown in red, PY in green. Note the co-localization of EphB2 and PY clusters in the EB2-treated cells (white arrows, color-merged image) that does not appear in the IgG Fc-treated cells denoting activated EphB2 kinase. (B) Spinal cord neurons from adult mouse were cultured and treated with EB2/Fc or IgG Fc for 12h, fixed, and incubated with goat anti-EphB2 and mouse anti-NMDAR1, followed by fluorescent secondary antibodies. Red is EphB2, green is NMDAR1 (NR1). Note the co-localization of EphB2 and NR1 clusters in the EB2-treated cells (white arrows, color-merged image) that does not appear in the IgG Fc-treated cells indicating that activated EphB2 is associating with glutamate receptors. (C) Collagen gels were prepared in 96-well plates, infused with EB2/Fc. The gels were maintained under liquid in culture conditions for up to 144h. The liquid was collected and replaced every 12h. Equal volumes of each fraction were evaluated by SDS-PAGE, followed by Western blot and immunodetection with anti-Fc. The +C lane is fresh, unincubated protein from the same lot. Note the lack of degradation of protein even after 5 days in culture.