Abstract
Background
Colorectal carcinoma (CRC) is the seventh-most common malignancy and is the main cause of death in Iraq. The incidence of this cancer has increased sharply after the invasion of Iraq in 2003.
Aim
To estimate immunohistochemical expression of vascular endothelial growth factor (VEGF) in CRC in relation to other parameters, such as grade and stage of tumour.
Methods
Formalin fixed, paraffin-embedded blocks from 52 patients (27 male and 25 female) with CRC were included in this study. A group of 22 patients with non-cancerous colonic tissues were included as a control group. Avidin–biotin complex method was employed for immunohistochemical detection of VEGF.
Results
VEGF immuno-expression was positive in 51.9% of CRC, while it was 18.2% in the normal colonic tissue (p <0.05). VEGF immunostaining was positively correlated with grade of colonic malignancy (p <0.05).
Conclusion
These findings provide further evidence for the role of VEGF in the carcinogenesis of CRC. However, VEGF could not be well correlated with stage of tumour and hence may be a poor prognostic parameter of state of malignancy of colonic carcinoma.
Keywords: colorectal carcinoma, VEGF, immunohistochemistry
Colorectal carcinoma (CRC) is the third-most common malignancy (1) and is the second-most common cause of death in the USA (2).
In Iraq, CRC is the seventh-most common type of malignancy and is the first cause of death due to a gastrointestinal malignancy. However, gastric carcinoma is the second-most frequent gastrointestinal malignancy (3). The sex incidence of CRC is about the same, though the incidence rises sharply with age in both sexes (4).
Furthermore, many other factors have been known to contribute significantly in the increased incidence of CRC. These factors include geographical variation, family history, diet, chronic inflammatory disease and polyposis syndrome (5–9).
Vascular endothelial growth factor (VEGF) is an important signalling protein involved in both vasculogenesis and angiogenesis (10–13). VEGF (usually refers to VEGF-A) gene which locates on chromosome 6p12, contains eight exons. The various VEGF-A coding regions give seven isoforms, the most common four are: VEGF-121, VEGF-165, VEGF-189 and VEGF-206 (12). The VEGF protein is a heparin-binding glycoprotein with 45 kDa. VEGF belongs to the Platelet-derived growth factor (PDGF) family. There are six types of VEGF: VEGF-A, B, C, D, E and F (12). The activity of VEGF protein is mainly on the vascular endothelial cells (so its name is derived from this fact) (13–15). The present study was designed to study the overexpression of VEGF in CRC in relation to grade and stage of the tumour among a group of Iraqi patients who referred to Kufa School of medicine teaching hospital for histopathological evaluation.
Patients and methods
During a one-year period starting from November 2006 to November 2007, 52 patients (27 male and 25 female) with CRC and 22 cases of non-cancerous lesion as a control group were subjected to the present investigation. All cases were referred to Kufa School of medicine teaching hospital from different regions of middle Euphrates area of Iraq for histopathological evaluation. All cases, whether malignant or non-cancerous lesions, were examined by two histopathologists independently and then subjected to the immunohistochemical method using the ABC technique. The total number of malignant cases was 52 (all were hemicolectomy or segment resection). Left side cases were 19, lower rectal tumours were 13 and right side were 20, while the total number of non-cancerous cases was 22 (all were endoscopic biopsies). The malignant colonic cases were staged according to the TNM (tumor size, lymph node involvement, distant metastasis) staging system (16). The mean age of patient was 58.1 years. The avidin–biotin complex (ABC) method was used for immunohistochemical detection of 0.2 mL (Clone VG1, Code M7273, LOT 00028659, Dako Denmark A/S Produktionsvej 42 DK-2600 Glostrup) as primary antibody for the detection of VEGF protein (Kit K5204, Dako Co). The antibody sensitivity and specificity were 98% and 100%, respectively. A Staining kit, Code K0673, from Dako Co, was also used. The criterion for a positive immune reaction was a dark-brown cytoplasmic precipitate. The intensity scoring of the staining was assessed quantitatively by counting the percentage of positive cells in 100 malignant cells at 40 total magnifications for at least 25 fields and qualitatively as dense and faint staining. A four-scaled scorings system was chosen in this study; Score 0 (negative): no stained malignant cells, Score 1 (weak): 5–10% of malignant cells, Score 2 (moderate): <25% of positive malignant cells, Score 3 (strong): 25–50% of malignant cells and Score 4 (very strong): >50% of malignant cells (16).
Statistical analysis
The results were statistically evaluated with the help of SSPS software by using Chi-square test and Correlation–Regression test.
Results
The positive results for VEGF immunohistochemical staining appear as brown cytoplasmic colour. Regarding the non-cancerous colonic tissue, 4 (18.2%) out of 22 cases showed positive immunohistochemical stain for VEGF, while 27 (51.9%) out of 52 malignant cases showed positive results with a significant difference between these two groups (p <0.05) (Table 1, Fig. 1).
Table 1.
VEGF overexpression in relation to grade and stage of colorectal carcinoma
| Pathological parameters | VEGF positive | VEGF negative | Total | p-Value |
|---|---|---|---|---|
| Non-cancerous | 4 (18.2%) | 18 (81.8) | 22 (29.7%) | <0.05 |
| Malignant | 27 (51.9%) | 25 (48.1%) | 52 (70.3%) | R = 0.81 |
| Adenocarcinoma | 26 (52%) | 24 (48%) | 50 (96.2%) | |
| Squamous carcinoma | 1 (50%) | 1 (50%) | 2 (3.8%) | |
| Grade | ||||
| Grade 1 | 9 (47.4%) | 10 (56.6%) | 19 (36.5%) | <0.05 |
| Grade 2 | 11 (52.4%) | 10 (47.6%) | 21 (40.4%) | R = 0.96 |
| Grade 3 | 7 (58.3%) | 5 (41.7%) | 12 (23%) | |
| TNM stage | ||||
| I | 7 (53.8%) | 6 (46.2%) | 13 (25%) | >0.05 |
| II | 11 (52.4%) | 10 (47.6%) | 21 (40.4%) | R = 0.05 |
| III | 9 (50%) | 9 (50%) | 18 (34.6%) | |
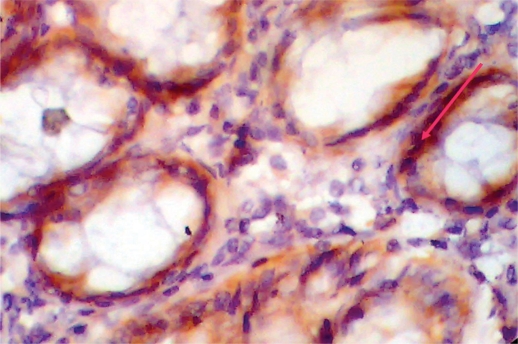
Fig. 1.
Normal colonic tissue with positive VEGF immunostaining, score 2.
VEGF overexpression was reported in 52% of adenocarcinoma and 50% of squamous cell carcinoma without any significant difference between the two groups (p >0.05) (Table 1, Figs. 2 and 3).
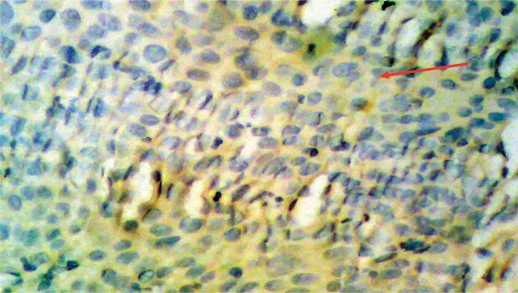
Fig. 2.
Squamous cell carcinoma of rectum, grade II exhibiting positive faint, score 1 VEGF immunostaining.
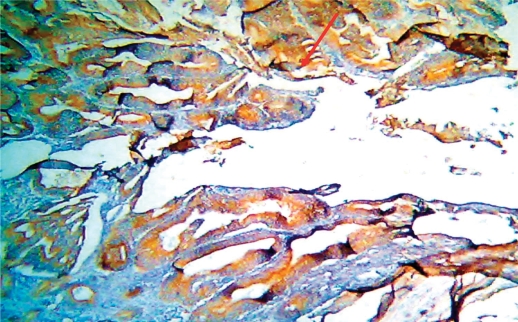
Fig. 3.
Adenocarcinoma of colon, grade I, stage II with positive dense VEGF immunostaining, score 4 (×10).
VEGF overexpression was found to be positive in 47.4% of grade I CRC, in 11 (52.4%) of grade II CRC, and in seven (58.3%) of grade III CRC. There was good correlation between the grade of tumour and VEGF overexpression (r = 0.96, p <0.05) (Table 1).
VEGF overexpression was found to be positive in seven (53.8%) of stage I CRC, in 11 (52.4%) of stage II CRC and in nine (50%) of stage III CRC. There was no correlation between the stage of tumour and VEGF overexpression (r = 0.96, p <0.05) (Table 1).
Regarding the intensity scorings of positive results of VEGF immunohistochemical staining, score 1 was found in 14 (51.9%) cases, score 2 in eight (19.65) cases, score 3 in four (14.8%) cases and score 4 was found only in one case (3.7%) of CRC. There was a strong correlation of VEGF immunohistochemical staining scoring with the grade of CRC (Table 2).
Table 2.
Intensity of vascular endothelial growth factors in relation to grade, stage and pattern of immunostaining in colorectal carcinoma
| Intensity of stain | Score 0 | Score +1 | Score +2 | Score +3 | Score +4 | Total |
|---|---|---|---|---|---|---|
| Grade | ||||||
| I | 10 (50%) | 3 (15%) | 2 (1%) | 4 (20%) | 1 (0.5%) | 20 (38.46%) |
| II | 10 (47.6%) | 6 (28.6%) | 5 (23.81%) | 0 (0%) | 0 (0%) | 21 (40.4%) |
| III | 5 (45.6%) | 4 (36.4%) | 2 (18%) | 0 (0%) | 0 (0%) | 11 (21.15%) |
| p <0.05 | R = 0.96 | |||||
| Stage | ||||||
| I | 6 (42.9%) | 5 (35.7%) | 2 (14.3%) | 1 (7.1%) | 0 (0%) | 14 (26.9%) |
| II | 10 (47.6%) | 4 (19%) | 3 (14.1%) | 3 (14.1%) | 1 (4.2%) | 21 (40.4%) |
| III | 9 (50%) | 5 (2.7%) | 3 (15.8%) | 1 (5.6%) | 0 (0%) | 17 (32.7%) |
| p <0.05 | R = 0.62 | |||||
| Pattern | ||||||
| Faint | 0 (0%) | 8 (72.7%) | 2 (18.2%) | 1 (9%) | 0 (0%) | 11 (40.7%) |
| Dense | 0 (0%) | 6 (36.5%) | 6 (36.5%) | 3 (18.8%) | 1 (6.3%) | 16 (59.3%) |
| p <0.05 | R = 0.95 | |||||
In relation to the staining pattern (dense or faint) of positive VEGF immunohistochemical staining, faint staining was seen in 40.7% (11 out of 27 cases) while the dense mode of immunostaining was reported in 59.3% (16 out of 27) (Table 2).
Discussion
Angiogenesis is crucial for tumour development. VEGF is considered as the most important, directly acting and potent angiogenic agent that has been shown to be over expressed in CRC. In the present investigation, VEGF was expressed in 18.2% of non-cancerous colonic tissue indicating that VEGF is an effective angiogenic factor in normal physiological conditions in the colon (17–21).
However, a positive VEGF immunostaining was found in 51.9% (27 out of 52 cases) of the study group of CRC with a significant difference of (p <0.05) as compared with the non-cancerous control samples. This observation indicates that VEGF has a fundamental role in the angiogenesis of CRC (18, 22–24).
A positive correlation between the intensity of VEGF immunostaining and histological types of CRC has been noted, with a significant difference among these three subtypes in relation to the VEGF over expression. This finding is in complete agreement with that reported elsewhere (25).
There was a positive correlation between the VEGF immunostaining and the pattern of staining (dense or faint) (r = 0.92, p <0.05) suggesting that colonic cancer is a highly vascular malignancy and the malignant colonic cancer cells are highly susceptible to the action of VEGF.
As grouped in Table 1, the present study shows that there was a gradual increase in the frequency of VEGF expression in parallel with the increase in the grade of tumour (in grade I there was 50% VEGF expression, in grade II 52% and in grade III 54.5%). However, no significant differences among the three degrees of differentiation (p >0.05) were noted. This observation is supported by previous investigators who reached the same conclusion (26, 27). Furthermore, there was a significant correlation between the stage of tumour and the intensity of VEGF immunostaining (p <0.05, r = 0.62). This finding indicates equivocally that VEGF is directly proportional to the degree of colorectal tumour spread as has been established elsewhere (18, 27–30). There is no significant difference among the three stages of CRC in relation to VEGF expression (p >0.05).
Conclusion
These findings unfold further evidence for the role of VEGF in the carcinogenesis of CRC. Accordingly, VEGF could be considered as a poor prognostic parameter of colonic carcinoma.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Cristi E, Peerrone G, Toscano G, Verzi A, Nori S, Santini D, et al. Tumor proliferation, angiogenesis, and polidy in human colon cancer. J Clin Pathol. 2005;58:1170–4. doi: 10.1136/jcp.2004.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson-Thompson S, Ahmed F, Germann RR, Lai SM, Friedman C. Descriptive epidemiology of colorectal cancer United States, 1998–2001. Cancer. 2006;107:1103–11. doi: 10.1002/cncr.22007. [DOI] [PubMed] [Google Scholar]
- 3.Iraqi Ministry of Health. Results of Iraqi cancer registry. 2004 Baghdad, Iraq.
- 4.Kayton ML. Cancer and pediatric inflammatory bowel disease. Semin Pediatr Surg. 2007;16:205–13. doi: 10.1053/j.sempedsurg.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Zarychanski R, Chen Y, Bernstein CN, Hebert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behavior. CMAJ. 2007;177:593–7. doi: 10.1503/cmaj.070558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khafagy W, El-Ghazaly M, El-Shobaky MT, Khafagy M. Colorectal cancer in Egypt – does it differ? Coloproctology. 2000;22:109–15. [Google Scholar]
- 7.Bronner MP. Gastrointestinal inherited polyposis syndromes. Mod Pathol. 2003;16:359–65. doi: 10.1097/01.MP.0000062992.54036.E4. [DOI] [PubMed] [Google Scholar]
- 8.Ahsan H, Neugut AI, Garbowski GC, Jacobson JS, Forde KA, Treat MR, et al. Family history of colorectal adenomatous polyps and increased risk for colorectal carcinoma. Ann Intern Med. 1998;128:900–5. doi: 10.7326/0003-4819-128-11-199806010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Eaden J. Colorectal carcinoma and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:24–30. doi: 10.1111/j.1365-2036.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 10.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal carcinoma: genetic of development and metastasis. J Gastroenterol. 2006;41:185–92. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 11.Hideki U, Ashley P, Wilkinson HK, Jermy JR, Hidetaka M, Ian TC. A new prognostic staging for rectal cancer. Ann Surg. 2004;240:832–9. doi: 10.1097/01.sla.0000143243.81014.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiota G, Ishida M, Noguchi N, Oyama K, Takano Y, Okubo M, et al. Circulating P53 antibody in patients with colorectal carcinoma: relation to clinicopathological features. Dig Dis Sci. 2000;45:122–8. doi: 10.1023/a:1005473729976. [DOI] [PubMed] [Google Scholar]
- 13.Takuya Y, Takao O, Tadashi S, Hiromitsu M, Kazuyuki T, Hirotaka I, et al. Vascular endothelial growth factor (VEGF) as a prognosticator for colorectal cancer. J Jpn Soc Colo-Proctol. 2000;53:27–34. [Google Scholar]
- 14.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DIR, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factor in health disease. Genome Biol. 2005;6:209–12. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton CC, Frderick LG. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 17.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:18040–5. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 18.Galizia G, Lieto E, Ferraraccio F, Orditura M, DeVita F, Castellano P, et al. Determination of molecular marker expression can predict clinical outcome in colon carcinomas. Clin Cancer Res. 2004;10:3490–9. doi: 10.1158/1078-0432.CCR-0960-03. [DOI] [PubMed] [Google Scholar]
- 19.Roberts J, Tomanek AS, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res. 2001;88:1127–34. doi: 10.1161/hh1101.091191. [DOI] [PubMed] [Google Scholar]
- 20.Hanabata N, Sasaki Y, Tanaka M, Tsuji T, Hatada Y, Munakata A. Vascular endothelial growth factor expression and micro vessel parameters of colonic mucosa correlate with sensitivity to steroid in patients with ulcerative colitis. Scand J Gastroenerol. 2005;40:188–93. doi: 10.1080/00365520410010580. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga T, Oshika Y, Aby Y, Ozeki Y, Sadahgiro S, Kijima H, et al. Vascular endothelial growth factor (VEGF) isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77:998–1002. doi: 10.1038/bjc.1998.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascinu S, Staccioli MP, Gasparini G, Giordani P, Catalano V, Ghiselli R, et al. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6:2803–7. [PubMed] [Google Scholar]
- 23.Kondo Y, Arii S, Furutani M, Isigami S, Mori A, Onodera H, et al. Implication of vascular endothelial growth factor and P53 status for angiogenesis in non-invasive colorectal carcinoma. Cancer. 2000;88:1820–7. [PubMed] [Google Scholar]
- 24.Zarychanski R, Chen Y, Bernstein CN, Hebert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behavior. CMAJ. 2007;177:573–7. doi: 10.1503/cmaj.070558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goethals L, Debucquoy A, Perneel C, Geboes K, Ectors N, De Schutter H, et al. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol. 2006;65:246–54. doi: 10.1016/j.ijrobp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Ono T, Miki C. Factors influencing tissue concentration of vascular endothelial growth factor in colorectal carcinoma. Am J Gastroenterol. 2000;95:1062–7. doi: 10.1111/j.1572-0241.2000.01909.x. [DOI] [PubMed] [Google Scholar]
- 27.Gunsilius E, Tswchmelitsch J, Eberweinm M, Schwelberger H, Spizzo G, Kahler CM, et al. In vivo release of vascular endothelial growth factor from colorectal carcinomas. Oncology. 2002;62:313–7. doi: 10.1159/000065062. [DOI] [PubMed] [Google Scholar]
- 28.Saad RS, Liu Y, Nathan G, Celerebezze J, Medich D, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17:197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]
- 29.Landriscina M, Casssano C, Ratto C, Longo R, Ippolit M, Palazzotti B, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal carcinoma. Br J Cancer. 1998;78:765–77. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakasaki T, Wada H, Shigemori C, Gabazza EC, Nobori T, Nakamura S, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247–54. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]