Abstract
Background. It is unknown whether sex and race influence clinical outcomes following primary human immunodeficiency virus type 1 (HIV-1) infection.
Methods. Data were evaluated from an observational, multicenter, primarily North American cohort of HIV-1 seroconverters.
Results. Of 2277 seroconverters, 5.4% were women. At enrollment, women averaged .40 log10 fewer copies/mL of HIV-1 RNA (P < .001) and 66 more CD4+ T cells/μL (P = .006) than men, controlling for age and race. Antiretroviral therapy (ART) was less likely to be initiated at any time point by nonwhite women and men compared to white men (P < .005), and by individuals from the southern United States compared to others (P = .047). Sex and race did not affect responses to ART after 6 months (P > .73). Women were 2.17-fold more likely than men to experience >1 HIV/AIDS-related event (P < .001). Nonwhite women were most likely to experience an HIV/AIDS-related event compared to all others (P = .035), after adjusting for intravenous drug use and ART. Eight years after diagnosis, >1 HIV/AIDS-related event had occurred in 78% of nonwhites and 37% of whites from the southern United States, and 24% of whites and 17% of nonwhites from other regions (P < .001).
Conclusions. Despite more favorable clinical parameters initially, female HIV-1-seroconverters had worse outcomes than did male seroconverters. Elevated morbidity was associated with being nonwhite and residing in the southern United States.
Women and minorities have been underrepresented in many studies of primary human immunodeficiency virus type 1 (HIV-1) infection in the United States [1–3]. Women have lower plasma viral loads and higher CD4+ T cell counts than do men following seroconversion [4–8] as well as during chronic HIV infection [9, 10]. Plasma viral loads have also been observed to be lower in nonwhites [11]. It is unknown, however, whether clinical presentations and outcomes of primary HIV infection differ by sex and race. A better understanding of the influence of sex and race on early HIV infection could potentially improve the diagnosis and care of HIV-infected women and minorities.
The Acute Infection and Early Disease Research Program (AIEDRP) was a multicenter, observational cohort of more than 2000 primarily North American individuals diagnosed with acute and recent HIV infection during the era of highly active antiretroviral therapy (ART). This data set provided a unique opportunity to evaluate whether sex or race influences clinical manifestations and outcomes of primary HIV infection. We hypothesized that sex and race differences in CD4+ T cell counts and viral loads result in differences in clinical outcomes of primary HIV infection.
MATERIALS AND METHODS
Study cohort
Subjects who enrolled in AIEDRP between 1997 and 2007 were evaluated. Follow-up in the study was initially open-ended, but it closed in 2008. Subjects were enrolled at multiple sites in the United States (26 sites), Australia (10 sites), Canada (2 sites), and Brazil (1 site) (see Acknowledgments). Enrollment criteria included one of the following: (1) negative or indeterminate HIV antibody and viral load of >15,000 copies/mL, (2) positive HIV antibody with documented negative HIV antibody within 12 months of enrollment, or (3) positive HIV antibody and an optical density of <1.0, as determined by a less sensitive enzyme immunoassay [12]. Individuals were further classified as acutely infected if they presented within 2 weeks and recently infected if they presented within between 3 weeks and 12 months of seroconversion. Study visits occurred at baseline and at weeks 2, 4, and 12, then every 12 weeks through week 168 and every 24 weeks for the remainder of the study. Baseline data were collected regarding race/ethnicity, age, HIV risk factors, seroconversion symptoms, viral load, and CD4+ T cell count. At each subsequent visit, viral load and CD4+ T cell count were obtained and subjects were queried regarding interim medical events and initiation of ART. Specific ART regimens and toxicities are not evaluated here, as these results have been published elsewhere [13]. The US region of recruitment was defined by Centers for Disease Control and Prevention criteria [14]. Data on insurance coverage, income, education, co-infection with hepatitis B or C virus, and mental illness were not available. The study was approved by each site’s institutional review board. All subjects gave written informed consent.
Statistical analysis
Analyses assume a 2-sided test with a .05 significance level and were performed in S-PLUS (version 8.0; Insightful) or SAS software (version 9.0; SAS Institute). The χ2 and Fisher exact tests were used for between-group comparisons of categorical outcomes. Continuous outcomes were compared using t tests and were log transformed for normality, as appropriate. Logistic regression was used to analyze dichotomous outcomes while adjusting for confounding covariates. Count data were analyzed using Poisson regression with an offset to account for follow-up time. Baseline CD4+ T cell count and HIV RNA level were modeled using ordinary least squares regression. These outcomes were also modeled longitudinally using a time-varying mixed effects model [10] with the final model chosen using the Akaike information criterion [11]. Kaplan-Meier analyses and extended Cox proportional hazards models, with time-varying covariates, were used in time-to-event analyses. In analyses including race, if the outcome for different races was similar, the races were collapsed into the categories of white and nonwhite individuals. Unless it is otherwise specified, nonwhite individuals included all individuals who identified as black, nonwhite Hispanic, or other.
RESULTS
Baseline characteristics of female and male study participants are shown in Table 1. Women constituted 124 (5.4%) of 2277 HIV seroconverters. No significant sex differences existed in the proportion of subjects identified as acutely or recently infected. The majority of men (77%) were white, whereas the majority of women (55%) were nonwhite. Women were younger and more likely to report intravenous drug use (IDU) and heterosexual contact as risk factors compared to men. The majority of subjects were recruited from North America, particularly the western United States. Almost half (45%) of nonwhite women, however, were recruited from the southern United States (also referred to herein as the South), and 79% of women recruited from the South were nonwhite. There were no sex differences in mean follow-up time.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristics | Women (n = 124) | Men (n = 2153) | P |
| Stage of infection | .28 | ||
| Acute seroconverter, no. (%) | 24 (19) | 514 (24) | |
| Recent seroconverter, no. (%)a | 100 (81) | 1639 (76) | |
| Age, geometric mean (95% CI), years | 32 (30.3–34.1) | 34 (34.1–34.8) | .03 |
| Follow-up time, mean (95% CI), years | 4.4 (3.07–6.68) | 4.1 (3.94–4.41) | .71 |
| Race/ethnicity, no. (%) | <.001 | ||
| White | 56 (45) | 1,654 (77) | |
| Black | 42 (34) | 119 (5) | |
| Hispanicb | 17 (14) | 260 (12) | |
| Other | 9 (7) | 120 (6) | |
| HIV risk factor, no. (%)c | |||
| Sex between men | … | 1948 (90) | |
| Sex between men and women | 104 (84) | 119 (6) | <.001 |
| Injection drug use | 20 (16) | 114 (5) | <.001 |
| Otherd | 17 (14) | 178 (8) | .05 |
| Recruitment by geographic region, no. (%) | <.001 | ||
| United States–West/Midweste | 60 (48) | 1246 (58) | |
| United States–South | 34 (27) | 141 (7) | |
| United States–Northeast | 16 (13) | 428 (20) | |
| Canada | 11 (9) | 112 (5) | |
| Australia and Brazilf | 3 (2) | 226 (11) | |
| Plasma HIV RNA concentration, mean (95% CI) log10 copies/mLg | 4.40 (4.18–4.62) | 4.85 (4.81–4.90) | <.001 |
| CD4+ T lymphocyte count, mean (95% CI) cells/μLh | 600 (552–648) | 546 (536–556) | .02 |
NOTE. According to Centers for Disease Control and Prevention (CDC) estimates [15], 27% of new HIV infections in the United States in 2006 occurred in women. Of women identified as white, black or Hispanic, 61% were black, 23% were white, and 16% were Hispanic. The corresponding racial/ethnic distribution of newly HIV-infected men in the United States in 2006 was estimated as 40% black, 41% white, and 19% Hispanic. The CDC did not provide estimates regarding geographic region of new infections. Estimated new AIDS diagnoses in 2008, which may be somewhat reflective of the epidemiology of individuals newly infected over the preceding 10 years, revealed that 46% occurred in the South, 22% in the Northeast, 19% in the West, and 11% in the Midwest [14]. CI, confidence interval.
Of recent seroconverters, 19% were diagnosed by negative HIV enzyme immunoassay (EIA) at <90 d; 68% by less sensitive EIA with optical density of <1 or negative EIA at 91–183 d; and 13% by negative EIA at 184–365 d. There were no sex differences in the criteria used to diagnose recent seroconversion (P = .14).
Includes 9 women and 110 men who also identified as nonwhite.
Some individuals reported >1 risk factor.
Includes blood transfusion and risk factor not reported or not identified.
Midwest accounted for 0 women and 8 men.
Includes 2 female and 5 male subjects from Brazil.
Baseline plasma HIV RNA concentrations were available for 111 women and 2022 men.
Baseline CD4+ T lymphocyte counts were available for 109 women and 2051 men.
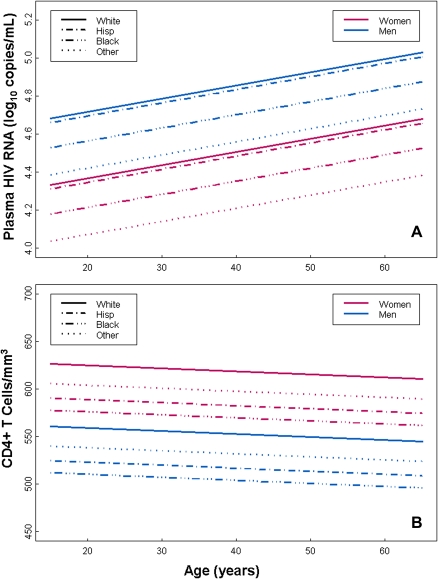
Baseline viral load was lower in women than in men (Table 1). A linear model was used to evaluate other factors that affect viral load. Viral load was higher by .07 log10 copies/mL (95% confidence interval [CI], .02–.12) per decade of life (P = .008), and higher in white, non-Hispanic individuals compared with all other races (.53 log10 copies/mL; 95% CI, .20–.86; P = .002). After adjusting for age and race, women had a lower viral load than men (mean difference .40 log10 copies/mL; 95% CI: .19–.60; P= .001) (Figure 1A).
Figure 1.
Linear model of baseline human immunodeficiency virus (HIV) RNA concentration (A) and CD4+ T cell count (B) as a function of age, race, and sex. Viral load was lower (P < .001) and CD4+ T cell count was higher (P = .005) in women than in men. Combining sexes, viral load was higher in Hispanics (P = .007) and whites (P < .001) compared with individuals of other races, whereas white individuals had higher CD4+ T cell counts than did black (P = .02) and Hispanic (P = .02) individuals. Other race/ethnicity pairwise differences were not statistically significant.
Women had higher baseline CD4+ T lymphocyte counts than men (Table 1). A linear model that included age and race was used to further evaluate baseline CD4+ T cell counts (Figure 1B). White, non-Hispanic individuals had higher CD4+ T cell counts (106 cells/μL; 95% CI, 32–180) than all other groups (P = .005), but no statistically significant differences were seen with increasing age (slope, −.32; 95% CI, −1.5 to .86; P = .6). After adjusting for age and race, women had more CD4+ T cells than men (mean difference, 66 cells/μL; 95% CI: 19–112; P = .006).
Frequently reported symptoms of acute retroviral syndrome included fever (78%), fatigue (72%), myalgia (57%), headache (50%), and pharyngitis (46%). Fewer women (62%) reported fatigue compared with men (73%, P = .02), but no sex differences were evident for other symptoms associated with primary infection. Higher viral load (difference, 1 log10 copy/mL) was predictive of 2 or more symptoms (odds ratio [OR], 1.46; 95% CI, 1.27–1.68; P < .001). Compared with individuals with a history of IDU, those without a history of IDU were more likely to present with 2 or more symptoms (OR, 3.62; 95% CI, 2.11–6.03; P < .001). Women were less likely than men to report 2 or more of the 6 most common symptoms (OR, .40; 95% CI, .24–.66; P < .001), but there were no differences in symptoms between nonwhites and whites (OR, .94; 95% CI, .63–1.38; P = .73). After controlling for baseline viral load, race, and IDU, women were still less likely to report 2 or more symptoms compared with men (OR, .48; 95% CI, .28–.84; P = .008).
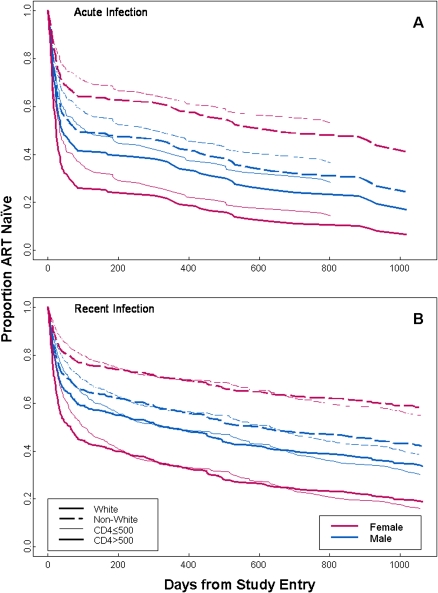
ART was started by 79 (63.7%) women and 1475 (68.5%; P = .28) men at some time during the study period. Many of the centers that participated in the AIEDRP database were conducting studies of ART in early HIV infection. Reasons for starting ART were not recorded in the database. Among subjects for whom clinical data were available near the time of treatment initiation (samples were collected an average of 9 days before treatment initiation), mean CD4+ T cells counts were similar in women (514 cells/μL; Standard Error [SE], 32; n = 64) and men (476 cells/μL; SE, 6; n = 1296; P = .19), but mean viral load was lower in women (4.7 log10 copies/mL; SE, .11; n = 70) than in men (5.0 log10 copies/mL; SE, .02; n = 1370; P = .002). Using the Kaplan-Meier method, we found that women were less likely to initiate ART at any time point (median, 271 d; 95% CI, 79–484) than men (median, 69 d; 95% CI, 57–89), although this difference was not statistically significant (P = .09). Acutely infected subjects initiated ART earlier (median, 16 d) than recently infected subjects (median, 191 d; P < .001). To adjust for this and other factors that influence time to ART initiation, a Cox proportional hazards model was used. This model stratified subjects by acute and recent infection and baseline CD4+ T cell count of <500 cells/μL and adjusted for baseline age, IDU, and time-varying: viral load, CD4+ T cell count, and CD4+ T cell count of ≤200 cells/μL before July 2004 or CD4+ T cell count of ≤350 cells/μL after July 2004 to account for changes in guidelines [16] for initiation of ART. Compared with white men, white women were more likely to initiate ART, and nonwhite men and women were less likely to start ART at any time point (Figure 2 and Table 2). Compared with subjects from the South, subjects from other regions were more likely to initiate treatment (Hazard Ratio [HR], 1.26, 95% CI, 1.0–1.57; P = .047). In a model evaluating race as an interactive effect with region, racial differences in time to initiation of ART persisted in other regions, but not in the South (Table 2).
Figure 2.
Extended Cox proportional hazards model of time to initiation of antiretroviral therapy (ART) in acute (A) and recent (B) human immunodeficiency virus (HIV) infection. All race sex pairwise comparisons are statistically significant (P < .04) except that for nonwhite women versus nonwhite men (P = .08).
Table 2.
Proportional Hazards Models for Time to Antiretroviral Therapy Initiation by Race and Sex and by Race Within Region
| HR | (95% CI) | P | |
| Race and sex | |||
| White men | 1.0 | Reference | … |
| White women | 1.42 | (1.01–1.99) | .040 |
| Nonwhite women | .55 | (.36–.83) | .004 |
| Nonwhite men | .80 | (.68–.93) | .005 |
| Race within region | |||
| South | |||
| Nonwhite | 1.0 | Reference | … |
| White | .80 | (.51–1.25) | .33 |
| All other regions | |||
| Nonwhite | 1.0 | Reference | … |
| White | .76 | (.64–.89) | <.001 |
NOTE. CI, confidence interval; HR, hazard ratio.
Similar percentages of women (77%) and men (81%) achieved a viral load of <400 copies/mL (P > .5) after 6 months of ART. After adjusting for CD4+ T cell count and viral load at the beginning of treatment, age, race, and IDU, no sex differences were detected in the probability of viral load of <400 copies/mL (OR, .90; 95% CI, .48–1.68; P = .73). A trend toward lower CD4+ T cell gains was seen during the first 6 months of ART in women (mean increase, 124 cells/μL; n = 33; 95% CI, 11–237) compared with men (mean increase, 206 cells/μL; n = 847; 95% CI, 190–222; P = .06). After adjustment for CD4+ T cell count and viral load at treatment initiation, race, and acute/recent infection status, no sex differences in CD4+ T cell reconstitution were detected (difference, 7 cells/μL; standard deviation, 31; P = .83).
Longitudinal viral load and CD4+ T cell counts were analyzed using a time-varying mixed effects model adjusting for age and race in individuals remaining off therapy for up to 3 years. Lower viral loads in women compared with men persisted through week 24 (viral load, .20 log10 copies/mL; 95% CI, .03–.36; P = .02), but by week 104, untreated women had higher viral loads compared with men (viral load, .24 log10 copies/mL; 95% CI, .07–.41; P < .01). Sex differences in CD4+ T cell counts persisted through week 24 with women having higher CD4+ T cell counts than men at that time (difference, 80 cells/μL; 95% CI, 43–117; P < .001). At subsequent time points, however, CD4+ T cell counts in treatment-naïve women and men were no longer significantly different.
Mortality in the cohort was low and did not differ between the sexes; 16 (.7%) deaths occurred in men, of which 2 (.1%) were attributed to HIV infection, and 1 (.8%) death that was not HIV-related occurred in a woman (P = .92). Similar proportions of women and men had at least one CD4+ T cell count of <200 cells/μL (P = .06; rate at 8 years, 16.5% and 25.3%, respectively). However, when evaluated by sex-race categories, a higher proportion of nonwhite women (40%; 95% CI, 25%–59%) had at least one CD4+ T cell count of <200 cells/μL compared with nonwhite men (20%; 95% CI, 14%–30%), white men (15%; 95% CI, 12%–20%), and white women (4.7%; 95% CI, 1.6%–14%; P < .001).
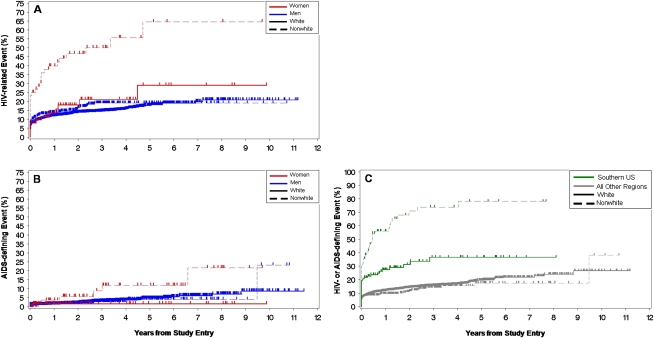
In an unadjusted time-to-event analysis, the occurrence of at least one non-AIDS HIV-related illness was higher in women (46%) compared with men (21%; P < .001) (Table 3), and this difference persisted when female-specific events were removed from the analysis (39%; P < .001). Women reported persistent generalized lymphadenopathy, diarrhea (otherwise undiagnosed), and peripheral neuropathy more often than men. Frequency of total AIDS-defining illnesses did not differ between the sexes, but women had higher rates of recurrent bacterial pneumonia and Candida esophagitis. Women had 2.17 times more combined HIV-related and AIDS-defining diseases per person than did men after adjusting for follow-up time (P < .001), and this difference remained after removing female-specific events and controlling for IDU (1.68 times more events per woman; P < .001). In an analysis of morbidity by sex and race, nonwhite women experienced more HIV-related and AIDS-defining illnesses than all other groups (Figures 3A and 3B). At 8 years’ follow-up, HIV-related events occurred in 64% (95% CI, 45%–83%) of nonwhite women and 21% (95% CI, 18%–24%) of other groups combined, and AIDS-defining events occurred in 22% (95% CI, 8%–50%) and 6% (95% CI, 4%–9%), respectively. IDU was associated with higher combined HIV- and AIDS-related diseases (36%; 95% CI, 23%–54%) compared to no IDU (25%; 95% CI, 21%–29%; P = .001). To further assess reasons for elevated morbidity in nonwhite women, relationships among sex and race and time to HIV- and AIDS-related illnesses were analyzed in a proportional hazards model censoring at time of ART initiation and stratified by IDU. Hazard ratios were reduced by 10%–20% compared to a sensitivity analysis that did not utilize stratification or censoring, but substantial unexplained morbidity in nonwhite women remained (Table 4).
Table 3.
Kaplan-Meier Failure Percentages for HIV-Related and AIDS-Defining Illnesses by Sex at 75th Percentile (8 Years) of Overall Follow-up
| Women |
Men |
||||
| Illness | % | (95% CI) | % | (95% CI) | Log-rankP |
| HIV-related illnessesa | |||||
| Seborrheic dermatitis | 1 | (0–8) | 3 | (2–4) | .23 |
| Persistent generalized lymphadenopathy | 23 | (15–33) | 3 | (3–4) | <.001 |
| Fever of >101.3°F or >38.5°C | 4 | (2–13) | 3 | (2–4) | .72 |
| Idiopathic thrombocytopenic purpura | 0 | … | <1 | (0–1) | .60 |
| Oral thrush | 7 | (3–19) | 6 | (4–8) | .90 |
| Oral hairy leukoplakia | 0 | … | 2 | (1–3) | .21 |
| Shingles | 5 | (1–15) | 6 | (4–9) | .93 |
| Diarrhea (otherwise undiagnosed) | 11 | (5–22) | 5 | (3–6) | .04 |
| Peripheral neuropathy (not drug-related/unknown) | 12 | (6–22) | 3 | (1–4) | <.001 |
| Cervical dysplasia or intraepithelial neoplasia | 4 | (1–16) | NA | NA | |
| Candida vaginitis | 10 | (5–18) | NA | NA | |
| Any HIV-related illnessbc | 46 | (34–61) | 21 | (18–24) | <.001 |
| AIDS-defining illnessesa | |||||
| HIV encephalopathy or dementia | 0 | … | <1 | (0–2) | .69 |
| Pneumocystis jiroveci pneumonia | 0 | … | 1 | (0–2) | .52 |
| Herpes simplex virus (mucocutaneous ulcer for >1 month) | 4 | (1–12) | 2 | (1–4) | .06 |
| Tuberculosis, pulmonary | 0 | … | <1 | (0–0) | .73 |
| Candida esophagitis | 7 | (2–27) | 1 | (0–2) | .003 |
| Bacterial pneumonia, recurrent | 8 | (3–20) | 2 | (1–4) | .02 |
| Lymphoma | 0 | … | 1 | (0–3) | .64 |
| Kaposi sarcoma, cutaneous | 0 | … | 1 | (0–2) | .47 |
| HIV-associated wasting syndrome | 0 | … | 1 | (0–2) | .53 |
| Cytomegalovirus infection (retinitis or colitis) | 0 | … | <1 | (0–1) | .72 |
| Cryptosporidiosis | 0 | … | <1 | (0–0) | .68 |
| Any AIDS-defining illnessc | 12 | (5–30) | 7 | (5–10) | .13 |
| Any HIV-related or AIDS-defining illnessc | 47 | (34–61) | 24 | (21–28) | <.001 |
NOTE. CI, confidence interval; HIV, human immunodeficiency virus; NA, not applicable to that group.
Recurrent events of the same type in an individual were only counted once.
When female-specific HIV-related events are excluded, women still had higher rates of HIV-related events (39%; P < .001) compared with men.
Individuals with > 1 event in any category were only counted once in summary calculations.
Figure 3.
Kaplan-Meier curves for time to human immunodeficiency virus (HIV)–related (A) and AIDS-defining events (B) by sex and race, and time to HIV-related and AIDS-defining events by region (C). Nonwhite women were more likely to experience >1 HIV-related (P < .001) (A) and AIDS-defining illness (P = .007) (B) than all others over the entire period of study follow-up. For HIV-related events, significant pairwise comparisons included nonwhite women versus white women (P < .001), nonwhite women versus nonwhite men (P = .02), nonwhite women versus white men (P < .001), white women versus white men (P < .001), and nonwhite men versus white men (P = .02). For AIDS-defining illnesses, nonwhite women were significantly more likely to experience >1 event than were white women (P = .02). Other pairwise comparisons were not statistically different. Geographic region combined with race significantly impacted likelihood of HIV- and/or AIDS-related events (C). Regions outside of the southern United States, including international regions, were combined because there were similar and not significant differences among them in terms of morbidity. Nonwhites and whites in the southern United States were significantly more likely to experience >1 HIV- and/or AIDS-related event than both race categories from all other regions (P < .001). No significant differences were seen between whites and nonwhites within the category “all other regions” (P = .2). Removal of international sites from the analysis did not substantively change the results in any way.
Table 4.
Proportional Hazards Models for Time to HIV-Related and AIDS-Defining Illnesses by Race and Sex
| Models |
||||||
| Stratified by IDU |
Censored at time of ART initiation and stratified by IDU |
|||||
| Participant group | HR | (95% CI) | P | HR | (95% CI) | P |
| Nonwhite women | 1.0 | References | … | 1.0 | Reference | … |
| White women | .37 | (.19–.71) | .003 | .44 | (.21–.94) | .035 |
| Nonwhite men | .31 | (.20–.49) | <.001 | .35 | (.21–.58) | <.001 |
| White men | .27 | (.40–.19) | <.001 | .31 | (.20–.48) | <.001 |
NOTE. ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; IDU, intravenous drug use.
Region of recruitment combined with race was strongly associated with HIV-related morbidity (Figure 3C). Approximately 8 years after diagnosis of acute or recent HIV infection, nonwhites from the South experienced the highest rate of at least 1 HIV- or AIDS-related event, (78%; 95% CI, 64%–89%) compared with whites from the South (37%; 95% CI, 26%–49%; P < .001), whites from other regions (24%; 95% CI, 19%–28%; P < .001), and nonwhites from other regions (17%; 95% CI, 12%–25%; P < .001). Whites from the South also experienced higher rates of events than did whites (P < .001) and nonwhites (P < .001) from other combined regions. Among whites, women had more morbidity than did men in the South (36% vs 22%; P = .006) and in other regions (21% vs 14%; P < .001). Similar to whites, nonwhite women had higher rates of events than men by region, (South, 81% vs 63%, p=1.0; other regions, 16% vs 12%, p=.19), but these differences did not reach statistical significance.
DISCUSSION
We evaluated data from more than 2000 primarily North American HIV seroconverters, the largest cohort ever recruited from this region, to determine whether sex and race influence clinical presentation, use of ART, and morbidity following primary HIV infection. Women had lower viral loads and higher CD4+ T cell counts at diagnosis compared with men, similar to what has previously been reported for HIV seroconverters [4–8]. Whites had higher viral loads and CD4+ T cell counts, whereas these values were lower in blacks at time of diagnosis, consistent with findings in studies of chronic HIV infection [11]. Women reported fewer symptoms of primary infection than men, but there were no race differences in reported symptoms. Nonwhites and individuals from the South initiated ART later than others. There were no sex or race differences in virologic or immunologic responses to ART. Unexpectedly, women experienced 2.17-fold more combined HIV- and AIDS-related illnesses than men. Morbidity was particularly elevated among nonwhite women, who were significantly more likely than others to have a CD4+ T cell count of <200 cells/μL and to experience HIV- and AIDS-related events. Individuals who lived in the South, particularly nonwhites, experienced substantially more HIV-related morbidity than those from other regions. Collectively, these data suggest that sex, race, and geographic region profoundly influence clinical outcomes following primary HIV infection.
Sex and race differences in viral loads and CD4+ T cell counts have been observed in intravenous drug users [4] and in individuals from diverse areas of the world who acquired HIV sexually [4–8, 17], which suggests these differences are biologically based rather than related to socioeconomic factors or modes of transmission. Mechanisms underlying sex and race differences in viral loads and CD4+ T cell counts, however, are unknown. Women in this study reported fewer symptoms of primary HIV infection. Some of the sex differences in reporting of symptoms of primary infection likely related to lower viral loads in women compared with men. Nevertheless, even after controlling for viral load, IDU, and race, sex differences in numbers of reported symptoms remained. It should be noted that fewer reported symptoms of acute retroviral syndrome could lead to less frequent diagnoses of primary infection in women than in men.
An unexpected interaction between sex and race was found in the timing of initiation of ART. White women started ART earlier than all others. Later initiation of ART by women compared with men has been observed in most [18–24], although not all [25, 26], studies of HIV-infected individuals. Importantly, these studies did not evaluate sex-race combinations and consequently may have failed to discover this interaction. The finding that nonwhite men and women initiated ART later than others is consistent with previous observations in chronically infected individuals from North America [18, 20, 21]. Reasons for differential use of ART within this cohort are unknown.
Despite the fact that women had higher CD4+ T cell counts and lower viral loads at study entry, they subsequently experienced significantly more combined HIV-related and AIDS-defining events. Nonwhite women in particular had higher rates of both HIV-related and AIDS-defining events than all others. These observations contrast with findings from a meta-analysis of seroconverter cohorts from Europe and Canada during the ART era that revealed lower HIV-related morbidity and mortality in women compared with men [24]. These findings are also distinct from studies of seroprevalent cohorts during the pre-ART era, which largely failed to detect sex differences in morbidity and mortality from HIV infection [5, 10, 27]. Notably, however, these findings are consistent with several studies from the United States [28, 29] that have reported sex differences in HIV-related morbidity and mortality in the period after ART was introduced. A retrospective analysis of clinical outcomes of HIV-infected individuals treated in a university-associated clinic in Tennessee [18] found that female sex and black race were associated with increased mortality. Collectively, these data suggest that sex differences in HIV-related morbidity observed in this study are not biologically based but are the result of socioeconomic conditions specific to the United States.
The increased HIV-associated morbidity in nonwhite women in this study could not be explained by differential effectiveness of ART, since there was no evidence of race-sex differences in responses to ART. Although IDU and later initiation of ART accounted for some events in nonwhite women, substantial unexplained morbidity remained. Region of residence combined with race emerged as a strong predictor of HIV-related morbidity, with 2.1–4.6-fold higher HIV-related morbidity in nonwhites from the South compared to other groups. Since a greater proportion of nonwhite women lived in the South, this likely accounts for their substantially worse clinical outcomes. Individuals from the southern United States, particularly blacks from this region, have significantly higher mortality from all causes compared to the general US population, and these disparities are related to socioeconomic factors [30]. Mechanisms underlying associations between socioeconomic status and health are thought to include access to health care, health behaviors, lifestyle, and environmental exposures [31]. Unfortunately, lack of information in the present study about these factors prevents further insights into the observed disparities in clinical outcomes. Nevertheless, the findings from this study, in conjunction with data on regional disparities in health outcomes in the United States, suggest that socioeconomic circumstances of nonwhite women in the South are a major determinant of elevated morbidity in this group.
This analysis has several limitations. Similar to other HIV seroconverter studies [32], bias was introduced by recruitment techniques that relied heavily on screening subjects who were viewed as at risk for HIV infection. Consequently, the cohort contained disproportionately more white, homosexual men and fewer women and nonwhites compared to the general population of persons with newly acquired HIV infection in North America. Furthermore, HIV seroconverters from the western United States were overrepresented, whereas those from the northeastern and southern United States were underrepresented. Because subjects received regular follow-up through the study, and because many of the recruitment sites concurrently offered ART in the context of clinical trials, subjects enrolled in the study may have initiated ART earlier and had better clinical outcomes compared to the general population of seroconverters in North America. Finally, outcome data were largely self-reported. Nevertheless, it is unlikely that these factors could account for the disparities in clinical outcomes relating to sex, race, and region of residence observed in this study. Indeed, this study likely underestimates sex differences in HIV-related morbidity in North America because HIV-infected women from the South, who experienced the worst clinical outcomes and constitute half of HIV-infected American women [33], were underrepresented in the cohort.
Although the introduction of potent ART in the mid-1990s initially led to dramatic improvements in survival of HIV-infected individuals [34–36], morbidity and mortality from HIV infection in the United States have not substantially declined in the past 7 years [37] and continue to be highest among black women and men [38, 39]. Major barriers to further improvements in outcomes are thought to include delays in diagnosis and limited access to medical care, leading to delays in initiation of ART and other HIV-associated therapies [40, 41]. The present study provides indirect evidence that primary HIV infection is less frequently diagnosed in nonwhites, as they are underrepresented in the study. This study also demonstrates that nonwhite women and men initiate ART later than whites. Importantly, however, the data from this study suggest that differential use of ART cannot entirely explain elevated morbidity in nonwhites and women, but that socioeconomic factors associated with residence in the South and nonwhite race may play a role. These results are consistent with findings from other studies from the United States and Europe that access to medical care explains only part of the socioeconomic variability in health outcomes [30, 42, 43]. A better understanding of the determinants of poor health outcomes for HIV-infected individuals in the South is critical to devising effective interventions. Strategies aimed at improving clinical outcomes among HIV-infected individuals in the southern United States, particularly nonwhites, are urgently needed to reduce HIV-related morbidity and mortality in North American women and men.
Funding
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant numbers AI41532, AI41531, AI41535, AI43638, AI41535 AI57005, AI41536, AI43271, AI41530, AI41534, AI52403, and AI57005).
Acknowledgments
We acknowledge the participating sites: United States: University of Minnesota, Minneapolis, MN; University of Cincinnati, Cincinnati, OH; Northwestern University, Chicago, IL; Rush University, Chicago, IL; SUNY Downstate, Brooklyn, NY; Columbia University, NY, NY; Fenway Community Health, Boston, MA; Community Research Initiative of New England, Boston, MA; Brigham and Women’s Hospital, Boston, MA; Beth Israel Medical Center, Boston, MA; University of Pennsylvania, Philadelphia, PA; Vanderbilt University, Nashville, TN; Duke University Medical Center, Durham, NC; University of Rochester, Rochester, NY; University of Texas Southwestern, Dallas, TX; University of Colorado Denver, Aurora, CO; Aaron Diamond AIDS Research Center, Rockefeller University, New York, NY; University of California, San Diego, San Diego, CA; Cedars-Sinai, Los Angeles, CA; University of California, San Francisco, San Francisco, CA; Los Angeles Biomedical Research Institute at Harbor–University of California, Los Angeles Medical Center, Torrance, CA; University of Washington Primary Infection Clinic, Seattle, WA; Johns Hopkins University, Baltimore, MD; University of Alabama, Birmingham, AL; Partners AIDS Research Center, Boston, MA; Canada: McGill University Health Centre, Montreal; University of British Columbia, Vancouver; Australia: The Centre Clinic, St Kilda, VIC; Prahran Market Clinic, St Kilda, VIC; Carlton Clinic, Carlton, VIC; Taylor Square Private Clinic, Darlinghurst, NSW; 407 Doctors, Darlinghurst, NSW; Holdsworth House General Practice, Darlinghurst, NSW; St Vincent’s Hospital, Darlinghurst, NSW; The Alfred Clinic, Prahran, VIC; Melbourne Sexual Health Clinic, Carlton, VIC; AIDS Research Initiative, Darlinghurst, NSW; Brazil: Centro de Referencia Estadual de AIDS, Salvador, Bahia.
References
- 1.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–80. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 2.Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection. Los Angeles County primary HIV infection recruitment network. Ann Intern Med. 2001;134:25–9. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 3.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 5.Hubert JB RC, Boufassa F, Delfraissy JF, Meyer L. Gender, disease progression and response to HAART. Int Conf AIDS. 2002 abstract no. ThOrC1448. [Google Scholar]
- 6.Delmas MC, Jadand C, De Vincenzi I, et al. Gender difference in CD4+ cell counts persist after HIV-1 infection. SEROCO Study Group. AIDS. 1997;11:1071–3. [PubMed] [Google Scholar]
- 7.Touloumi G, Pantazis N, Babiker AG, et al. Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS. 2004;18:1697–705. doi: 10.1097/01.aids.0000131395.14339.f5. [DOI] [PubMed] [Google Scholar]
- 8.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 10.Prins M, Robertson JR, Brettle RP, et al. Do gender differences in CD4 cell counts matter? AIDS. 1999;13:2361–4. doi: 10.1097/00002030-199912030-00007. [DOI] [PubMed] [Google Scholar]
- 11.Smith PR, Sarner L, Murphy M, et al. Ethnicity and discordance in plasma HIV-1 RNA viral load and CD4+ lymphocyte count in a cohort of HIV-1-infected individuals. J Clin Virol. 2003;26:101–7. doi: 10.1016/s1386-6532(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 12.Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–8. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Apuzzo LG, Vaida F, Gallant JE, et al. Tolerability and efficacy of PI versus NNRTI-based regimens in subjects receiving HAART during acute or early HIV infection. J Acquir Immune Defic Syndr. 2009;50:267–75. doi: 10.1097/QAI.0b013e3181963ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HIV/AIDS Surveillance—General Epidemiology (through 2007) Vol. 2010. Atlanta, GA: Centers for Disease Control Prevention; 2010. [Google Scholar]
- 15.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 17.Gray RH, Li X, Wawer MJ, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004;189:1209–15. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 18.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-Infected persons in care. J Infect Dis. 2009;199:991–8. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNaghten AD, Hanson DL, Dworkin MS, Jones JL. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 22.Giordano TP, White AC, Jr, Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 23.McNaghten AD. The XV International AIDS Conference. Bangkok, Thailand: 2004. Gender disparity in HIV treatment and AIDS opportunistic illnesses (OI) [Google Scholar]
- 24.Jarrin I, Geskus R, Bhaskaran K, et al. Gender differences in HIV progression to AIDS and death in industrialized countries: slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168:532–40. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 25.Changes in the uptake of antiretroviral therapy and survival in people with known duration of HIV infection in Europe: results from CASCADE. HIV Med. 2000;1:224–31. doi: 10.1046/j.1468-1293.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 26.Murri R, Lepri AC, Phillips AN, et al. Access to antiretroviral treatment, incidence of sustained therapy interruptions, and risk of clinical events according to sex: evidence from the I.Co.N.A. Study. J Acquir Immune Defic Syndr. 2003;34:184–90. doi: 10.1097/00126334-200310010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Cozzi Lepri A, Pezzotti P, Dorrucci M. Phillips AN, Rezza G. HIV disease progression in 854 women and men infected through injecting drug use and heterosexual sex and followed for up to nine years from seroconversion. Italian Seroconversion Study. BMJ. 1994;309:1537–42. doi: 10.1136/bmj.309.6968.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woldemichael G, Christiansen D, Thomas S, Benbow N. Demographic characteristics and survival with AIDS: health disparities in Chicago, 1993-2001. Am J Public Health. 2009;99:S118–23. doi: 10.2105/AJPH.2007.124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15:1115–23. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3:e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 32.Connick E, MaWhinney S, Wilson CC, Campbell TB. Challenges in the study of patients with HIV type 1 seroconversion. Clin Infect Dis. 2005;40:1355–7. doi: 10.1086/429339. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Fact sheet: MMWR Analysis Provides New Details on HIV Incidence in U.S. Populations. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/mmwr-incidence.pdf. Accessed March 2009. [Google Scholar]
- 34.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 35.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–9. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 36.Ewings FM, Bhaskaran K, McLean K, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS. 2008;22:89–95. doi: 10.1097/QAD.0b013e3282f3915e. [DOI] [PubMed] [Google Scholar]
- 37.HIV AIDS in the United States: a picture of today’s epidemic. http://www.cdc.gov/hiv/topics/surveillance/united_states.htm. Centers for Disease Control and Prevention. 2008. [Google Scholar]
- 38.AIDS surveillance—general epidemiology (through 2006) http://www.cdc.gov/hiv/topics/surveillance/resources/slides/epidemiology/index.htm. Centers for Disease Control and Prevention. 2008. [Google Scholar]
- 39.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America–forgotten but not gone. N Engl J Med. 2010;362:967–70. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 41.Fleishman JA, Gebo KA, Reilly ED, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000-2002. Med Care. 2005;43:III 40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 42.Berkman L, Epstein AM. Beyond health care–socioeconomic status and health. N Engl J Med. 2008;358:2509–10. doi: 10.1056/NEJMe0802773. [DOI] [PubMed] [Google Scholar]
- 43.Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–81. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]