Abstract
Introduction
Since their initial discovery in 1989, grapefruit juice-drug interactions have received extensive interest from the scientific, medical, regulatory, and lay communities. Although knowledge regarding the effects of grapefruit juice on drug disposition continues to expand, the list of drugs studied in the clinical setting remains relatively limited.
Areas covered
This article reviews the in vitro effects of grapefruit juice and its constituents on the activity of cytochrome P450 enzymes, organic anion-transporting polypeptides, P-glycoprotein, esterases and sulfotransferases. The translational applicability of the in vitro findings to the clinical setting is discussed for each drug metabolizing enzyme and transporter. Reported area under the plasma concentration-time curve ratios for available grapefruit juice-drug interaction studies are also provided. Relevant investigations were identified by searching the Pubmed electronic database from 1989 to 2010.
Expert opinion
Grapefruit juice increases the bioavailability of some orally-administered drugs that are metabolized by CYP3A and normally undergo extensive presystemic extraction. In addition, grapefruit juice can decrease the oral absorption of a few drugs that rely on organic anion-transporting polypeptides in the gastrointestinal tract for their uptake. The number of drugs shown to interact with grapefruit juice in vitro is far greater than the number of clinically relevant grapefruit juice-drug interactions. For the majority of patients, complete avoidance of grapefruit juice is unwarranted.
Keywords: CYP3A, drug-fruit juice interactions, flavonoids, furanocoumarins, grapefruit OATP, P-glycoprotein
1. Introduction
The first grapefruit juice (GFJ)-drug interaction was serendipitously identified by Bailey and colleagues in 1989 [1]. As a result of extensive work conducted since this initial discovery, the scientific literature is now replete with information regarding the effects of GFJ and its constituents on drug disposition, both in vitro and in vivo [2-6]. Pharmacokinetic studies have demonstrated that GFJ can increase the bioavailability of drugs from an array of therapeutic classes. Examples include some calcium channel blockers, benzodiazepines, and statins – Table 1. The elevation in a drug's area under the plasma concentration-time curve (AUC) with GFJ ingestion is the result of irreversible inhibition of cytochrome P450 (CYP) 3A by furanocoumarins present in the juice [7, 8]. Unlike other known CYP3A inhibitors, normal consumption of GFJ only inhibits CYP3A in the enterocyte cells lining the small intestine – hepatic CYP3A activity remains unaffected, except with unrealistically large ingestion of GFJ.
Table 1.
In vivo effects of grapefruit juice on drug exposure.
| Central Nervous System Drugs | ||
|---|---|---|
| Benzodiazepines | ||
| Drug | AUC Ratio | Reference |
| Alprazolam | 1.12* | [70] |
| Diazepam | 3.24 | [71] |
| Midazolam | 1.53 | [68] |
| 1.65 | [29] | |
| 1.52 | [72] | |
| 2.31 | [73] | |
| 2.00 2.15* 5.95* |
[74] | |
| 2.39 | [75] | |
| 1.49* | [76] | |
| 1.73 | [77] | |
| 1.36 – mean concentration at 2 hour ratio | [78] | |
| Quazepam | 1.31* | [79] |
| Triazolam | 1.48 | [80] |
| 1.53 1.49* 2.43* |
[81] | |
| 1.73* | [79] | |
| 1.51 – acute GFJ exposure 1.60 – extended GFJ exposure |
[40] | |
| Antiepileptics | ||
| Drug | AUC Ratio | Reference |
| Carbamazepine | 1.41 | [82] |
| Phenytoin | 0.89 – male volunteers 0.95 – epileptic patients |
[83] |
| Other Central Nervous System Drugs | ||
| Drug | AUC Ratio | Reference |
| Buspirone | 9.21* | [84] |
| Clozapine | 1.01 – mean plasma concentration ratio | [85] |
| 1.15* – mean trough level ratio | [86] | |
| 0.92 | [87] | |
| Fluvoxamine | 1.60* | [88] |
| Haloperidol | 0.99* – mean trough level ratio | [89] |
| Sertraline | 1.47 – mean trough level ratio | [90] |
| 2.04* | [91] | |
| Opioids | ||
| Drug | AUC Ratio | Reference |
| Alfentanil | 1.62 | [77] |
| Fentanyl | 0.99 | [92] |
| Methadone | 1.19 | [93] |
| 1.17 | [94] | |
| Oxycodone | 1.62* | [95] |
| Antiinfectives | ||
| Antibacterials | ||
| Drug | AUC Ratio | Reference |
| Clarithromycin | 1.15* | [96] |
| Erythromycin | 1.49 | [97] |
| Telithromycin | 1.05 | [98] |
| Antimalarials | ||
| Drug | AUC Ratio | Reference |
| Artemether | 1.90* | [99] |
| 2.44* – acute GFJ exposure 3.51*–extended GFJ exposure |
[100] | |
| Halofantrine | 2.21 | [101] |
| Quinine | 0.96* 0.77 |
[102] |
| Primaquine | 1.19 | [103] |
| Antihelmintics | ||
| Drug | AUC Ratio | Reference |
| Albendazole sulfoxide | 3.13* | [104] |
| Praziquantel | 1.84 | [105] |
| Antiretrovirals | ||
| Drug | AUC Ratio | Reference |
| Amprenavir | 0.90 | [106] |
| Indinavir | 0.98* | [107] |
| 0.94 | [108] | |
| Saquinavir | 1.50 | [109] |
| Antifungals | ||
| Drug | AUC Ratio | Reference |
| Itraconazole | 1.03 | [110] |
| 0.57* | [111] | |
| 1.20* | [112] | |
| 1.30* – female subjects 1.11* – male subjects |
[113] | |
| Cardiovascular Agents | ||
| 6-blockers | ||
| Drug | AUC Ratio | Reference |
| Acebutolol | 0.94* | [114] |
| Celiprolol | 0.15* | [115] |
| Talinolol | 0.70 – acute GFJ exposure 0.73* – extended GFJ exposure |
[59] |
| Calcium channel blockers | ||
| Drug | AUC Ratio | Reference |
| Amlodipine | 1.14 | [116] |
| 1.08 | [117] | |
| Azelnidipine | 3.28 | [118] |
| Diltiazem | 1.10* | [119] |
| 1.18 | [120] | |
| Felodipine | 2.51* | [121] |
| 1.86 | [14] | |
| 1.43 | [42] | |
| 1.72 | [122] | |
| 2.16* – acute GFJ exposure 3.11* – extended GFJ expsoure |
[37] | |
| 1.73 – acute GFJ exposure 1.57 – extended GFJ exposure |
[123] | |
| 2.45 | [124] | |
| 2.16 | [125] | |
| 2.88 – single dose study 2.86 – steady-state study |
[126] | |
| 1.94 | [127] | |
| 1.81 | [35] | |
| 2.30 – median AUC ratio | [128] | |
| 2.04 – median AUC ratio | [7] | |
| 2.01 | [129] | |
| 1.35 | [36] | |
| 1.93 | [130] | |
| 2.85 3.34* |
[131] | |
| Manidipine | 2.62 | [132] |
| Nicardipine | 1.56 | [133] |
| Nifedipine | 1.47* | [15] |
| 2.02* | [134] | |
| 1.58* | [135] | |
| 1.10 | [136] | |
| 1.35* | [121] | |
| Nimodipine | 1.51 | [137] |
| Nisoldipine | 1.76 | [13] |
| 4.11 | [41] | |
| Nitrendipine | 2.25* | [138] |
| Pranidipine | 1.68 | [139] |
| Verapamil | 1.09 | [140] |
| 1.29* | [141] | |
| 1.43* | [142] | |
| Antiarrhythmics | ||
| Drug | AUC Ratio | Reference |
| Amiodarone | 1.50* | [143] |
| Quinidine | 1.08 | [144] |
| 1.05* – median Cmax ratio | [145] | |
| HMG-CoA Reductase Inhibitors | ||
| Drug | AUC Ratio | Reference |
| Atorvastatin | 1.83* | [146] |
| 1.33* | [147] | |
| 2.46* | [148] | |
| Lovastatin | 15.3* | [149] |
| 1.91 | [75] | |
| Pitavastatin | 1.13* | [146] |
| Pravastatin | 1.00* | [147] |
| 0.92* | [148] | |
| Simvastatin | 16.1* | [150] |
| 13.5* | [43] | |
| 3.56 | [151] | |
| Other Cardiovascular Agents | ||
| Drug | AUC Ratio | Reference |
| Aliskiren | 0.39* | [152] |
| Digoxin | 1.03* | [62] |
| 1.09* | [61] | |
| Losartan | 1.17 | [153] |
| Warfarin | No difference in INR values* | [154] |
| Proton pump inhibitors | ||
| Drug | AUC Ratio | Reference |
| Lansoprazole | 1.21 | [155] |
| 1.15 | [156] | |
| Omeprazole | 1.11 | [157] |
| Immunosuppressants | ||
| Drug | AUC Ratio | Reference |
| Cyclosporine | 1.23 | [158] |
| 1.37* | [159] | |
| 1.60* – African-American subjects 1.44* – Caucasian subjects |
[160] | |
| 1.60* | [161] | |
| 1.85* | [34] | |
| 1.38 – median AUC ratio | [8] | |
| 1.55* | [39] | |
| 1.42* | [162] | |
| 1.24 | [163] | |
| 1.08* | [164] | |
| 1.43 | [165] | |
| 1.41* – oral solution 1.38* – microemulsion capsules |
[166] | |
| 1.43 | [167] | |
| 1.24 – mean trough level ratio | [168] | |
| Increased AUC reported* | [169] | |
| Tacrolimus | 2.10* – mean trough level ratio | [170] |
| Insulin secretagogues | ||
| Drug | AUC Ratio | Reference |
| Glyburide (glibenclamide) | 1.05* | [49] |
| Repaglinide | 1.13 | [171] |
| Antineoplastics | ||
| Drug | AUC Ratio | Reference |
| Etoposide | 0.76 | [172] |
| Nilotinib | 1.18* | [173] |
| Sunitinib | 1.11* | [76] |
| Steroids and Hormones | ||
| Drug | AUC Ratio | Reference |
| Budesonide | 1.70* – immediate release capsules 2.29* – extended release capsules |
[174] |
| 17β-estradiol | 1.16* | [175] |
| Ethinylestradiol | 1.28* | [176] |
| Levothyroxine | 0.91* | [177] |
| Methylprednisolone | 1.72* | [178] |
| Prednisone | 1.50* | [164] |
| Antihistamines | ||
| Drug | AUC Ratio | Reference |
| Desloratadine | 1.07* | [179] |
| Fexofenadine | 0.67* | [179] |
| 0.64 0.39* |
[180] | |
| 0.37* | [46] | |
| 0.48 | [38] | |
| 0.58 0.59 |
[10] | |
| Terfenadine | 2.88 | [181] |
| 1.28* – active metabolite (fexofenadine) ratio | [182] | |
| 1.54* – active metabolite (fexofenadine) ratio | [183] | |
| 1.53 – active metabolite (fexofenadine) ratio 1.57* – active metabolite (fexofenadine) ratio |
[184] | |
| Other Drugs | ||
| Drug | AUC Ratio | Reference |
| Caffeine | 1.33* | [185] |
| 1.04 | [186] | |
| Chlorzoxazone | No change in 2 hour 6-OH-chlorzoxazone/chlorzoxazone ratio | [187] |
| Cisapride | 1.37 | [188] |
| 2.44* | [189] | |
| 1.49 | [190] | |
| 2.55* | [191] | |
| Dextromethorphan | Plasma levels not measured | [192] |
| Plasma levels not measured | [193] | |
| Montelukast | 0.93* | [194] |
| Scopolamine | 1.35* | [195] |
| Sildenafil | 1.23 | [196] |
| Theophylline | 1.02* | [197] |
AUC: area under the plasma concentration-time curve; AUC ratio: AUC with GFJ ingestion divided by AUC with control beverage ingestion; Cmax: maximum observed plasma concentration.
AUC ratio computed using mean values unless otherwise specified.
Indicates administration of GFJ in a manner deemed to be inconsistent with usual dietary consumption – for example, use of double-strength juice or excessive volumes of juice.
Interested readers are encouraged to review the original research reports for specific study details.
Grapefruit juice can also decrease the bioavailability of some drugs (e.g., fexofenadine) [9]. The putative mechanism underlying such interactions is a reduction in drug uptake transport via inhibition of organic anion transporting polypeptides (OATPs) by GFJ flavonoids [10]. Other in vitro studies have suggested that GFJ is capable of inhibiting P-glycoprotein (P-gp), esterases, and sulfotransferases (SULT). However, a lack of clinical evidence makes it difficult to assess the actual impact, if any, GFJ has on these enzymes and transporter. The need for controlled clinical studies is especially important because an in vitro-in vivo disconnect has been noted for many natural products and beverages [3, 5].
This article provides a thorough review of the published data regarding GFJ-drug interactions, with an emphasis on the clinical significance of in vitro findings. Since the interaction potential of GFJ will vary based upon the specific product tested, a discussion of GFJ chemistry has been included. To facilitate clinical decision making, data from published pharmacokinetic investigations have been collated and are presented in Table 1.
2. Grapefruit Juice Composition
Grapefruit juice is rich in a number of phytochemicals, including flavonoids and furanocoumarins. The most abundant flavonoid in the juice is naringin, with reported concentrations ranging from approximately 200 to 2000 μmol/L [11, 12]. Due to their abundance and in vitro inhibition of CYP3A, the flavonoids were originally presumed to be the GFJ constituents responsible for mediating drug interactions. However, administration of naringin capsules, in the same amounts found in GFJ, failed to alter the pharmacokinetics of the CYP3A substrates, felodipine and nisoldipine [13, 14]. A similar study involving another flavonoid, quercetin, also failed to demonstrate an effect on nifedipine disposition [15]. Based on these findings and more recent work, the flavonoids are no longer thought to play a major role in GFJ-drug interactions involving CYP3A substrates.
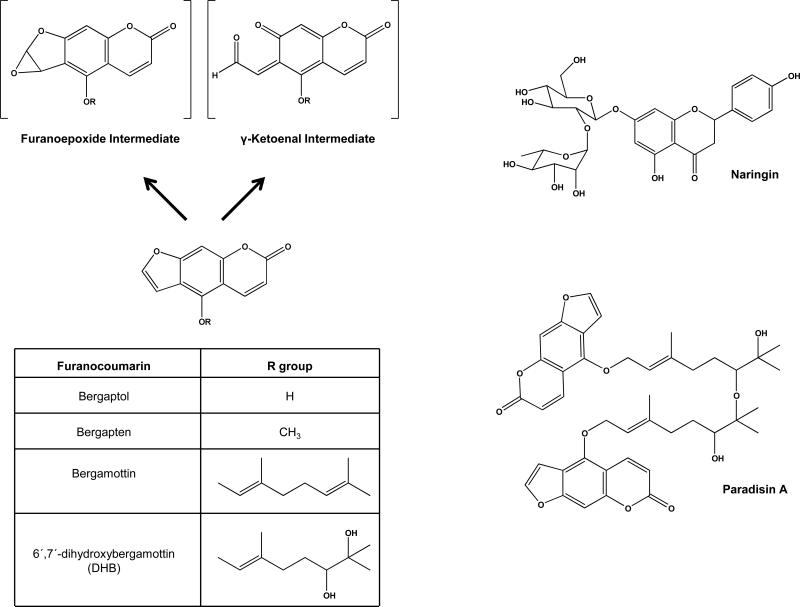
The furanocoumarins (FCs, Figure 1) are a structurally distinct class of compounds found in GFJ. All FCs contain a three-ring “head” and, except for bergaptol and bergapten, an aliphatic “tail.” The FCs differ in the presence and composition of their aliphatic tail, which in turn influences their inhibitory potency toward CYP3A. In addition, the furan ring moiety has an important role in the generation of a reactive intermediate – a furanoepoxide or γ-ketoenal – that irreversibly binds to CYP apoprotein and eliminates enzymatic activity [16].
Figure 1.
Structures of GFJ constituents implicated in clinical drug interactions. Left panel: The furanocoumarins are potent, mechanism-based, inhibitors of CYP3A. Mechanism-based inhibition of CYP3A is thought to result after binding of CYP protein to either a furanoepoxide or γ-ketoenal intermediate. Right panel: Naringin is the most abundant flavonoid in GFJ and inhibits OATP transport in vivo. Paradisin A is a representative member of a class of dimeric compounds present in GFJ formed by linkage of 6′,7′-dihydroxybergamottin to itself, or to bergamottin.
Of the various FCs present in GFJ, bergamottin and 6′,7′-dihydroxybergamottin (DHB) have been the most-extensively studied regarding their capacity to mediate GFJ-drug interactions (Figure 1). Reported concentration ranges for bergamottin and DHB in GFJ are 1-37 μM and 0.2-52.5 μM, respectively [11, 17, 18]. In addition, GFJ contains FC dimers – also known as spiroesters or paradisins – that are formed through either head-to-tail or tail-to-tail linkage of DHB to itself, or to bergamottin. Although present at lower concentrations than bergamottin and DHB, these dimers are potent inhibitors of CYP3A in vitro [18-21].
The variability in the concentrations of flavonoids and FCs in GFJ may result from factors such as (1) the type, origin, and quality of the grapefruits used to make the juice; (2) the manufacturing process; and, (3) storage conditions [11]. Exposure of GFJ to ultraviolet (UV) light or heat has been shown to alter the concentrations of FCs in the juice [22, 23]. The concentrations of bergamottin and DHB rapidly declined after being irradiated with UV light, whereas the bergaptol levels decreased more slowly. After 6 hours of UV exposure, the concentrations of bergaptol, DHB, and bergamottin were reduced to 6%, 2%, and 2% of their baseline values, respectively [22]. The same investigators also demonstrated that bergamottin and DHB are unstable at elevated temperatures. Although exposure to 4°C and 37°C for one hour failed to change the bergamottin and DHB concentrations in the juice, their levels declined when the juice was heated to 62°C, 72°C, and 95°C [23]. More specifically, 95°C exposure for one hour caused the bergamottin concentration to decrease from 17.9 μM to 3.14 μM, and the DHB concentration to fall from 7.85 μM to 0.16 μM. Bergaptol concentrations concurrently increased by 14.1 μM under the same treatment conditions, suggesting that bergamottin and DHB are degraded to bergaptol by exposure to heat. The UV- and heat-treated GFJ did not interact with nifedipine after administration to rats [22, 23].
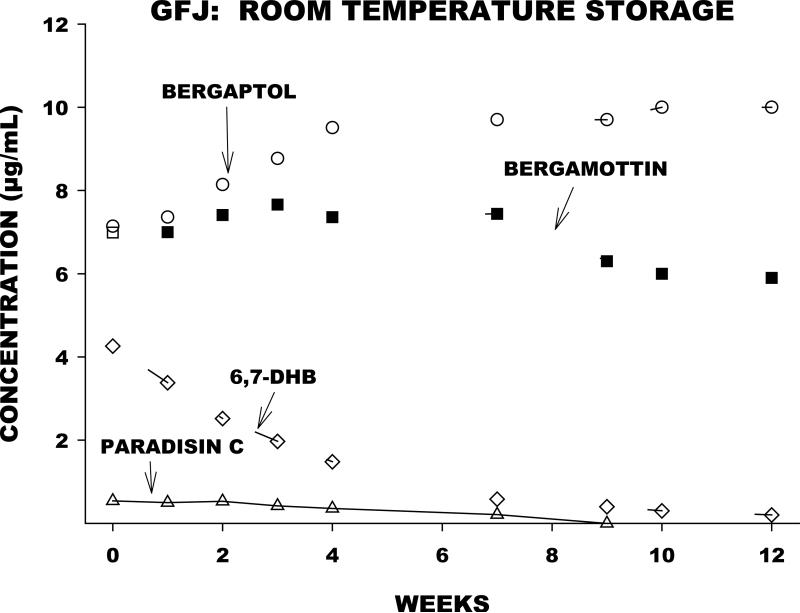
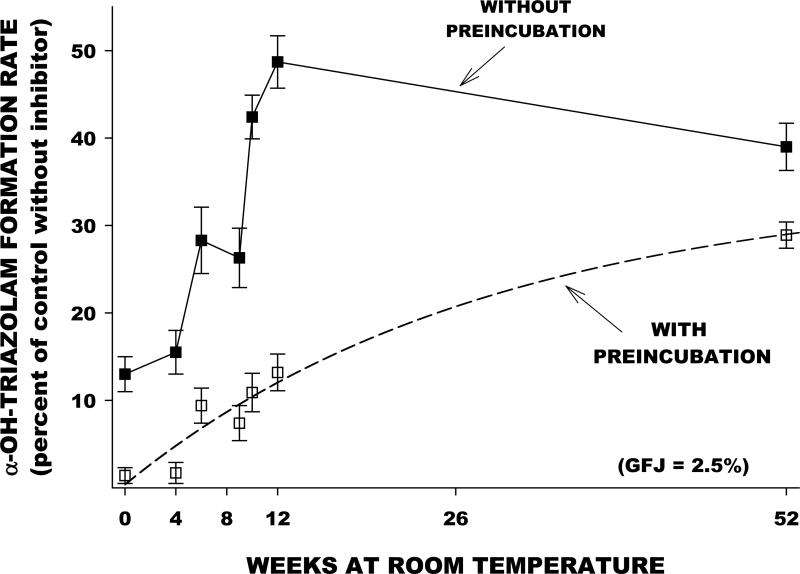
We evaluated the effect of prolonged storage at room temperature on the furanocoumarin content and the CYP3A-inhibiting capacity of a single GFJ sample. Aliquots were removed and analyzed at multiple time points over a one year period. Furanocoumarin concentrations were determined by liquid chromatography-mass spectroscopy, and in vitro inhibition of CYP3A in human liver microsomes was determined using the index substrate triazolam. Concentrations of DHB and paradisin C declined substantially with time (Figure 2). Bergamottin concentrations were relatively stable, while bergaptol concentrations increased. In the same samples, reversible and irreversible CYP3A inhibition declined with time in parallel with the decrement in DHB and paradisin C concentrations (Figure 3). The findings confirm the assumption that DHB and paradisins in GFJ are mainly responsible for CYP3A inhibition. The results also suggest that drug interactions with GFJ are less likely with GFJ that has undergone extensive storage at room temperature.
Figure 2.
Time-dependent changes in concentrations of bergaptol, bergamottin, DHB, and paradisin C in aliquots of a GFJ sample stored at room temperature for one year.
Figure 3.
In the same GFJ sample described in Figure 2, time-dependent changes in the capacity of the aliquots to inhibit CYP3A activity – represented as triazolam hydroxylation activity – by human liver microsomes in vitro. Lower values on the y-axis indicate greater inhibitory capacity. Each point is the mean ± SE from four different human liver samples. At all time points, the irreversible component of CYP3A inhibition (indicated by the values obtained following preincubation of GFJ with microsomes prior to addition of substrate) exceeds the reversible component. Note that the irreversible component of inhibition – thought to be responsible for clinical drug interactions – decreases substantially over the one year period. For a detailed description of methodology see references [29, 68, 69].
The complex chemistry of GFJ makes its use in drug-interaction studies challenging. The majority of investigations to date have not provided a phytochemical analysis of the juice, instead reporting that “the same lot of juice was used throughout the study.” This assures some degree of product quality control, but does not allow comparison of results from different studies. Ideally, future studies should report the chemical composition of the GFJ being administered, especially the bergamottin, DHB, and paradisin concentrations when CYP3A inhibition is anticipated. When the mechanism of interaction is presumed to be OATP inhibition, quantifying the amount of naringin in the juice appears warranted [10].
3. Interpretation of Grapefruit Juice-Drug Interaction Studies
Drug interaction studies with GFJ have usually employed a crossover design, in which the substrate drug of interest is given on one occasion under control conditions (e.g., with water), and on another occasion with GFJ. In both trials, the primary outcome is the drug's AUC. The mean AUC ratio – AUC with GFJ ingestion divided by AUC under control conditions – is then calculated from the individual study participant data. A mean AUC ratio that significantly differs from 1.0 indicates the potential for a GFJ-drug interaction. For AUC ratios greater than 1.0, the following ranges are generally utilized to describe the qualitative degree of inhibition: strong, AUC ratio ≥ 5.0; moderate, 2.0 ≤ AUC ratio < 5.0; weak, 1.25 ≤ AUC ratio < 2.0; and, negligible, AUC ratio < 1.25 [24].
Two critical points regarding GFJ-drug investigations are: (1) statistical significance does not necessarily imply clinical significance, and (2) an AUC ratio alone is insufficient to draw conclusions regarding the clinical relevance of an interaction. This judgment can only be made with additional information regarding the drug's exposure-response relationship. Generally speaking, a GFJ-drug interaction is likely to be of clinical importance if the substrate drug has a narrow therapeutic index, or when the drug's pharmacokinetics are dramatically altered by GFJ administration.
4. Effects of Grapefruit Juice on Drug Metabolizing Enzymes and Transporters
4.1 Cytochrome P450 Enzymes
The cytochrome P450 enzyme family is responsible for the phase I metabolism of the majority of drugs used in clinical practice. Of the various CYP isoforms, CYP3A (including CYP3A4 and CYP3A5) is the most important because it is responsible, either entirely or in part, for the metabolism of over 50% of the most commonly prescribed drugs [25]. CYP3A exhibits a broad substrate specificity and comprises the majority of CYP content in both enterocytes and hepatocytes. Since an orally-administered CYP3A substrate is sequentially exposed to enteric and then hepatic CYP3A, the amount of drug that reaches the systemic circulation can be markedly different than the administered dose – a process known as presystemic extraction or first-pass metabolism. In turn, inhibition of CYP3A at the enteric and/or hepatic level will increase the oral bioavailability of drugs that normally undergo extensive presystemic extraction.
4.1.1 In vitro Interactions with Grapefruit Juice
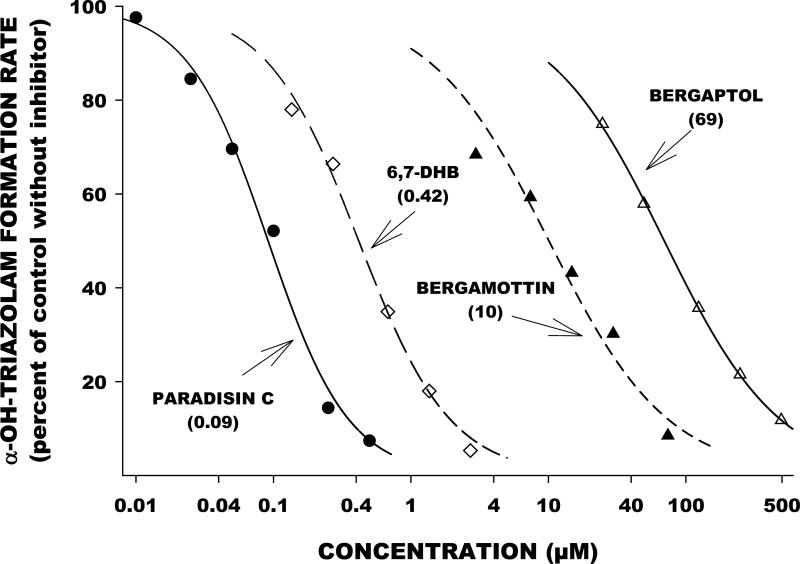
Many in vitro investigations have reported on the ability of the FCs to inhibit CYP activity, both reversibly and irreversibly, with inhibitory constants in the nanomolar to low micromolar range [16-18, 20, 21, 26-33]. Bergamottin inhibits multiple CYP isoforms, including CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. The paradisins and DHB inhibit the in vitro activity of CYP1A2, CYP1B1, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Some notable differences amongst the FCs exist, especially for inhibition of CYP3A4. The order of potency for in vitro inhibition of CYP3A4 activity has been shown to be: paradisins > DHB > bergamottin > bergaptol. This has been demonstrated in studies from our own laboratory (Figure 4). In another in vitro study, CYP3A4-mediated nifedipine oxidation was inhibited by the paradisins, DHB, and bergamottin with IC50 values of 3, 650, and 1500 nanomolar, respectively [21]. In a study exploring the effects of paradisin A, DHB, bergamottin and bergaptol on CYP3A activity, only bergaptol failed to significantly inhibit testosterone hydroxylation at concentrations up to 20 μM [30].
Figure 4.
Inhibition of triazolam hydroxylation activity – an index of CYP3A activity – in human liver microsomes in relation to varying concentrations of paradisin C, DHB, bergamottin, and bergaptol. All studies represent preincubation of inhibitors with microsomal protein prior to addition of the substrate, thereby indicating irreversible (mechanism-based) inhibition. The numbers next to the inhibitor lines are the 50% inhibitory concentrations (IC50). Smaller numbers indicate greater inhibitory potency. For a detailed description of methodology see references [29, 68, 69].
Mechanism-based inhibition of CYP3A by bergamottin and DHB is substrate-independent for both compounds [27]. For reversible inhibition, DHB inhibited midazolam and testosterone hydroxylation by human intestinal microsomes with nearly identical Ki values – 0.9 and 0.7 μM, respectively. In contrast, the reversible inhibition for bergamottin was substrate-dependent, with the Ki value for midazolam hydroxylation (13.3 μM) significantly greater than the Ki for testosterone hydroxylation (1.6 μM). A subsequent study verified the importance of DHB over bergamottin in the inhibition of CYP3A4 [28]. Using Caco-2 cells modified to express CYP3A4, it was found that DHB and bergamottin differ in their time courses for CYP3A4 inhibition. While maximal CYP3A4 inhibition by DHB occurred within 30 minutes, up to 3 hours was needed for bergamottin to significantly reduce midazolam hydroxylation. Similar data for the paradisins are not available. These in vitro studies suggest that the concentration of DHB in GFJ is more important for in vivo inhibition of CYP3A than the presence of bergamottin. The lack of clinically significant interactions with two beverages known to contain high levels of bergamottin corroborates this contention [34, 35]. Furthermore, when pure bergamottin was administered to 11 volunteers, mean felodipine AUC0-24 was not significantly increased by a 2 mg, 6 mg, or 12 mg bergamottin dose [36].
4.1.2 Clinical Relevance
Definitive evidence that the FCs are the constituents in GFJ responsible for mediating in vivo drug interactions has recently been obtained. Processing of commercial GFJ with a citrus-debittering system yielded a product in which roughly 99% of the FCs were removed [7]. The median AUC for felodipine after administration with 240 mL of this juice was 48 nmol · h/L. This was not significantly different from the value (54 nmol · h/L) obtained with the control beverage, orange juice. In constrast, the median AUC was significantly greater when felodipine was ingested with unprocessed GFJ – 110 nmol · h/L. The furanocoumarin-free GFJ also did not alter the disposition of cyclosporine in a more recent pharmacokinetic trial [8].
Although the FCs inhibit multiple CYP enzymes in vitro, clinically significant inhibition by GFJ has only been unequivocally shown for CYP3A. For example, GFJ does not interact with theophylline (CYP1A2 substrate) or warfarin (CYP2C9 substrate) – Table 1. The vast majority of CYP3A substrate drugs have AUC ratios with GFJ ingestion that are below the “strong inhibition” cut-off value of 5.0. More importantly, AUC ratios > 5 often occurred in studies where GFJ was administered in quantities exceeding usual levels of consumption – for instance, thrice daily ingestion of double-strength GFJ.
Grapefruit juice is unique among CYP3A inhibitors because, when consumed in usual dietary volumes, only enteric CYP3A is affected. Therefore, GFJ has no impact on the pharmacokinetics of CYP3A substrate drugs administered intravenously, and does not prolong the plasma half-life of orally administered agents. Ingestion of GFJ causes an irreversible loss of enteric CYP3A protein, without a decrease in CYP3A mRNA [37-39]. Clinically, implies that prior exposure to GFJ is sufficient to cause an interaction – i.e., GFJ does not have to be physically present in the gastrointestinal tract for an interaction to occur [29, 40-43]. Recovery of CYP3A activity requires de novo enzyme synthesis, and the half-life for this process is approximately 23 hours [29].
4.2 Organic Anion-Transporting Polypeptides
Organic anion-transporting polypeptides (OATPs) are a family of proteins involved in the transport of bile acids, hormones, and drugs. The OATPs contribute to the pharmacokinetic behavior of many drugs. OATPs are found throughout the body, including sites relevant to drug absorption, distribution, metabolism, and excretion. In the small intestine, OATP1A2 and OATP2B1 are the principal OATPs; however, OATP1B1 and OATP1B3 mRNA has been detected in human intestinal biopsy samples [38].
4.2.1 In vitro Interactions with Grapefruit Juice
Flavonoids are capable of inhibiting the OATP1B1-mediated uptake of dehydroepiandrosterone sulfate in stably transfected HeLa cells [44]. When tested at the same concentration (50 μM), naringin and its aglycone, naringenin, decreased dehydroepiandrosterone sulfate uptake to 44% and 47% of control values, respectively.
Another study examined the effects of GFJ and its constituents on OATP2B1 activity using estrone-3-sulfate and glyburide (glibenclamide) as probe substrates [45]. In transfected HEK293 cells, 5% GFJ decreased estrone-3-sulfate uptake by 82%, and glyburide uptake by approximately 60%. Of the GFJ constituents tested at a concentration of 10 μM (naringin, naringenin, quercetin, bergamottin, DHB), only bergamottin reduced estrone-3-sulfate uptake by at least 50%. However, bergamottin did not inhibit glyburide uptake. For this substrate, DHB (10 μM) and naringenin (10 μM) exhibited the greatest inhibitory capacity, reducing transport by approximately 65% and 50%, respectively.
The uptake of fexofenadine by OATP1A2-expressing HeLa cells was inhibited by GFJ in a concentration-dependent manner, with 0.5% juice causing a 50% decrease in transport [46]. In a subsequent study, naringin inhibited OATP1A2-mediated fexofenadine uptake with an IC50 value of 3.6 μM [10]. In a Xenopus laevis oocyte system, naringin inhibited talinolol transport by OATP1A2 (IC50 = 343 μM), but not by OATP2B1 [47].
4.2.2 Clinical Relevance
The number of reported drugs whose AUC values are reduced in the presence of GFJ is limited – Table 1 [9]. Fexofenadine was the first drug reported to interact with GFJ through OATP inhibition and has been the most extensively studied [46]. The reported AUC ratios for fexofenadine are between 0.37 and 0.67, with lower values associated with higher intake of GFJ (1200 mL). Unlike its effect on CYP3A, GFJ-mediated inhibition of OATP is short-lived. One study demonstrated that the AUC ratio of fexofenadine was 0.48 when coadministered with 300 mL of GFJ [38]. However, when the same volume of juice was given 2 hours and 4 hours before fexofenadine, the ratios were 0.62 and 0.96, respectively. Furthermore, immunohistochemical analysis of intestinal biopsy samples from study volunteers revealed no changes in OATP1A2 expression as a result of GFJ ingestion, in contrast to the observed reduction in intestinal CYP3A protein levels [38].
In vitro and in vivo data indicate that the flavonoids are the constituents in GFJ primarily responsible for OATP inhibition. Administration of a 1,210 μM aqueous solution of naringin with fexofenadine resulted in an AUC ratio of 0.78 [10] . The AUC ratio was 0.58 when fexofenadine was ingested with GFJ (naringin concentration of 1,234 μM). A furanocoumarin-rich fraction of GFJ concomitantly with, or two hours prior to, fexofenadine failed to alter drug exposure – AUC ratios of 0.98 and 0.95, respectively [10]. As further evidence implicating the flavonoids in the inhibition of OATP, orange juice and apple juice, which do not contain FCs, also reduce plasma concentrations of OATP substrates [46, 48].
Other OATP substrates that have their bioavailability notably reduced by GFJ include celiprolol, talinolol, aliskiren, and etoposide – Table 1. However, Table 1 also contains many drugs that are putative OATP substrates, but do not exhibit reduced exposure when ingested with GFJ. An example is glyburide. Although OATP-mediated uptake of glyburide is inhibited by GFJ in vitro, the only clinical study to date reported an AUC ratio of 1.05 [49]. The statins are also known OATP substrates, but coadministration with GFJ either causes no change, or an increase, in their exposure.
There are many reasons for these discrepancies. The rate and extent of drug absorption is influenced by a number of factors related to drug dissolution, drug transport, and enteric/hepatic first-pass metabolism. Furthermore, many drugs are substrates for OATPs, P-glycoprotein, and CYP3A – all of whom are reported to be influenced by GFJ. As a result, OATP-mediated transport may only play a small role in determining the systemic exposure for a given drug [9]. This implies that the only way to identify the potential of GFJ to interact with a particular therapeutic agent is to conduct a clinical pharmacokinetic study.
4.3 P-glycoprotein
P-glycoprotein (P-gp) is a 170 kDa transmembrane protein that belongs to the ATP-binding cassette superfamily of transporters. It is located throughout the body, including multiple sites involved in drug disposition. P-gp is localized to the apical membrane of hepatocytes and enterocytes, where it serves as a barrier to drug absorption by transporting drugs into the bile and intestinal lumen, respectively. It colocalizes with OATP1A2 to the apical brush border membrane of small bowel enterocytes [38]. P-glycoprotein possesses a broad substrate specificity, and there is considerable overlap with known CYP3A substrates and inhibitors [50]. Consequently, it is thought that GFJ-mediated inhibition of P-gp efflux transport might augment the increase in drug exposure seen for dual P-gp/CYP3A substrates.
4.3.1 In vitro Interactions with Grapefruit Juice
The majority of in vitro investigations have reported an inhibition of P-gp activity by GFJ, extracts of the juice, and GFJ constituents [8, 30, 31, 39, 46, 47, 51-57]. Nevertheless, the use of different P-gp substrates, inhibitor concentrations, and experimental systems has made it difficult to clearly define the in vitro effects of GFJ and its constituents on this transporter.
Grapefruit juice is a potent inhibitor of P-gp-mediated colchicine transport [56]. GFJ inhibited the secretory transport (basal-to-apical) of colchicine across Caco-2 cell monolayers with an IC50 value of 0.46%. Secretory colchicine transport was also dose-dependently reduced by naringenin, DHB, and naringin – IC50 values of 12, 90, and 592 μM, respectively. However, it should be noted that DHB decreased P-gp activity to a greater extent than naringin or naringenin. Naringin and naringenin are weak inhibitors of P-gp-mediated talinolol transport, with calculated IC50 values in the millimolar range [47, 54]. Although the mechanism of P-gp inhibition by naringin has not been directly examined, the fact that naringin is a substrate for this transporter suggests it is a competitive inhibitor [58].
A study by Ohnishi and colleagues [30] compared the effect of five FCs on vinblastine uptake by Caco-2 cells. Paradisin A, bergamottin, DHB, bergapten and bergaptol all increased vinblastine uptake – by decreasing P-gp-mediated vinblastine efflux – at concentrations ranging from 0.1 to 20 μM. The estimated concentrations necessary to increase uptake by 100% over baseline values were 0.035 μM for paradisin A and bergamottin, 0.040 μM for DHB, 0.050 μM for bergapten, and 0.30 μM for bergaptol. While paradisin A demonstrated a marked concentration-dependent increase in vinblastine uptake, the concentration-response relationship for the other FCs appeared to plateau in concentration range tested. Additional studies with other cell systems and substrates will be necessary to further determine if the paradisins are the primary P-gp inhibitors in GFJ.
4.3.2 Clinical Relevance
Definitive evidence supporting in vivo inhibition of P-gp by GFJ is limited. Three studies of intestinal biopsy samples demonstrated that GFJ ingestion does not alter the mRNA and protein expression levels of P-gp [37-39, 59]. This is similar to GFJ's effect on OATP expression, and suggests that GFJ-mediated inhibition of P-gp would be short-lived.
Cyclosporine is a well-known P-gp/CYP3A substrate, but P-gp may be a more important determinant of cyclosporine absorption than enteric CYP3A [60]. Numerous studies have examined GFJ's effect on cyclosporine disposition, with reported AUC ratios ranging from 1.08 to 1.85 – Table 1. Therefore, it is possible that GFJ inhibition of P-gp may be occurring – albeit in the context of enteric CYP3A inhibition. The results of one study in seven healthy volunteers support this hypothesis [39]. When cyclosporine was coadministered with Seville orange juice or GFJ, only GFJ significantly increased the mean AUC of cyclosporine, even though the concentration of DHB was similar in the two juices and both juices reduced intestinal CYP3A content. The authors concluded that the elevated levels of cyclosporine were a result of P-gp inhibition by constituents in GFJ that were not found in Seville orange juice. However, Paine and Oberlies [3] noted that the study may have been underpowered (n=7) and/or the Seville orange juice administered may have differed from GFJ in total furanocoumarin content. Thus, the findings might reflect less robust inhibition of CYP3A by Seville orange juice as compared with GFJ.
Considerable evidence indicates that GFJ has minimal effect on P-gp. The mean AUC ratios for the P-gp/CYP3A substrates amprenavir and indinavir did not significantly differ from 1 (Table 1). Digoxin transport has also been extensively used as an in vitro P-gp activity marker, and GFJ reduces digoxin transport in cell culture systems. Nevertheless, GFJ had a negligible effect on the pharmacokinetics of digoxin in the two studies published to date [61, 62]. Fexofenadine, talinolol, and celiprolol are transported by both P-gp and OATP. Since GFJ decreases the bioavailability of all three of these drugs, it is likely that GFJ's effect on OATPs is more pronounced than its effect (if any) on enteric P-gp for these compounds.
It is not fully established whether GFJ inhibits in vivo P-gp activity, largely because an ideal P-gp marker substrate has not been identified. Most P-gp transported drugs are also metabolized by CYP3A, thereby making it difficult to assign causality when the levels of a P-gp/CYP3A substrate drug are elevated with GFJ ingestion. Furthermore, many non-metabolized P-gp substrates (e.g. fexofenadine) are transported by OATPs.
4.4 Esterases
Esterases are a ubiquitous class of enzymes present in many body tissues, including those important for drug metabolism – e.g., liver and small intestine. These enzymes are involved in the activation of a number of prodrugs used clinically.
4.4.1 In vitro Interactions with Grapefruit Juice
The hydrolysis of the ester prodrugs, enalapril and lovastatin, was reduced in the presence of GFJ [63]. At a concentration of 40%, GFJ reduced purified porcine esterase-mediated hydrolysis of enalapril and lovatstatin to 31% and 26% of control values, respectively. In similar studies with liver S9 fractions, 10%, 20%, and 40% GFJ reduced the hydrolysis of lovastatin to 54%, 46%, and 38% of control, respectively. Conversely, the liver S9 fraction-mediated hydrolysis of enalapril was resistant to inhibition – 40% GFJ reduced activity to 78% of the level observed in the absence of juice. A subsequent study demonstrated that naringin (1000 μM), hesperidin (200 μM), bergamottin (100 μM), DHB (100 μM), and bergapten (100 μM) were weak inhibitors of esterase activity [64]. However, the flavonoid aglycones inhibited the hydrolysis of p-nitrophenylacetate by human liver microsomes with IC50 values in the micromolar range: naringenin (30 μM), quercetin (43 μM), kaempferol (62 μM), morin (80 μM), and galangin (81 μM).
4.4.2 Clinical Relevance
At the present time, the effect of GFJ on esterase activity in humans is not established. Of the two drugs tested in vitro – enalapril and lovastatin – only the GFJ-lovastatin interaction has been explored in vivo (Table 1). Although the AUC ratio for lovastatin and lovastatin acid were increased with GFJ exposure, the fact that lovastatin is also a CYP3A substrate makes it difficult to characterize the role, if any, of esterase inhibition.
4.5 Sulfotransferases
The sulfotransferase enzymes are involved in the phase II conjugation of both endo- and xenobiotics. SULT1A1 has extensive tissue distribution and is the most abundant SULT isoform in the adult liver. Levels of SULT1A3, on the other hand, are highest in the intestine and are very low in the liver.
4.5.1 In vitro Interactions with Grapefruit Juice
GFJ inhibited SULT1A1 and SULT1A3 activity in a concentration-dependent manner [65, 66]. Ritodrine sulfation by SULT1A1was reduced by approximately 50%, 90%, and 100% in the presence of 1%, 5%, and 10% GFJ, respectively. The degree of inhibition of ritodrine sulfation by SULT1A3 was markedly lower – 10% GFJ reduced activity by only 50%. Similar results were observed with other substrates – p-nitrophenol (SULT1A1) and dopamine (SULT1A3) – indicating the inhibition by GFJ was not substrate dependent [65, 66]. Among individual GFJ constituents, quercetin was the most potent inhibitor of SULT, virtually abolishing SULT1A1 activity at the three concentrations (0.1, 1, 10 μM) tested. The resistance of SULT1A3 to inhibition was again noted, with 10 μM quercetin decreasing activity by only 50%. The effects of naringin, naringenin, bergamottin, and DHB on SULT1A1 and SULT1A3 activity were less pronounced [66]. These findings for recombinant SULT isoforms are consistent with earlier work that characterized the inhibitory effects of quercetin, naringenin, and naringin on human liver cytosol sulfotransferase activity [67].
4.5.2 Clinical Relevance
The potential inhibition of sulfotransferases by GFJ has not been directly studied in humans. Nevertheless, the in vitro observation that the predominant intestinal isoform (SULT1A3) is relatively resistant to GFJ-mediated inhibition would suggest that a clinical interaction is unlikely.
5. Conclusion
Grapefruit juice and its constituents alter the activity of CYP enzymes, esterases, sulfotransferases, OATPs, and P-gp in vitro. Although in vitro studies can be informative, the in vivo environment is complex and cannot be fully replicated in vitro. Consequently, the question of whether GFJ interacts with a given drug can only be definitively answered with data from controlled pharmacokinetic studies.
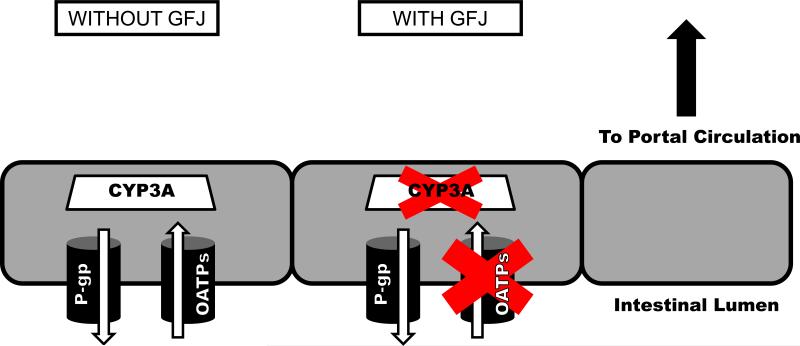
Currently, clinical evidence for significant GFJ-drug interactions exists for CYP3A and OATPs – Figure 5. For CYP3A substrates, grapefruit juice will only cause a clinically relevant interaction if all of the following conditions are met: (1) the drug is given orally; (2) the drug is significantly metabolized by CYP3A; and (3), the drug normally undergoes extensive first-pass metabolism by enteric CYP3A. Because the effect of GFJ on CYP3A is irreversible, up to 3 days may be necessary for enzymatic activity to return after GFJ ingestion. The number of drugs where GFJ causes a strong degree of CYP3A inhibition (AUC ratio ≥ 5) is quite small – Table 1. In fact, the majority of affected drugs exhibit AUC ratios between 1.0 and 2.0 in the presence of GFJ. Nevertheless, avoidance of GFJ may be prudent in some clinical contexts.
Figure 5.
Schematic representation of intestinal enterocyte cells. In the control condition (without GFJ, left), substrate drugs in the intestinal lumen (below) may undergo uptake transport by OATPs, efflux transport by P-gp, and/or metabolism by CYP3A, all of which may influence the extent to which the drug reaches the portal circulation (above). With exposure to GFJ (middle), uptake transport by OATPs and metabolism by CYP3A are potentially inhibited.
The flavonoids are the GFJ constituents responsible for clinically significant OATP inhibition. In contrast to CYP3A, OATP inhibition by GFJ is short-lived, suggesting that the GFJ-mediated reduction in drug bioavailability can be avoided by separating drug administration from GFJ ingestion. This type of GFJ-mediated interaction has only recently been identified and, as a result, examined only for a few drugs. Since OATPs are increasingly recognized for their role in mediating drug disposition, an increase in the number of GFJ-OATP investigations can be anticipated.
The variability in reported AUC ratios among studies using the same drug should also be appreciated. This variability can be attributed to intrinsic biological variation, as well as, variable concentrations of bioactive constituents in the juices tested. Since the furanocoumarins are the substances responsible for CYP3A inhibition, future GFJ-drug interaction studies will be most useful if the concentrations of these constituents are measured and reported. Similarly, measurement of flavonoids should be conducted for GFJ-OATP interaction studies. Phytochemical characterization of the juice will enable between-study comparisons and could potentially identify the minimum constituent concentrations necessary to cause clinically significant effects.
6. Expert Opinion
The fortuitous finding of pharmacokinetic interactions of prescription drugs with GFJ over 20 years ago precipitated a great deal of important basic and translational research at the interface of nutrition science, molecular pharmacology, and clinical therapeutics. We also have learned some cautionary lessons about the interpretation and misinterpretation of biomedical science as it relates to a widely-consumed and popular beverage.
In vitro metabolic models are useful in providing valuable data for estimating the probability of drug interactions involving prescription drugs, but are far less useful for predicting interactions of prescription drugs with GFJ and other fruit beverages. CYP3A is inhibited in vitro by many fruit beverages and their components, but only GFJ has the potential to produce clinically important drug interactions in vivo. This may be explained by the unique presence in GFJ of furanocoumarin derivatives that irreversibly inhibit CYP3A.
Among clinical pharmacokinetic studies that demonstrate statistically significant drug interactions with GFJ, only a few show interactions large enough to be clinically important, and many of these involve exposure to GFJ that exceed quantities typically ingested by consumers. Although the use of concentrated or unrealistically large quantities of juice increases the likelihood of finding a GFJ-drug interaction should one exist, it makes it difficult to translate the applicability of the findings to the usual clinical setting. As a result, future clinical investigations should involve administration of GFJ in a manner consistent with “real world” consumption by the general public. Investigators might also consider studying the effects of high-dose and low-dose GFJ administration within the same clinical trial.
Variability among studies in the magnitude of drug interactions with GFJ may be partly explained by variations in furanocoumarin content. The value of future research will be enhanced by quantitation of furanocoumarin levels in the GFJ products used. Moreover, our knowledge could be enhanced by drug interaction studies involving administration of individual furanocoumarins. These types of trials might lead to the identification of the furanocoumarin(s) necessary to cause an interaction, and the minimum concentration required for in vivo CYP3A inhibition. To date, only bergamottin has been studied in this manner [36].
The recent findings that GFJ can be rendered “interaction-free” by removal of furanocoumarins via methods such as filtration, heating, or UV exposure suggests that we are one step closer toward eliminating the risk of GFJ-drug interactions. If methods of furanocoumarin removal can be adapted for large-scale production of taste-acceptable GFJ products consumed by the public, the therapeutic problem of drug interactions with GFJ would diminish or disappear. Ideally, this modified GFJ would also provide the same health benefits to the consumer as the unprocessed juice.
Acknowledgments
Declaration of Interest
MJ Hanley is supported by grant F31AT006068 from the National Center for Complementary and Alternative Medicine, National Institutes of Health, USA. P Cancelon is an employee of the Florida Department of Citrus while DJ Greenblatt has consulted for them. WW Widmer is an employee of US Department of Agriculture.
List of abbreviations
- AUC
area under the plasma concentration-time curve
- CYP
cytochrome P450
- DHB
6′,7′-dihydroxybergamottin
- FC
furanocoumarin
- GFJ
grapefruit juice
- OATP
organic anion-transporting polypeptide
- P-gp
P-glycoprotein
- SULT
sulfotransferase
- UV
ultraviolet
Footnotes
- Grapefruit juice is a complex matrix that contains an array of phytochemicals. The compounds most relevant for grapefruit juice-drug interactions are the furanocoumarins and the flavonoid, naringin.
- In vitro investigations have shown that grapefruit juice and its constituents can inhibit the activity of multiple drug metabolizing enzymes and transporters.
- Clinical pharmacokinetic studies have identified several furanocoumarin derivatives in grapefruit juice as mechanism-based inhibitors of enteric CYP3A. Consequently, ingestion of grapefruit juice with certain CYP3A substrates can increase their plasma concentrations.
- Grapefruit juice can also decrease the bioavailability of drugs by inhibiting intestinal uptake transporters belonging to the organic anion-transporting polypeptides family.
- Drug interactions with grapefruit juice are likely to be clinically significant for drugs with a narrow therapeutic index and/or in cases where the magnitude of the interaction is large.
References
- 1 **.Bailey DG, Spence JD, Edgar B, et al. Ethanol enhances the hemodynamic effects of felodipine. Clin Invest Med. 1989 Dec;12(6):357–62. [The first report of a GFJ-drug interaction.] [PubMed] [Google Scholar]
- 2.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998 Aug;46(2):101–10. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 *.Paine MF, Oberlies NH. Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol. 2007 Feb;3(1):67–80. doi: 10.1517/17425255.3.1.67. [This review article describes the importance of the small intestine in drug disposition.] [DOI] [PubMed] [Google Scholar]
- 4.Mertens-Talcott SU, Zadezensky I, De Castro WV, et al. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 2006 Dec;46(12):1390–416. doi: 10.1177/0091270006294277. [DOI] [PubMed] [Google Scholar]
- 5 *.Farkas D, Greenblatt DJ. Influence of fruit juices on drug disposition: discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol. 2008 Apr;4(4):381–93. doi: 10.1517/17425255.4.4.381. [This review describes the effects of various fruit juices on drug disposition and highlights the frequently observed disconnect between in vitro and in vivo studies.] [DOI] [PubMed] [Google Scholar]
- 6.Greenblatt DJ. Update on drug interactions with grapefruit juice: an evidence-based review. Pharmacy Times. 2010 January;:95–104. [Google Scholar]
- 7 **.Paine MF, Widmer WW, Hart HL, et al. A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. Am J Clin Nutr. 2006 May;83(5):1097–105. doi: 10.1093/ajcn/83.5.1097. [This study detailed a process for removing furanocoumarins from GFJ and established the furanocoumarins as the clinically relevant CYP3A inhibitors in the juice.] [DOI] [PubMed] [Google Scholar]
- 8 *.Paine MF, Widmer WW, Pusek SN, et al. Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am J Clin Nutr. 2008 Apr;87(4):863–71. doi: 10.1093/ajcn/87.4.863. [This investigation provided further evidence that the furanocoumarins are responsible for in vivo GFJ-drug interactions with cyclosporine, a dual P-gp/CYP3A substrate.] [DOI] [PubMed] [Google Scholar]
- 9.Greenblatt DJ. Analysis of drug interactions involving fruit beverages and organic anion-transporting polypeptides. J Clin Pharmacol. 2009 Dec;49(12):1403–7. doi: 10.1177/0091270009342251. [DOI] [PubMed] [Google Scholar]
- 10 *.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007 Apr;81(4):495–502. doi: 10.1038/sj.clpt.6100104. [This work identifies naringin as a clinically significant inhibitor of OATP-mediated drug uptake.] [DOI] [PubMed] [Google Scholar]
- 11 *.De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006 Jan 11;54(1):249–55. doi: 10.1021/jf0516944. [This study demonstrates the inherent variability in GFJ constituents within and between different brands of juice.] [DOI] [PubMed] [Google Scholar]
- 12.Ho PC, Saville DJ, Coville PF, Wanwimolruk S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm Acta Helv. 2000 Apr;74(4):379–85. doi: 10.1016/s0031-6865(99)00062-x. [DOI] [PubMed] [Google Scholar]
- 13.Bailey DG, Arnold JM, Strong HA, et al. Effect of grapefruit juice and naringin on nisoldipine pharmacokinetics. Clin Pharmacol Ther. 1993 Dec;54(6):589–94. doi: 10.1038/clpt.1993.195. [DOI] [PubMed] [Google Scholar]
- 14.Bailey DG, Arnold JM, Munoz C, Spence JD. Grapefruit juice--felodipine interaction: mechanism, predictability, and effect of naringin. Clin Pharmacol Ther. 1993 Jun;53(6):637–42. doi: 10.1038/clpt.1993.84. [DOI] [PubMed] [Google Scholar]
- 15.Rashid J, McKinstry C, Renwick AG, et al. Quercetin, an in vitro inhibitor of CYP3A, does not contribute to the interaction between nifedipine and grapefruit juice. Br J Clin Pharmacol. 1993 Nov;36(5):460–3. doi: 10.1111/j.1365-2125.1993.tb00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HL, Kent UM, Hollenberg PF. The grapefruit juice effect is not limited to cytochrome P450 (P450) 3A4: evidence for bergamottin-dependent inactivation, heme destruction, and covalent binding to protein in P450s 2B6 and 3A5. J Pharmacol Exp Ther. 2005 Apr;313(1):154–64. doi: 10.1124/jpet.104.079608. [DOI] [PubMed] [Google Scholar]
- 17.Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997 Nov;25(11):1228–33. [PubMed] [Google Scholar]
- 18.Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Role of furanocoumarin derivatives on grapefruit juice-mediated inhibition of human CYP3A activity. Drug Metab Dispos. 2000 Jul;28(7):766–71. [PubMed] [Google Scholar]
- 19.Ohta T, Maruyama T, Nagahashi M, et al. Paradisin C: a new CYP3A4 inhibitor from grapefruit juice. Tetrahedron. 2002;58:6631–5. [Google Scholar]
- 20.Girennavar B, Poulose SM, Jayaprakasha GK, et al. Furocoumarins from grapefruit juice and their effect on human CYP 3A4 and CYP 1B1 isoenzymes. Bioorg Med Chem. 2006 Apr 15;14(8):2606–12. doi: 10.1016/j.bmc.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 21 *.Tassaneeyakul W, Guo LQ, Fukuda K, et al. Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys. 2000 Jun 15;378(2):356–63. doi: 10.1006/abbi.2000.1835. [This comprehensive investigation demonstrated that the furanocoumarins are potent inhibitors of multiple CYP isoforms in vitro.] [DOI] [PubMed] [Google Scholar]
- 22.Uesawa Y, Mohri K. UV-irradiated grapefruit juice loses pharmacokinetic interaction with nifedipine in rats. Biol Pharm Bull. 2006 Jun;29(6):1286–9. doi: 10.1248/bpb.29.1286. [DOI] [PubMed] [Google Scholar]
- 23 *.Uesawa Y, Mohri K. The use of heat treatment to eliminate drug interactions due to grapefruit juice. Biol Pharm Bull. 2006 Nov;29(11):2274–8. doi: 10.1248/bpb.29.2274. [This study showed that bergamottin and DHB are heat-sensitive and possibly degrade to bergaptol.] [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Reynolds KS, Zhao P, Huang SM. Drug interactions evaluation: an integrated part of risk assessment of therapeutics. Toxicol Appl Pharmacol. 2010 Mar 1;243(2):134–45. doi: 10.1016/j.taap.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Greenblatt DJ, He P, von Moltke LL, Court MH. The CYP3 family. In: Ioannides C, editor. Cytochrome P450: Role in the Metabolism and Toxicology of Drugs and Other Xenobiotics. Royal Society of Chemistry; Cambridge, United Kingdom: 2008. pp. 354–83. [Google Scholar]
- 26.Guo LQ, Yamazoe Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol Sin. 2004 Feb;25(2):129–36. [PubMed] [Google Scholar]
- 27 *.Paine MF, Criss AB, Watkins PB. Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos. 2004 Oct;32(10):1146–53. doi: 10.1124/dmd.104.000547. [This in vitro investigation systematically characterized the inhibition kinetics of DHB and bergamottin toward CYP3A4.] [DOI] [PubMed] [Google Scholar]
- 28 *.Paine MF, Criss AB, Watkins PB. Two major grapefruit juice components differ in time to onset of intestinal CYP3A4 inhibition. J Pharmacol Exp Ther. 2005 Mar;312(3):1151–60. doi: 10.1124/jpet.104.076836. [This in vitro study showed that DHB and bergamottin differ in their ability to enter Caco-2 cells and cause CYP3A inhibition.] [DOI] [PubMed] [Google Scholar]
- 29 *.Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin Pharmacol Ther. 2003 Aug;74(2):121–9. doi: 10.1016/S0009-9236(03)00118-8. [This clinical investigation determined the recovery half-life for CYP3A activity after a single ingestion of GFJ to be approximately 23 hours.] [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi A, Matsuo H, Yamada S, et al. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br J Pharmacol. 2000 Jul;130(6):1369–77. doi: 10.1038/sj.bjp.0703433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eagling VA, Profit L, Back DJ. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol. 1999 Oct;48(4):543–52. doi: 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda K, Ohta T, Yamazoe Y. Grapefruit component interacting with rat and human P450 CYP3A: possible involvement of non-flavonoid components in drug interaction. Biol Pharm Bull. 1997 May;20(5):560–4. doi: 10.1248/bpb.20.560. [DOI] [PubMed] [Google Scholar]
- 33.Edwards DJ, Bellevue FH, 3rd, Woster PM. Identification of 6',7'-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996 Dec;24(12):1287–90. [PubMed] [Google Scholar]
- 34.Schwarz UI, Johnston PE, Bailey DG, et al. Impact of citrus soft drinks relative to grapefruit juice on ciclosporin disposition. Br J Clin Pharmacol. 2006 Oct;62(4):485–91. doi: 10.1111/j.1365-2125.2005.02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey DG, Dresser GK, Bend JR. Bergamottin, lime juice, and red wine as inhibitors of cytochrome P450 3A4 activity: comparison with grapefruit juice. Clin Pharmacol Ther. 2003 Jun;73(6):529–37. doi: 10.1016/S0009-9236(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 36.Goosen TC, Cillie D, Bailey DG, et al. Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in humans. Clin Pharmacol Ther. 2004 Dec;76(6):607–17. doi: 10.1016/j.clpt.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37 **.Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997 May 15;99(10):2545–53. doi: 10.1172/JCI119439. [This clinical study showed that GFJ ingestion causes a loss of intestinal CYP3A4 protein, but does not alter CYP3A4 mRNA levels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38 **.Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007 Mar;81(3):362–70. doi: 10.1038/sj.clpt.6100056. [This report showed that various drug metabolizing enzymes and transporters are expressed in the small intestine. It also demonstrated that OATP1A2 and P-gp colocalize in enterocytes, but their expression is unaltered by GFJ ingestion. Finally, the article suggests that the impact of GFJ on OATP transport is short-lived.] [DOI] [PubMed] [Google Scholar]
- 39.Edwards DJ, Fitzsimmons ME, Schuetz EG, et al. 6',7'-Dihydroxybergamottin in grapefruit juice and Seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin Pharmacol Ther. 1999 Mar;65(3):237–44. doi: 10.1016/S0009-9236(99)70102-5. [DOI] [PubMed] [Google Scholar]
- 40.Culm-Merdek KE, von Moltke LL, Gan L, et al. Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther. 2006 Mar;79(3):243–54. doi: 10.1016/j.clpt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Takanaga H, Ohnishi A, Murakami H, et al. Relationship between time after intake of grapefruit juice and the effect on pharmacokinetics and pharmacodynamics of nisoldipine in healthy subjects. Clin Pharmacol Ther. 2000 Mar;67(3):201–14. doi: 10.1067/mcp.2000.104215. [DOI] [PubMed] [Google Scholar]
- 42.Lundahl J, Regardh CG, Edgar B, Johnsson G. Relationship between time of intake of grapefruit juice and its effect on pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur J Clin Pharmacol. 1995;49(1-2):61–7. doi: 10.1007/BF00192360. [DOI] [PubMed] [Google Scholar]
- 43.Lilja JJ, Kivisto KT, Neuvonen PJ. Duration of effect of grapefruit juice on the pharmacokinetics of the CYP3A4 substrate simvastatin. Clin Pharmacol Ther. 2000 Oct;68(4):384–90. doi: 10.1067/mcp.2000.110216. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Wolkoff AW, Morris ME. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos. 2005 Nov;33(11):1666–72. doi: 10.1124/dmd.105.005926. [DOI] [PubMed] [Google Scholar]
- 45.Satoh H, Yamashita F, Tsujimoto M, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005 Apr;33(4):518–23. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 46 **.Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002 Jan;71(1):11–20. doi: 10.1067/mcp.2002.121152. [The first study to show that GFJ, and other fruit juices, can reduce the bioavailability of drugs that are OATP substrates.] [DOI] [PubMed] [Google Scholar]
- 47.Shirasaka Y, Kuraoka E, Spahn-Langguth H, et al. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther. 2010 Jan;332(1):181–9. doi: 10.1124/jpet.109.159756. [DOI] [PubMed] [Google Scholar]
- 48.Lilja JJ, Juntti-Patinen L, Neuvonen PJ. Orange juice substantially reduces the bioavailability of the beta-adrenergic-blocking agent celiprolol. Clin Pharmacol Ther. 2004 Mar;75(3):184–90. doi: 10.1016/j.clpt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Lilja JJ, Niemi M, Fredrikson H, Neuvonen PJ. Effects of clarithromycin and grapefruit juice on the pharmacokinetics of glibenclamide. Br J Clin Pharmacol. 2007 Jun;63(6):732–40. doi: 10.1111/j.1365-2125.2006.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995 Jul;13(3):129–34. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 51.Takanaga H, Ohnishi A, Matsuo H, Sawada Y. Inhibition of vinblastine efflux mediated by P-glycoprotein by grapefruit juice components in caco-2 cells. Biol Pharm Bull. 1998 Oct;21(10):1062–6. doi: 10.1248/bpb.21.1062. [DOI] [PubMed] [Google Scholar]
- 52.Soldner A, Christians U, Susanto M, et al. Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm Res. 1999 Apr;16(4):478–85. doi: 10.1023/a:1011902625609. [DOI] [PubMed] [Google Scholar]
- 53.Spahn-Langguth H, Langguth P. Grapefruit juice enhances intestinal absorption of the P-glycoprotein substrate talinolol. Eur J Pharm Sci. 2001 Feb;12(4):361–7. doi: 10.1016/s0928-0987(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 54.Ofer M, Wolffram S, Koggel A, et al. Modulation of drug transport by selected flavonoids: Involvement of P-gp and OCT? Eur J Pharm Sci. 2005 Jun;25(2-3):263–71. doi: 10.1016/j.ejps.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Lim SL, Lim LY. Effects of citrus fruit juices on cytotoxicity and drug transport pathways of Caco-2 cell monolayers. Int J Pharm. 2006 Jan 3;307(1):42–50. doi: 10.1016/j.ijpharm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Dahan A, Amidon GL. Grapefruit juice and its constituents augment colchicine intestinal absorption: potential hazardous interaction and the role of p-glycoprotein. Pharm Res. 2009 Apr;26(4):883–92. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 57.de Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Grapefruit juice-drug interactions: Grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci. 2007 Oct;96(10):2808–17. doi: 10.1002/jps.20975. [DOI] [PubMed] [Google Scholar]
- 58.Tourniaire F, Hassan M, Andre M, et al. Molecular mechanisms of the naringin low uptake by intestinal Caco-2 cells. Mol Nutr Food Res. 2005 Oct;49(10):957–62. doi: 10.1002/mnfr.200500088. [DOI] [PubMed] [Google Scholar]
- 59.Schwarz UI, Seemann D, Oertel R, et al. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005 Apr;77(4):291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 60.Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997 Sep;62(3):248–60. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- 61.Becquemont L, Verstuyft C, Kerb R, et al. Effect of grapefruit juice on digoxin pharmacokinetics in humans. Clin Pharmacol Ther. 2001 Oct;70(4):311–6. [PubMed] [Google Scholar]
- 62.Parker RB, Yates CR, Soberman JE, Laizure SC. Effects of grapefruit juice on intestinal P-glycoprotein: evaluation using digoxin in humans. Pharmacotherapy. 2003 Aug;23(8):979–87. doi: 10.1592/phco.23.8.979.32881. [DOI] [PubMed] [Google Scholar]
- 63.Li P, Callery PS, Gan LS, Balani SK. Esterase inhibition attribute of grapefruit juice leading to a new drug interaction. Drug Metab Dispos. 2007 Jul;35(7):1023–31. doi: 10.1124/dmd.106.013268. [DOI] [PubMed] [Google Scholar]
- 64.Li P, Callery PS, Gan LS, Balani SK. Esterase inhibition by grapefruit juice flavonoids leading to a new drug interaction. Drug Metab Dispos. 2007 Jul;35(7):1203–8. doi: 10.1124/dmd.106.013904. [DOI] [PubMed] [Google Scholar]
- 65.Nishimuta H, Tsujimoto M, Ogura K, et al. Inhibitory effects of various beverages on ritodrine sulfation by recombinant human sulfotransferase isoforms SULT1A1 and SULT1A3. Pharm Res. 2005 Aug;22(8):1406–10. doi: 10.1007/s11095-005-5263-y. [DOI] [PubMed] [Google Scholar]
- 66.Nishimuta H, Ohtani H, Tsujimoto M, et al. Inhibitory effects of various beverages on human recombinant sulfotransferase isoforms SULT1A1 and SULT1A3. Biopharm Drug Dispos. 2007 Dec;28(9):491–500. doi: 10.1002/bdd.579. [DOI] [PubMed] [Google Scholar]
- 67.Walle T, Eaton EA, Walle UK. Quercetin, a potent and specific inhibitor of the human P-form phenosulfotransferase. Biochem Pharmacol. 1995 Aug 25;50(5):731–4. doi: 10.1016/0006-2952(95)00190-b. [DOI] [PubMed] [Google Scholar]
- 68.Farkas D, Oleson LE, Zhao Y, et al. Pomegranate juice does not impair clearance of oral or intravenous midazolam, a probe for cytochrome P450-3A activity: comparison with grapefruit juice. J Clin Pharmacol. 2007 Mar;47(3):286–94. doi: 10.1177/0091270006298359. [DOI] [PubMed] [Google Scholar]
- 69.Hanley MJ, Cerundolo R, Radwanski N, Court MH. Grapefruit juice, lyophilized grapefruit juice, and powdered whole grapefruit inhibit cytochrome P450-mediated triazolam hydroxylation by beagle dog liver microsomes. J Vet Pharmacol Ther. 2010 Apr;33(2):189–95. doi: 10.1111/j.1365-2885.2009.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl) 2000 Jun;150(2):185–90. doi: 10.1007/s002130000438. [DOI] [PubMed] [Google Scholar]
- 71.Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998 Jan-Mar;23(1):55–9. doi: 10.1007/BF03189827. [DOI] [PubMed] [Google Scholar]
- 72.Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995 Jul;58(1):20–8. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 73.Andersen V, Pedersen N, Larsen NE, et al. Intestinal first pass metabolism of midazolam in liver cirrhosis --effect of grapefruit juice. Br J Clin Pharmacol. 2002 Aug;54(2):120–4. doi: 10.1046/j.1365-2125.2002.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veronese ML, Gillen LP, Burke JP, et al. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003 Aug;43(8):831–9. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]
- 75.Rogers JD, Zhao J, Liu L, et al. Grapefruit juice has minimal effects on plasma concentrations of lovastatin-derived 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Clin Pharmacol Ther. 1999 Oct;66(4):358–66. doi: 10.1053/cp.1999.v66.a101208. [DOI] [PubMed] [Google Scholar]
- 76.van Erp NP, Baker SD, Zandvliet AS, et al. Marginal increase of sunitinib exposure by grapefruit juice. Cancer Chemother Pharmacol. 2010 May 29; doi: 10.1007/s00280-010-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: noninvasive assessment by use of pupillary miosis. Clin Pharmacol Ther. 2004 Nov;76(5):452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Vanakoski J, Mattila MJ, Seppala T. Grapefruit juice does not enhance the effects of midazolam and triazolam in man. Eur J Clin Pharmacol. 1996;50(6):501–8. doi: 10.1007/s002280050148. [DOI] [PubMed] [Google Scholar]
- 79.Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006 Mar;62(3):209–15. doi: 10.1007/s00228-005-0071-1. [DOI] [PubMed] [Google Scholar]
- 80.Hukkinen SK, Varhe A, Olkkola KT, Neuvonen PJ. Plasma concentrations of triazolam are increased by concomitant ingestion of grapefruit juice. Clin Pharmacol Ther. 1995 Aug;58(2):127–31. doi: 10.1016/0009-9236(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 81.Lilja JJ, Kivisto KT, Backman JT, Neuvonen PJ. Effect of grapefruit juice dose on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000 Aug;56(5):411–5. doi: 10.1007/s002280000156. [DOI] [PubMed] [Google Scholar]
- 82.Garg SK, Kumar N, Bhargava VK, Prabhakar SK. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998 Sep;64(3):286–8. doi: 10.1016/S0009-9236(98)90177-1. [DOI] [PubMed] [Google Scholar]
- 83.Kumar N, Garg SK, Prabhakar S. Lack of pharmacokinetic interaction between grapefruit juice and phenytoin in healthy male volunteers and epileptic patients. Methods Find Exp Clin Pharmacol. 1999 Nov;21(9):629–32. [PubMed] [Google Scholar]
- 84.Lilja JJ, Kivisto KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998 Dec;64(6):655–60. doi: 10.1016/S0009-9236(98)90056-X. [DOI] [PubMed] [Google Scholar]
- 85.Vandel S, Netillard C, Perault MC, Bel AM. Plasma levels of clozapine and desmethylclozapine are unaffected by concomitant ingestion of grapefruit juice. Eur J Clin Pharmacol. 2000 Jul;56(4):347–8. doi: 10.1007/s002280000126. [DOI] [PubMed] [Google Scholar]
- 86.Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine's steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001 Oct;62(10):812–7. doi: 10.4088/jcp.v62n1010. [DOI] [PubMed] [Google Scholar]
- 87.Lane HY, Chiu CC, Kazmi Y, et al. Lack of CYP3A4 inhibition by grapefruit juice and ketoconazole upon clozapine administration in vivo. Drug Metabol Drug Interact. 2001;18(3-4):263–78. doi: 10.1515/dmdi.2001.18.3-4.263. [DOI] [PubMed] [Google Scholar]
- 88.Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction--is it risky or not? J Clin Psychopharmacol. 2003 Aug;23(4):422–4. doi: 10.1097/01.jcp.0000085423.74359.f2. [DOI] [PubMed] [Google Scholar]
- 89.Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999 Mar;14(2):113–8. doi: 10.1097/00004850-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999 Nov;21(11):1890–9. doi: 10.1016/S0149-2918(00)86737-5. [DOI] [PubMed] [Google Scholar]
- 91.Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009 Oct;4(Pt 3):832–5. doi: 10.1080/15622970802688069. [DOI] [PubMed] [Google Scholar]
- 92.Kharasch ED, Whittington D, Hoffer C. Influence of hepatic and intestinal cytochrome P4503A activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology. 2004 Sep;101(3):729–37. doi: 10.1097/00000542-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 93.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004 Sep;76(3):250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Benmebarek M, Devaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004 Jul;76(1):55–63. doi: 10.1016/j.clpt.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Nieminen TH, Hagelberg NM, Saari TI, et al. Grapefruit Juice Enhances the Exposure to Oral Oxycodone. Basic Clin Pharmacol Toxicol. 2010 Apr 15; doi: 10.1111/j.1742-7843.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- 96.Cheng KL, Nafziger AN, Peloquin CA, Amsden GW. Effect of grapefruit juice on clarithromycin pharmacokinetics. Antimicrob Agents Chemother. 1998 Apr;42(4):927–9. doi: 10.1128/aac.42.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanazawa S, Ohkubo T, Sugawara K. The effects of grapefruit juice on the pharmacokinetics of erythromycin. Eur J Clin Pharmacol. 2001 Jan-Feb;56(11):799–803. doi: 10.1007/s002280000229. [DOI] [PubMed] [Google Scholar]
- 98.Shi J, Montay G, Leroy B, Bhargava VO. Effects of itraconazole or grapefruit juice on the pharmacokinetics of telithromycin. Pharmacotherapy. 2005 Jan;25(1):42–51. doi: 10.1592/phco.25.1.42.55621. [DOI] [PubMed] [Google Scholar]
- 99.van Agtmael MA, Gupta V, van der Wosten TH, et al. Grapefruit juice increases the bioavailability of artemether. Eur J Clin Pharmacol. 1999 Jul;55(5):405–10. doi: 10.1007/s002280050648. [DOI] [PubMed] [Google Scholar]
- 100.van Agtmael MA, Gupta V, van der Graaf CA, van Boxtel CJ. The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999 Oct;66(4):408–14. doi: 10.1053/cp.1999.v66.a101946. [DOI] [PubMed] [Google Scholar]
- 101.Charbit B, Becquemont L, Lepere B, et al. Pharmacokinetic and pharmacodynamic interaction between grapefruit juice and halofantrine. Clin Pharmacol Ther. 2002 Nov;72(5):514–23. doi: 10.1067/mcp.2002.128148b. [DOI] [PubMed] [Google Scholar]
- 102.Ho PC, Chalcroft SC, Coville PF, Wanwimolruk S. Grapefruit juice has no effect on quinine pharmacokinetics. Eur J Clin Pharmacol. 1999 Jul;55(5):393–8. doi: 10.1007/s002280050646. [DOI] [PubMed] [Google Scholar]
- 103.Cuong BT, Binh VQ, Dai B, et al. Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? Br J Clin Pharmacol. 2006 Jun;61(6):682–9. doi: 10.1111/j.1365-2125.2006.02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagy J, Schipper HG, Koopmans RP, et al. Effect of grapefruit juice or cimetidine coadministration on albendazole bioavailability. Am J Trop Med Hyg. 2002 Mar;66(3):260–3. doi: 10.4269/ajtmh.2002.66.260. [DOI] [PubMed] [Google Scholar]
- 105.Castro N, Jung H, Medina R, et al. Interaction between grapefruit juice and praziquantel in humans. Antimicrob Agents Chemother. 2002 May;46(5):1614–6. doi: 10.1128/AAC.46.5.1614-1616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demarles D, Gillotin C, Bonaventure-Paci S, et al. Single-dose pharmacokinetics of amprenavir coadministered with grapefruit juice. Antimicrob Agents Chemother. 2002 May;46(5):1589–90. doi: 10.1128/AAC.46.5.1589-1590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shelton MJ, Wynn HE, Hewitt RG, DiFrancesco R. Effects of grapefruit juice on pharmacokinetic exposure to indinavir in HIV-positive subjects. J Clin Pharmacol. 2001 Apr;41(4):435–42. doi: 10.1177/00912700122010140. [DOI] [PubMed] [Google Scholar]
- 108.Penzak SR, Acosta EP, Turner M, et al. Effect of Seville orange juice and grapefruit juice on indinavir pharmacokinetics. J Clin Pharmacol. 2002 Oct;42(10):1165–70. doi: 10.1177/009127002401382650. [DOI] [PubMed] [Google Scholar]
- 109.Kupferschmidt HH, Fattinger KE, Ha HR, et al. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br J Clin Pharmacol. 1998 Apr;45(4):355–9. doi: 10.1046/j.1365-2125.1998.t01-1-00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kawakami M, Suzuki K, Ishizuka T, et al. Effect of grapefruit juice on pharmacokinetics of itraconazole in healthy subjects. Int J Clin Pharmacol Ther. 1998 Jun;36(6):306–8. [PubMed] [Google Scholar]
- 111.Penzak SR, Gubbins PO, Gurley BJ, et al. Grapefruit juice decreases the systemic availability of itraconazole capsules in healthy volunteers. Ther Drug Monit. 1999 Jun;21(3):304–9. doi: 10.1097/00007691-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 112.Gubbins PO, McConnell SA, Gurley BJ, et al. Influence of grapefruit juice on the systemic availability of itraconazole oral solution in healthy adult volunteers. Pharmacotherapy. 2004 Apr;24(4):460–7. doi: 10.1592/phco.24.5.460.33350. [DOI] [PubMed] [Google Scholar]
- 113.Gubbins PO, Gurley BJ, Williams DK, et al. Examining sex-related differences in enteric itraconazole metabolism in healthy adults using grapefruit juice. Eur J Clin Pharmacol. 2008 Mar;64(3):293–301. doi: 10.1007/s00228-007-0417-y. [DOI] [PubMed] [Google Scholar]
- 114.Lilja JJ, Raaska K, Neuvonen PJ. Effects of grapefruit juice on the pharmacokinetics of acebutolol. Br J Clin Pharmacol. 2005 Dec;60(6):659–63. doi: 10.1111/j.1365-2125.2005.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lilja JJ, Backman JT, Laitila J, et al. Itraconazole increases but grapefruit juice greatly decreases plasma concentrations of celiprolol. Clin Pharmacol Ther. 2003 Mar;73(3):192–8. doi: 10.1067/mcp.2003.26. [DOI] [PubMed] [Google Scholar]
- 116.Josefsson M, Zackrisson AL, Ahlner J. Effect of grapefruit juice on the pharmacokinetics of amlodipine in healthy volunteers. Eur J Clin Pharmacol. 1996;51(2):189–93. doi: 10.1007/s002280050183. [DOI] [PubMed] [Google Scholar]
- 117.Vincent J, Harris SI, Foulds G, et al. Lack of effect of grapefruit juice on the pharmacokinetics and pharmacodynamics of amlodipine. Br J Clin Pharmacol. 2000 Nov;50(5):455–63. doi: 10.1046/j.1365-2125.2000.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirashima H, Uchida N, Fukazawa I, et al. Effect of a single glass of grapefruit juice on the apparent oral bioavailability of the dihydropyridine calcium channel antagonist, azelnidipine, in healthy Japanese volunteers. Jpn J Clin Pharmacol Ther. 2006;37(3):127–33. [Google Scholar]
- 119.Sigusch H, Henschel L, Kraul H, et al. Lack of effect of grapefruit juice on diltiazem bioavailability in normal subjects. Pharmazie. 1994 Sep;49(9):675–9. [PubMed] [Google Scholar]
- 120.Christensen H, Asberg A, Holmboe AB, Berg KJ. Coadministration of grapefruit juice increases systemic exposure of diltiazem in healthy volunteers. Eur J Clin Pharmacol. 2002 Nov;58(8):515–20. doi: 10.1007/s00228-002-0516-8. [DOI] [PubMed] [Google Scholar]
- 121.Bailey DG, Spence JD, Munoz C, Arnold JM. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991 Feb 2;337(8736):268–9. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 122.Lundahl J, Regardh CG, Edgar B, Johnsson G. Effects of grapefruit juice ingestion--pharmacokinetics and haemodynamics of intravenously and orally administered felodipine in healthy men. Eur J Clin Pharmacol. 1997;52(2):139–45. doi: 10.1007/s002280050263. [DOI] [PubMed] [Google Scholar]
- 123.Lundahl JU, Regardh CG, Edgar B, Johnsson G. The interaction effect of grapefruit juice is maximal after the first glass. Eur J Clin Pharmacol. 1998 Mar;54(1):75–81. doi: 10.1007/s002280050424. [DOI] [PubMed] [Google Scholar]
- 124.Bailey DG, Kreeft JH, Munoz C, et al. Grapefruit juice-felodipine interaction: effect of naringin and 6',7'-dihydroxybergamottin in humans. Clin Pharmacol Ther. 1998 Sep;64(3):248–56. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 125.Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000 Nov;68(5):468–77. doi: 10.1067/mcp.2000.110774. [DOI] [PubMed] [Google Scholar]
- 126.Dresser GK, Bailey DG, Carruthers SG. Grapefruit juice--felodipine interaction in the elderly. Clin Pharmacol Ther. 2000 Jul;68(1):28–34. doi: 10.1067/mcp.2000.107524. [DOI] [PubMed] [Google Scholar]
- 127.Malhotra S, Bailey DG, Paine MF, Watkins PB. Seville orange juice-felodipine interaction: comparison with dilute grapefruit juice and involvement of furocoumarins. Clin Pharmacol Ther. 2001 Jan;69(1):14–23. doi: 10.1067/mcp.2001.113185. [DOI] [PubMed] [Google Scholar]
- 128.Kakar SM, Paine MF, Stewart PW, Watkins PB. 6'7'-Dihydroxybergamottin contributes to the grapefruit juice effect. Clin Pharmacol Ther. 2004 Jun;75(6):569–79. doi: 10.1016/j.clpt.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 129.Bailey DG, Arnold JM, Bend JR, et al. Grapefruit juice-felodipine interaction: reproducibility and characterization with the extended release drug formulation. Br J Clin Pharmacol. 1995 Aug;40(2):135–40. [PMC free article] [PubMed] [Google Scholar]
- 130.Bailey DG, Bend JR, Arnold JM, et al. Erythromycin-felodipine interaction: magnitude, mechanism, and comparison with grapefruit juice. Clin Pharmacol Ther. 1996 Jul;60(1):25–33. doi: 10.1016/S0009-9236(96)90163-0. [DOI] [PubMed] [Google Scholar]
- 131.Edgar B, Bailey D, Bergstrand R, et al. Acute effects of drinking grapefruit juice on the pharmacokinetics and dynamics of felodipine--and its potential clinical relevance. Eur J Clin Pharmacol. 1992;42(3):313–7. doi: 10.1007/BF00266354. [DOI] [PubMed] [Google Scholar]
- 132.Uno T, Ohkubo T, Motomura S, Sugawara K. Effect of grapefruit juice on the disposition of manidipine enantiomers in healthy subjects. Br J Clin Pharmacol. 2006 May;61(5):533–7. doi: 10.1111/j.1365-2125.2006.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uno T, Ohkubo T, Sugawara K, et al. Effects of grapefruit juice on the stereoselective disposition of nicardipine in humans: evidence for dominant presystemic elimination at the gut site. Eur J Clin Pharmacol. 2000 Dec;56(9-10):643–9. doi: 10.1007/s002280000235. [DOI] [PubMed] [Google Scholar]
- 134.Sigusch H, Hippius M, Henschel L, et al. Influence of grapefruit juice on the pharmacokinetics of a slow release nifedipine formulation. Pharmazie. 1994 Jul;49(7):522–4. [PubMed] [Google Scholar]
- 135.Rashid TJ, Martin U, Clarke H, et al. Factors affecting the absolute bioavailability of nifedipine. Br J Clin Pharmacol. 1995 Jul;40(1):51–8. doi: 10.1111/j.1365-2125.1995.tb04534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Odou P, Ferrari N, Barthelemy C, et al. Grapefruit juice-nifedipine interaction: possible involvement of several mechanisms. J Clin Pharm Ther. 2005 Apr;30(2):153–8. doi: 10.1111/j.1365-2710.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 137.Fuhr U, Maier-Bruggemann A, Blume H, et al. Grapefruit juice increases oral nimodipine bioavailability. Int J Clin Pharmacol Ther. 1998 Mar;36(3):126–32. [PubMed] [Google Scholar]
- 138.Soons PA, Vogels BA, Roosemalen MC, et al. Grapefruit juice and cimetidine inhibit stereoselective metabolism of nitrendipine in humans. Clin Pharmacol Ther. 1991 Oct;50(4):394–403. doi: 10.1038/clpt.1991.156. [DOI] [PubMed] [Google Scholar]
- 139.Hashimoto K, Shirafuji T, Sekino H, et al. Interaction of citrus juices with pranidipine, a new 1,4-dihydropyridine calcium antagonist, in healthy subjects. Eur J Clin Pharmacol. 1998 Nov-Dec;54(9-10):753–60. doi: 10.1007/s002280050547. [DOI] [PubMed] [Google Scholar]
- 140.Zaidenstein R, Dishi V, Gips M, et al. The effect of grapefruit juice on the pharmacokinetics of orally administered verapamil. Eur J Clin Pharmacol. 1998 Jun;54(4):337–40. doi: 10.1007/s002280050470. [DOI] [PubMed] [Google Scholar]
- 141.Ho PC, Ghose K, Saville D, Wanwimolruk S. Effect of grapefruit juice on pharmacokinetics and pharmacodynamics of verapamil enantiomers in healthy volunteers. Eur J Clin Pharmacol. 2000 Dec;56(9-10):693–8. doi: 10.1007/s002280000189. [DOI] [PubMed] [Google Scholar]
- 142.Fuhr U, Muller-Peltzer H, Kern R, et al. Effects of grapefruit juice and smoking on verapamil concentrations in steady state. Eur J Clin Pharmacol. 2002 Apr;58(1):45–53. doi: 10.1007/s00228-002-0436-7. [DOI] [PubMed] [Google Scholar]
- 143.Libersa CC, Brique SA, Motte KB, et al. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br J Clin Pharmacol. 2000 Apr;49(4):373–8. doi: 10.1046/j.1365-2125.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Min DI, Ku YM, Geraets DR, Lee H. Effect of grapefruit juice on the pharmacokinetics and pharmacodynamics of quinidine in healthy volunteers. J Clin Pharmacol. 1996 May;36(5):469–76. doi: 10.1002/j.1552-4604.1996.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 145.Damkier P, Hansen LL, Brosen K. Effect of diclofenac, disulfiram, itraconazole, grapefruit juice and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol. 1999 Dec;48(6):829–38. doi: 10.1046/j.1365-2125.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ando H, Tsuruoka S, Yanagihara H, et al. Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin. Br J Clin Pharmacol. 2005 Nov;60(5):494–7. doi: 10.1111/j.1365-2125.2005.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fukazawa I, Uchida N, Uchida E, Yasuhara H. Effects of grapefruit juice on pharmacokinetics of atorvastatin and pravastatin in Japanese. Br J Clin Pharmacol. 2004 Apr;57(4):448–55. doi: 10.1046/j.1365-2125.2003.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lilja JJ, Kivisto KT, Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther. 1999 Aug;66(2):118–27. doi: 10.1053/cp.1999.v66.100453001. [DOI] [PubMed] [Google Scholar]
- 149.Kantola T, Kivisto KT, Neuvonen PJ. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1998 Apr;63(4):397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- 150.Lilja JJ, Kivisto KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther. 1998 Nov;64(5):477–83. doi: 10.1016/S0009-9236(98)90130-8. [DOI] [PubMed] [Google Scholar]
- 151.Lilja JJ, Neuvonen M, Neuvonen PJ. Effects of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin. Br J Clin Pharmacol. 2004 Jul;58(1):56–60. doi: 10.1111/j.1365-2125.2004.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tapaninen T, Neuvonen PJ, Niemi M. Grapefruit juice greatly reduces the plasma concentrations of the OATP2B1 and CYP3A4 substrate aliskiren. Clin Pharmacol Ther. 2010 Sep;88(3):339–42. doi: 10.1038/clpt.2010.101. [DOI] [PubMed] [Google Scholar]
- 153.Zaidenstein R, Soback S, Gips M, et al. Effect of grapefruit juice on the pharmacokinetics of losartan and its active metabolite E3174 in healthy volunteers. Ther Drug Monit. 2001 Aug;23(4):369–73. doi: 10.1097/00007691-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 154.Sullivan DM, Ford MA, Boyden TW. Grapefruit juice and the response to warfarin. Am J Health Syst Pharm. 1998 Aug 1;55(15):1581–3. doi: 10.1093/ajhp/55.15.1581. [DOI] [PubMed] [Google Scholar]
- 155.Uno T, Yasui-Furukori N, Takahata T, et al. Lack of significant effect of grapefruit juice on the pharmacokinetics of lansoprazole and its metabolites in subjects with different CYP2C19 genotypes. J Clin Pharmacol. 2005 Jun;45(6):690–4. doi: 10.1177/0091270005275430. [DOI] [PubMed] [Google Scholar]
- 156.Miura M, Kagaya H, Tada H, et al. Intestinal CYP3A4 is not involved in the enantioselective disposition of lansoprazole. Xenobiotica. 2006 Jan;36(1):95–102. doi: 10.1080/00498250500485065. [DOI] [PubMed] [Google Scholar]
- 157.Tassaneeyakul W, Vannaprasaht S, Yamazoe Y. Formation of omeprazole sulphone but not 5-hydroxyomeprazole is inhibited by grapefruit juice. Br J Clin Pharmacol. 2000 Feb;49(2):139–44. doi: 10.1046/j.1365-2125.2000.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hermann M, Asberg A, Reubsaet JL, et al. Intake of grapefruit juice alters the metabolic pattern of cyclosporin A in renal transplant recipients. Int J Clin Pharmacol Ther. 2002 Oct;40(10):451–6. doi: 10.5414/cpp40451. [DOI] [PubMed] [Google Scholar]
- 159.Bistrup C, Nielsen FT, Jeppesen UE, Dieperink H. Effect of grapefruit juice on Sandimmun Neoral absorption among stable renal allograft recipients. Nephrol Dial Transplant. 2001 Feb;16(2):373–7. doi: 10.1093/ndt/16.2.373. [DOI] [PubMed] [Google Scholar]
- 160.Lee M, Min DI, Ku YM, Flanigan M. Effect of grapefruit juice on pharmacokinetics of microemulsion cyclosporine in African American subjects compared with Caucasian subjects: does ethnic difference matter? J Clin Pharmacol. 2001 Mar;41(3):317–23. doi: 10.1177/00912700122010131. [DOI] [PubMed] [Google Scholar]
- 161.Ducharme MP, Warbasse LH, Edwards DJ. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther. 1995 May;57(5):485–91. doi: 10.1016/0009-9236(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 162.Ku YM, Min DI, Flanigan M. Effect of grapefruit juice on the pharmacokinetics of microemulsion cyclosporine and its metabolite in healthy volunteers: does the formulation difference matter? J Clin Pharmacol. 1998 Oct;38(10):959–65. doi: 10.1002/j.1552-4604.1998.tb04393.x. [DOI] [PubMed] [Google Scholar]
- 163.Min DI, Ku YM, Perry PJ, et al. Effect of grapefruit juice on cyclosporine pharmacokinetics in renal transplant patients. Transplantation. 1996 Jul 15;62(1):123–5. doi: 10.1097/00007890-199607150-00023. [DOI] [PubMed] [Google Scholar]
- 164.Hollander AA, van Rooij J, Lentjes GW, et al. The effect of grapefruit juice on cyclosporine and prednisone metabolism in transplant patients. Clin Pharmacol Ther. 1995 Mar;57(3):318–24. doi: 10.1016/0009-9236(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 165.Yee GC, Stanley DL, Pessa LJ, et al. Effect of grapefruit juice on blood cyclosporin concentration. Lancet. 1995 Apr 15;345(8955):955–6. doi: 10.1016/s0140-6736(95)90700-9. [DOI] [PubMed] [Google Scholar]
- 166.Brunner LJ, Pai KS, Munar MY, et al. Effect of grapefruit juice on cyclosporin A pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2000 Nov;4(4):313–21. doi: 10.1034/j.1399-3046.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 167.Ioannides-Demos LL, Christophidis N, Ryan P, et al. Dosing implications of a clinical interaction between grapefruit juice and cyclosporine and metabolite concentrations in patients with autoimmune diseases. J Rheumatol. 1997 Jan;24(1):49–54. [PubMed] [Google Scholar]
- 168.Ducharme MP, Provenzano R, Dehoorne-Smith M, Edwards DJ. Trough concentrations of cyclosporine in blood following administration with grapefruit juice. Br J Clin Pharmacol. 1993 Nov;36(5):457–9. doi: 10.1111/j.1365-2125.1993.tb00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brunner LJ, Munar MY, Vallian J, et al. Interaction between cyclosporine and grapefruit juice requires long-term ingestion in stable renal transplant recipients. Pharmacotherapy. 1998 Jan-Feb;18(1):23–9. [PubMed] [Google Scholar]
- 170.Liu C, Shang YF, Zhang XF, et al. Co-administration of grapefruit juice increases bioavailability of tacrolimus in liver transplant patients: a prospective study. Eur J Clin Pharmacol. 2009 Sep;65(9):881–5. doi: 10.1007/s00228-009-0702-z. [DOI] [PubMed] [Google Scholar]
- 171.Bidstrup TB, Damkier P, Olsen AK, et al. The impact of CYP2C8 polymorphism and grapefruit juice on the pharmacokinetics of repaglinide. Br J Clin Pharmacol. 2006 Jan;61(1):49–57. doi: 10.1111/j.1365-2125.2005.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reif S, Nicolson MC, Bisset D, et al. Effect of grapefruit juice intake on etoposide bioavailability. Eur J Clin Pharmacol. 2002 Oct;58(7):491–4. doi: 10.1007/s00228-002-0495-9. [DOI] [PubMed] [Google Scholar]
- 173.Yin OQ, Gallagher N, Li A, et al. Effect of grapefruit juice on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol. 2010 Feb;50(2):188–94. doi: 10.1177/0091270009336137. [DOI] [PubMed] [Google Scholar]
- 174.Seidegard J, Randvall G, Nyberg L, Borga O. Grapefruit juice interaction with oral budesonide: equal effect on immediate-release and delayed-release formulations. Pharmazie. 2009 Jul;64(7):461–5. [PubMed] [Google Scholar]
- 175.Schubert W, Cullberg G, Edgar B, Hedner T. Inhibition of 17 beta-estradiol metabolism by grapefruit juice in ovariectomized women. Maturitas. 1994 Dec;20(2-3):155–63. doi: 10.1016/0378-5122(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 176.Weber A, Jager R, Borner A, et al. Can grapefruit juice influence ethinylestradiol bioavailability? Contraception. 1996 Jan;53(1):41–7. doi: 10.1016/0010-7824(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 177.Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005 Sep;60(3):337–41. doi: 10.1111/j.1365-2125.2005.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Varis T, Kivisto KT, Neuvonen PJ. Grapefruit juice can increase the plasma concentrations of oral methylprednisolone. Eur J Clin Pharmacol. 2000 Sep;56(6-7):489–93. doi: 10.1007/s002280000171. [DOI] [PubMed] [Google Scholar]
- 179.Banfield C, Gupta S, Marino M, et al. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin Pharmacokinet. 2002;41(4):311–8. doi: 10.2165/00003088-200241040-00004. [DOI] [PubMed] [Google Scholar]
- 180.Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005 Mar;77(3):170–7. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 181.Clifford CP, Adams DA, Murray S, et al. The cardiac effects of terfenadine after inhibition of its metabolism by grapefruit juice. Eur J Clin Pharmacol. 1997;52(4):311–5. doi: 10.1007/s002280050296. [DOI] [PubMed] [Google Scholar]
- 182.Honig PK, Wortham DC, Lazarev A, Cantilena LR. Grapefruit juice alters the systemic bioavailability and cardiac repolarization of terfenadine in poor metabolizers of terfenadine. J Clin Pharmacol. 1996 Apr;36(4):345–51. doi: 10.1002/j.1552-4604.1996.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 183.Benton RE, Honig PK, Zamani K, et al. Grapefruit juice alters terfenadine pharmacokinetics, resulting in prolongation of repolarization on the electrocardiogram. Clin Pharmacol Ther. 1996 Apr;59(4):383–8. doi: 10.1016/S0009-9236(96)90105-8. [DOI] [PubMed] [Google Scholar]
- 184.Rau SE, Bend JR, Arnold MO, et al. Grapefruit juice-terfenadine single-dose interaction: magnitude, mechanism, and relevance. Clin Pharmacol Ther. 1997 Apr;61(4):401–9. doi: 10.1016/S0009-9236(97)90190-9. [DOI] [PubMed] [Google Scholar]
- 185.Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993 Apr;35(4):431–6. doi: 10.1111/j.1365-2125.1993.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maish WA, Hampton EM, Whitsett TL, et al. Influence of grapefruit juice on caffeine pharmacokinetics and pharmacodynamics. Pharmacotherapy. 1996 Nov-Dec;16(6):1046–52. [PubMed] [Google Scholar]
- 187.Lucas D, Ferrara R, Gonzalez E, et al. Chlorzoxazone, a selective probe for phenotyping CYP2E1 in humans. Pharmacogenetics. 1999 Jun;9(3):377–88. doi: 10.1097/00008571-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 188.Gross AS, Goh YD, Addison RS, Shenfield GM. Influence of grapefruit juice on cisapride pharmacokinetics. Clin Pharmacol Ther. 1999 Apr;65(4):395–401. doi: 10.1016/S0009-9236(99)70133-5. [DOI] [PubMed] [Google Scholar]
- 189.Kivisto KT, Lilja JJ, Backman JT, Neuvonen PJ. Repeated consumption of grapefruit juice considerably increases plasma concentrations of cisapride. Clin Pharmacol Ther. 1999 Nov;66(5):448–53. doi: 10.1016/S0009-9236(99)70007-X. [DOI] [PubMed] [Google Scholar]
- 190.Offman EM, Freeman DJ, Dresser GK, et al. Red wine-cisapride interaction: comparison with grapefruit juice. Clin Pharmacol Ther. 2001 Jul;70(1):17–23. doi: 10.1067/mcp.2001.116892. [DOI] [PubMed] [Google Scholar]
- 191.Desta Z, Kivisto KT, Lilja JJ, et al. Stereoselective pharmacokinetics of cisapride in healthy volunteers and the effect of repeated administration of grapefruit juice. Br J Clin Pharmacol. 2001 Oct;52(4):399–407. doi: 10.1046/j.0306-5251.2001.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Di Marco MP, Edwards DJ, Wainer IW, Ducharme MP. The effect of grapefruit juice and seville orange juice on the pharmacokinetics of dextromethorphan: the role of gut CYP3A and P-glycoprotein. Life Sci. 2002 Jul 26;71(10):1149–60. doi: 10.1016/s0024-3205(02)01799-x. [DOI] [PubMed] [Google Scholar]
- 193.Strauch K, Lutz U, Bittner N, Lutz WK. Dose-response relationship for the pharmacokinetic interaction of grapefruit juice with dextromethorphan investigated by human urinary metabolite profiles. Food Chem Toxicol. 2009 Aug;47(8):1928–35. doi: 10.1016/j.fct.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 194.Mougey EB, Lang JE, Wen X, Lima JJ. Effect of Citrus Juice and SLCO2B1 Genotype on the Pharmacokinetics of Montelukast. J Clin Pharmacol. 2010 Oct 25; doi: 10.1177/0091270010374472. [DOI] [PubMed] [Google Scholar]
- 195.Ebert U, Oertel R, Kirch W. Influence of grapefruit juice on scopolamine pharmacokinetics and pharmacodynamics in healthy male and female subjects. Int J Clin Pharmacol Ther. 2000 Nov;38(11):523–31. doi: 10.5414/cpp38523. [DOI] [PubMed] [Google Scholar]
- 196.Jetter A, Kinzig-Schippers M, Walchner-Bonjean M, et al. Effects of grapefruit juice on the pharmacokinetics of sildenafil. Clin Pharmacol Ther. 2002 Jan;71(1):21–9. doi: 10.1067/mcp.2002.121236. [DOI] [PubMed] [Google Scholar]
- 197.Fuhr U, Maier A, Keller A, et al. Lacking effect of grapefruit juice on theophylline pharmacokinetics. Int J Clin Pharmacol Ther. 1995 Jun;33(6):311–4. [PubMed] [Google Scholar]