Abstract
Background. It is well established that the prevalence of human papillomavirus (HPV) infection is increased among human immunodeficiency virus (HIV)–positive individuals, but the temporal relationships between these infections are unclear.
Methods. During a South African cervical cancer screening trial, 5595 women 35–65 years of age were followed up for 36 months; 577 women were HIV positive at enrollment, HIV seroconversion occurred in 123 women, and 4895 women remained HIV negative throughout. Tests for high-risk HPV DNA and cytology were performed on cervical samples, and a colposcopy/biopsy was performed at each visit. The effects of early HIV infection on the risk of HPV infection and HPV-related disease were evaluated.
Results. Among seroconverters, HPV infection prevalence was 20.3% before seroconversion, 23.6% at seroconversion (P = .4), and 49.1% after seroconversion (P = .01). Seroconverters had significantly lower HPV infection prevalence than women with prevalent HIV infection before and at seroconversion (41.8% and 45.9%, respectively) but had similar HPV infection prevalence to women with prevalent HIV infection after seroconversion (49.4%). HIV seroconversion was associated with newly detected HPV infection (adjusted hazard ratio [AHR], 4.02; 95% confidence interval [CI], 2.26–7.13) and increased risk of low-grade cytological abnormalities (AHR, 2.53; 95% CI, 1.16–5.51) compared with HIV-negative women.
Conclusion. Detection of HPV infection increases rapidly within the first years after HIV seroconversion, suggesting that mucosal immune dysfunction occurring at an early stage of HIV infection may influence HPV-related diseases.
It is well established that individuals infected with human immunodeficiency virus (HIV) have an increased risk of human papillomavirus (HPV) infection and HPV-related disease, including cervical intraepithelial neoplasia (CIN) and cervical cancer [1–3]. Among HIV-infected women, the prevalence of HPV infection is higher [3–8], new detection of HPV infection is greater [6, 8], and HPV infections are more likely to persist [4, 8]. Markers of more advanced HIV disease, including low CD4 cell counts and higher plasma HIV RNA levels, are usually associated with higher risks of HPV infection and HPV-related disease [9, 10]. However, because most studies have not considered the duration of HIV infection, the temporal relationship between these 2 infections is not well defined. Studies of newly HIV-infected persons would be informative to better delineate the interactions between these 2 viruses over time.
Primary HIV infection is characterized by an acute symptomatic period in most incident infections [11–13]. Viremia is high and there is a transient drop in CD4 cell counts. Thereafter, viremia declines to reach its individualized set point and CD4 cell counts return to normal levels [14]. The progression of HIV infection occurs over the ensuing years as most clearly tracked by CD4 cell counts. There is now growing appreciation of the damage done to the host by HIV during the initial stages of infection [15]. Animal models have demonstrated a rapid and almost complete loss of CD4+ T cells from the intestinal lamina propria and genital tissues during acute simian immunodeficiency virus (SIV) infection in rhesus macaques [16–19]. Consistent phenomena have been observed among HIV-infected humans, and there is an acute and apparently irreversible reduction in CD4+ cells in the gut mucosa [20]. Despite the significant loss in mucosal CD4+ cells shortly after infection, the clinical impact of this acute reduction is unclear. Because mucosal immune responses are believed to play an important role in regulating anogenital HPV infections, we hypothesized that early damage to the mucosal immune response soon after HIV infection would result in increased detection of HPV infections.
Here we present data collected as part of a prospective cervical cancer screening trial conducted in Cape Town, South Africa. During this trial, we identified women in whome HIV seroconversion had occurred during follow-up. As HPV infection and cervical disease were also measured in these women during follow-up, it allowed for the evaluation of the risk of cervical HPV infection and cytologic abnormalities occurring among women with incident HIV infection.
METHODS
Cervical Cancer Prevention Trial
As part of a cervical cancer prevention trial, 6555 black women aged 35–65 years were recruited in Khayelitsha, Cape Town, South Africa, between June 2000 and December 2002 [21]. All women were screened at enrollment with cytology (Papanicolaou tests), tests for cervical high-risk HPV DNA, and visual inspection with acetic acid (VIA). On a follow-up visit 2–6 d later, the participants were randomized to 1 of 3 study arms: (1) for cryrotherapy if the baseline HPV DNA test was positive; (2) for cryotherapy if the baseline VIA test was positive; or (3) to a control group of delayed treatment, among which cryotherapy was not undertaken regardless of baseline HPV and VIA results. All women were followed up, and at 6 months a purposively selected sample of about two-thirds of the participants (all women who were HPV-positive or VIA-positive at baseline during all years of the study, and all women who were negative on both tests at baseline but who enrolled during 2002) were enrolled in extended follow-up 12, 24, and 36 months postrandomization. At these follow-up visits, cytology and HPV DNA testing were repeated, and all participants underwent colposcopy with biopsy if indicated. HIV tests were performed at all visits. When CIN of grade 2 or higher (CIN2+) was diagnosed by biopsy in a participant in any group, she was treated using the loop electrosurgical excision procedure (LEEP; or large loop excision of the transformation zone [LLETZ]) and exited the study. All participants provided written informed consent. A data safety monitoring committee monitored the trial during the fieldwork phase. A detailed description of the trial can be found elsewhere [21, 22].
Study Cohorts Stratified by HIV Status
During this trial, we identified 170 women who were HIV seronegative at enrollment and who HIV seroconverted during the follow-up period. Because cryotherapy interacts with HPV infection [23], we restricted our analysis to participants who did not undergo cryotherapy. There were 949 participants who underwent cryotherapy by the study design of the original trial. The 5595 untreated women were categorized into 3 cohorts based on their HIV serostatus: (1) incident HIV infection cohort (n = 123)—women who were HIV seronegative at enrollment and who seroconverted during follow-up; (2) prevalent HIV infection cohort (n = 577)—women who were HIV seropositive at enrollment; and (3) HIV-negative control cohort (n = 4895)—women who remained HIV seronegative through the study. This study took place between 2000 and 2005, at which time antiretroviral therapy was not available in the public sector in the communities where the study took place.
Laboratory Tests and Clinical Procedures
All participants received counseling for confidential HIV testing and anonymous HIV testing at each visit. HIV testing was performed using the Abbott HIV 1/2g 0 kit on the Abbott AXSYM system (Abbott Laboratories). Positive results were confirmed using the Vironsticka HIV uniform 2 plus 0 kit (Organon Teknika). Cervical samples (ThinPrep; Cytyc) were collected at each visit for HPV DNA testing and cytology classification. Hybrid Capture 2 DNA assay (Qiagen) and probes for 13 high–carcinogenic risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) were used for HPV testing. Samples were considered positive for high-risk HPV DNA if ≥1 pg/mL HPV was detected. HPV DNA–positive samples collected at baseline, 6 months, and 12 months in the control group and HPV-and-treat group in which sufficient material remained were genotyped to determine which specific high-risk HPV type was present by use of linear array on the prototype Line Blot HPV assay (Roche Molecular Diagnostics). Liquid-based cytology was done and classified based on the 1991 modification of the Bethesda System [24]. At baseline, cervical samples were also tested for Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas infections (Hybrid Capture for CT/GC; Qiagen). At each follow-up visit, a colposcopy was done and any abnormal lesions were biopsied. All participants also had endocervical curettage (ECC) samples collected. Histologic diagnoses were reached based on an adjudicated and consensus review of biopsy and ECC results by a gynecologic pathologist (T.C.W.) at Columbia University as described elsewhere [21, 22].
Statistical Analysis
Among participants with incident HIV infection, the HPV prevalence at the visit in which HIV seroconversion was detected was compared with the HPV prevalence at the visit prior to and following seroconversion using the McNemar test. At the visit, the HPV prevalence in the incident HIV infection cohort was calculated before, at, and after seroconversion and was compared with the prevalent HIV-infected and HIV-negative cohorts at comparable time points (0–24 months for the visit before HIV seroconversion, 6–36 months for the visit at HIV seroconversion, and 12–36 months for the visit after HIV seroconversion). Logistic regression was done using a generalized estimating equation (GEE) with independent correlations to adjust for within-subject correlation. Multivariate models were fit to adjust for potential confounders such as age, smoking, marital status, having sex before 16 years of age, having ≥5 lifetime sex partners, having ≥2 sex partners during the previous month, presence of Trichomonas vaginalis, and infection with N. gonorrhoeae or C. trachomatis at baseline. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to describe differences in HPV prevalence between the cohorts.
To determine the HPV type present, the first available type pertinent to the time point of interest was selected. Each high-risk type was reported, and HPV types were grouped into clade α6 (HPV types 51, 56, and 66), clade α7 (HPV types 18, 39, 45, and 59), and clade α9 (HPV types 16, 31, 33, 35, 52, and 58) [25], and classified as infection with multiple types or a single type of HPV. For comparisons of proportions, χ2 tests were used.
Longitudinal analyses were done using the Kaplan-Meier method and Cox proportional hazards model. First, among women who were HPV negative at the baseline, the cumulative detection of new HPV infections (HPV incidence) was compared between the 3 study cohorts and tested using log-rank tests. Next, only among women who were HIV negative at enrollment (prevalent HIV-positive women were excluded), HIV positivity was included as a time-dependent variable to evaluate seroconversion as a risk factor for a series of HPV-related conditions, including newly detected HPV infections, HPV clearance of prevalent HPV infections, cytological abnormalities, and biopsy-confirmed cervical disease. Cox regression models were fit and analyses were adjusted for the potential confounders listed above. Crude and adjusted hazard ratios and 95% CIs were reported.
In the original trial, women received LEEP and exited the study if CIN2+ was diagnosed. These women were more likely to be HPV positive and to have cytological abnormalities than women without CIN2+. To evaluate the effect of this potential bias, we performed a sensitivity analysis, including women with CIN2+, while imputing them as HPV positive or with positive high-grade squamous intraepithelial lesion cytology results after their censor date. All analyses were performed using SAS software (version 9.2; SAS Institute).
RESULTS
HIV Infection Incidence and Prevalence in Study Population
At enrollment, 782 of 6555 participants were HIV seropositive (ie, HIV prevalence of 11.9% in this population). During the follow-up period, 170 participants who were HIV seronegative at enrollment converted to HIV seropositive. The cumulative HIV incidence was 1.65% by 12 months, 3.35% by 24 months, and 7.04% by 36 months. Of the incident infections, 32.9% were first detected as HIV seropositive at 6 months, 10.6% at 12 months, 27.7% at 24 month, and 28.8% at 36 months. The mean time between the visit before and at HIV seroconversion was 351 d, and the mean time between the visit after and at HIV seroconversion was 315 d.
After restricting the analysis to participants who did not receive cryotherapy, those who had incident HIV infection had similar characteristics to those with prevalent HIV infection (Table 1). Compared with the HIV-negative cohort, those with incident HIV infection were younger (mean age, 41.5 years with incident HIV infection vs 44.0 years for the HIV-negative cohort), were less likely to be married (34.1% vs 54.5%), were more likely to start sexual intercourse before 16 years of age (45.5% vs 33.2%), and were more likely to have >5 lifetime sex partners (40.7% vs 31.6%). They were also more likely to use contraceptive methods and less likely to have >5 live births. Participants with prevalent and incident HIV infection were more likely to have C. trachomatis or N. gonorrhoeae infections, or cytologic abnormalities at baseline, compared with HIV-negative women. The differences between cohorts were similar if the treated women were included (data not shown).
Table 1.
Characteristics of Participants in the HIV-Negative, Incident HIV Infection, and Prevalent HIV Infection Cohorts at Enrollment
| No. (%) of participants |
|||
| Characteristic | HIV-negative cohort (n = 4895) | Incident HIV infection cohort (n = 123) | Prevalent HIV infection cohort(n = 577) |
| Age, years, mean (SD) | 44.0 (7.3) | 41.5 (6.2) | 40.5 (5.9) |
| Age, years 35–39 40–49 50–65 |
1714 (35.0)2103 (43.0)1078 (22.0) | 60 (48.8)a50 (40.6)13 (10.6) | 340 (58.9)b188 (32.6)49 (8.5) |
| Education level No school Some primary school Some middle school Middle school graduate |
487 (10.0)1848 (37.7)1924 (39.3)636 (13.0) | 13 (10.6)43 (35.0)48 (39.0)19 (15.5) | 30 (5.2)b196 (34.0)229 (39.7)122 (21.1) |
| Currently employed | 1235 (25.2) | 23 (18.7) | 140 (24.3) |
| Married | 2670 (54.5) | 42 (34.1)a | 180 (31.2)b |
| Age <16 years at first sexual intercourse | 1626 (33.2) | 56 (45.5)a | 225 (39.0)b |
| ≥5 lifetime sex partners | 1549 (31.6) | 50 (40.7)a | 281 (48.7)b |
| ≥2 sex partners during previous month | 57 (1.2) | 3 (2.4) | 17 (2.9)b |
| Current smoker | 354 (7.2) | 11 (8.9) | 49 (8.5) |
| Current contraceptive user Injectable Oral |
682 (13.9)81 (1.6) | 31 (25.2)a5 (4.1)a | 107 (18.5)b15 (2.6) |
| No. of live births None1–4 ≥5 |
177 (3.6)3057 (62.5)1661 (33.9) | 5 (4.1)a90 (73.2)28 (22.7) | 27 (4.7)b456 (79.0)94 (16.3) |
| Presence of Trichomonas vaginalis | 505 (10.3) | 12 (9.8) | 73 (12.7) |
| Chlamydia trachomatis or Neisseria gonorrhoeae infection | 208 (4.3) | 10 (8.1)a | 47 (8.1)b |
| Cytology ASCUS+ | 427 (8.7) | 17 (13.8)a | 98 (17.0)b |
NOTE. Data are no. (%) of participants, unless otherwise indicated. ASCUS+, positive atypical squamous cells of undetermined significance; HIV, human immunodeficiency virus; SD, standard deviation.
P < .05 comparing incident HIV-infected women with HIV-negative women.
P < .05 comparing prevalent HIV-infected women with HIV-negative women.
HPV Infection Prevalence Before and After HIV Seroconversion
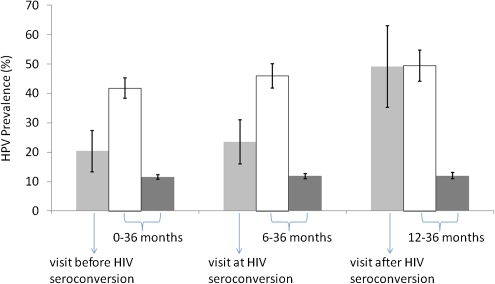
HPV infection prevalence in the incident HIV infection cohort was similar at the visit in which seroconversion was detected and at the visit before HIV seroconversion was detected (23.58% and 20.33%, respectively; P = .45) but increased to 49.09% at the first visit after HIV seroconversion was detected (P = .01) (Figure 1). HPV prevalence in the prevalent HIV infection cohort was 41.78%, 45.94%, and 49.43% at the visits equivalent to the before, at, and after HIV seroconversion time points, respectively. HPV infection prevalence among the HIV-negative cohort remained at a lower level at the visits equivalent to the before, at, and after HIV seroconversion time points (11.61%, 11.90%, and 12.00%, respectively).
Figure 1.
Impact of HIV seroconversion on HPV infection prevalence. This bar chart shows the HPV infection prevalence (with 95% confidence interval [CI]) in participants with incident HIV infection at the visit before, at, and after HIV seroconversion, and in those with prevalent HIV infection and no HIV infection at the equivalent visits. The light gray bars represent the HPV infection prevalence for participants with incident HIV infection; the white bars are for those with prevalent HIV infection; and the dark gray bars are for HIV-negative participants. The error bars represent the 95% CIs.
After adjusting for age, smoking, marital status, having sex before 16 years of age, having ≥5 lifetime sex partners, having ≥2 sex partners during the previous month, presence of T. vaginalis, infection with N. gonorrhoeae, or infection with C. trachomatis, participants in the incident HIV infection cohort were ∼2 times more likely to have HPV infection than those in the HIV-negative cohort at the visits equivalent to the before and at HIV seroconversion time points (adjusted odds ratio [AOR], 1.57; 95% CI, 1.08–2.27; and AOR, 2.09; 95% CI, 1.33–3.27; respectively), but were >5 times more likely to have HPV infection than those in the HIV-negative cohort at the visit after HIV seroconversion (AOR, 5.76; 95% CI, 3.05–10.85) (Table 2). At all 3 equivalent time points, participants in the prevalent HIV infection cohort had significantly higher HPV infection prevalence than those in the HIV-negative cohort (AOR, 4.86; 95% CI, 4.05–5.84; AOR, 5.47; 95% CI, 4.48–6.68; and AOR, 5.55; 95% CI, 4.03–7.64; respectively).
Table 2.
Odds Ratios for the Association Between Human Immunodeficiency Virus (HIV) Infection Status and Human Papillomavirus (HPV) Infection Prevalence
| Odds ratio (95% confidence interval) |
|||
| Visit in relationship to HIV seroconversiona |
|||
| Odds ratio type | Prior to detection | When detected | Following detection |
| Crude | |||
| HIV-negative cohort | Reference | Reference | Reference |
| Incident HIV infection cohort | 2.08 (1.43–3.04) | 2.28 (1.49–3.49) | 6.16 (3.57–10.64) |
| Prevalent HIV infection cohort | 5.46 (4.63–6.43) | 6.29 (5.23–7.57) | 6.39 (4.78–8.55) |
| Adjustedb | |||
| HIV-negative cohort | Reference | Reference | Reference |
| Incident HIV infection cohort | 1.57 (1.08–2.27) | 2.09 (1.33–3.27) | 5.76 (3.05–10.85) |
| Prevalent HIV infection cohort | 4.86 (4.05–5.84) | 5.47 (4.48–6.68) | 5.55 (4.03–7.64) |
NOTE.
For the HIV incident infection cohort, HPV infection prevalence was determined at the visit when HIV seroconversion was detected and the visit prior to and following that visit. For the HIV-negative and prevalent HIV infection cohorts, comparable time points were used.
Adjusted for age, smoking, marital status, having sex before 16 years of age, having ≥5 lifetime sex partners, having ≥2 sex partners during the previous month, presence of Trichomonas vaginalis, infection with Neisseria gonorrhoeae or Chlamydia trachomatis, and baseline HPV status and visual inspection with acetic acid status.
The distribution of specific HPV types among those with high-risk HPV infections differed slightly between HIV-positive and HIV-negative participants. HIV-positive participants were more likely to have HPV types 18, 33, 35, and 66 than HIV-negative participants (P < .05) (Table 3). HIV-positive participants were more likely to have clade α6 infections than those who were HIV negative (24.14% vs 17.38%, respectively; P < .05), as well as infection with multiple types (37.44% vs 16.60%, respectively; P < .05).
Table 3.
Distribution of 13 High-risk Human Papillomavirus (HPV) Types in Human Immunodeficiency Virus (HIV)–Positive and HIV-Negative Women
| HPV genotype | No. (%) of HIV-positive women (n = 201) |
No. (%) of HIV-negative women(n = 512) |
||
| 16 | 29 | (14.29) | 84 | (16.41) |
| 18 a | 34 | (16.75) | 45 | (8.79) |
| 31 | 13 | (6.40) | 40 | (7.81) |
| 33 a | 26 | (12.81) | 35 | (6.84) |
| 35 a | 41 | (20.20) | 69 | (13.48) |
| 39 | 16 | (7.88) | 22 | (4.30) |
| 45 | 25 | (12.32) | 59 | (11.52) |
| 51 | 20 | (9.85) | 40 | (7.81) |
| 52 | 33 | (16.26) | 70 | (13.67) |
| 56 | 13 | (6.40) | 30 | (5.86) |
| 58 | 24 | (11.82) | 60 | (11.72) |
| 59 | 8 | (3.94) | 37 | (7.23) |
| 66 a | 19 | (9.36) | 24 | (4.69) |
| Clade α6a | 49 | (24.14) | 89 | (17.38) |
| Clade α7 | 78 | (38.42) | 159 | (31.05) |
| Clade α9 | 137 | (67.49) | 326 | (63.67) |
| Multiple high-risk HPV types a | 76 | (37.44) | 85 | (16.60) |
NOTE.a P < .05 comparing HIV-infected participants with HIV-negative participants.
Longitudinal Analyses
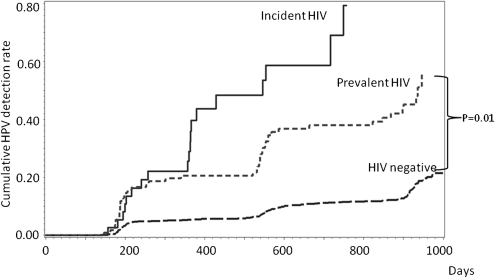
The risk of newly detected HPV infections was compared among the cohorts. Among 94 participants in the incident HIV infection cohort who were HPV negative at the HIV seroconversion visit, the cumulative risk of newly detected HPV infection by 36 months after randomization was 79.34%. In the prevalent HIV infection cohort (n = 297) and HIV-negative cohort (n = 3799), participants who were HPV negative at the 6-month visit had a cumulative risk of newly detected HPV infection by 36 months of 45.14% and 12.79%, respectively. Participants with incident HIV infection had significantly higher cumulative rates of newly detected HPV infection than those with prevalent HIV infection (P = .01) and those who were HIV negative (P < .001) (Figure 2).
Figure 2.
Cumulative Probability of Newly detected HPV infection by study cohort. These Kaplan-Meier curves show the cumulative detection rate of HPV infection among participants who were HPV negative at the time of HIV seroconversion (incident HIV cohort; n = 94) and among those who were HPV negative at 6 months in the prevalent HIV infection group (n = 297) and in the HIV-negative group (n = 3799).
HIV seroconversion, as a time-dependent variable, was associated with a higher risk of newly detected HPV infection and HPV persistence (Table 4). Among participants who were both HIV and HPV negative at enrollment, 92 HIV seroconverted during the study and 4351 did not. The risk of HPV detection was significantly increased among the 92 who seroconverted (AHR, 4.02; 95% CI, 2.26–7.13). Among those who were HIV negative but HPV positive at enrollment, 31 HIV seroconverted and 544 did not. In these participants, HIV seroconversion tended to be associated with a decreased likelihood of clearing HPV infection, although this was not statistically significant (AHR, 0.25; 95% CI, 0.06–1.03).
Table 4.
Time-dependent Associations Between Human Immunodeficiency Virus (HIV) Seroconversion and Human Papillomavirus (HPV)–related Diseases Among a Subset of Participants Who Were HIV Negative at Enrollment
| No. of events/no. of participants |
||||
| Outcome | Participants with incident HIV infection | Participants who remained HIV negative | Hazard ratio (95% CI) | Adjusteda hazardratio (95% CI) |
| HPV infection | ||||
| Newly detected HPVb | 37/92 | 438/4351 | 4.36 (2.48–7.64) | 4.02 (2.26–7.13) |
| HPV clearancec | 22/31 | 319/544 | .28 (0.07–1.15) | .25 (0.06–1.03) |
| Abnormal cytologyd | ||||
| ASCUS+ | 41/99 | 691/4310 | 1.42 (0.67–3.00) | 1.44 (0.68–3.07) |
| LSIL+ | 18/99 | 240/4310 | 2.67 (1.24–5.75) | 2.53 (1.16–5.51) |
| HSIL+ | 2/99 | 73/4310 | 1.25 (0.17–9.12) | 1.03 (0.14–7.58) |
| Cervical neoplasiae | ||||
| CIN1+ | 9/123 | 102/4895 | 1.68 (0.40–7.05) | 1.82 (0.39–8.37) |
| CIN2+ | 1/123 | 65/4895 | N/A | N/A |
NOTE. ASCUS+, positive atypical squamous cells of undetermined significance; CI, confidence interval; CIN1+, cervical intraepithelial neoplasia of grade 1 or higher; CIN2+, cervical intraepithelial neoplasia of grade 2 or higher; HSIL+, positive high-grade squamous intraepithelial lesion; LSIL+, positive low-grade squamous intraepithelial lesion.
Adjusted for age, smoking, marital status, having sex before age 16 years, having ≥5 lifetime sex partners, having ≥2 sex partners during the previous month, presence of Trichomonas vaginalis, infection with Neisseria gonorrhoeae or Chlamydia trachomatis, and baseline HPV status and visual inspection with acetic acid status.
Restricted to participants who were HPV negative at enrollment.
Restricted to participants who were HPV positive at enrollment.
Restricted to participants with a cytology result that was negative for intraepithelial lesion or malignancy at enrollment.
Among all women.
HIV seroconversion was also associated with an increased risk of developing low-grade cytological abnormalities (Table 4). Among participants who had normal cytology at baseline, HIV seroconversion was associated with an increased risk of developing low-grade squamous intraepithelial lesions or more severe lesions (LSIL+) (AHR, 2.53; 95% CI, 1.16–5.51). HIV seroconversion remained significantly associated with LSIL after adjusting for HPV infection (AHR, 2.30; 95% CI, 1.05–5.03). There was no increased risk of high-grade squamous intraepithelial lesions (HSILs) (AHR, 1.03; 95% CI, 0.14–7.58). No significant associations were observed in biopsy-confirmed cervical disease.
In sensitivity analyses evaluating the effects of selective loss of women with CIN2+ because of the study design, no appreciable differences in the results were observed (data not shown).
DISCUSSION
By analyzing data from 123 women with incident HIV infection enrolled in a cervical cancer prevention trial in South Africa, we found that within ∼1 year after HIV seroconversion, HPV infection prevalence more than doubled and reached levels similar to that observed among women with prevalent HIV infection. Low-grade cytological abnormalities were also significantly increased. These findings provide new insights that may help in the understanding of the interactions between HPV and HIV during the early stage of HIV infection.
Our results are consistent with those of a recently published report of 26 young female South African sex workers who HIV seroconverted during a microbicide trial. In that study, the prevalence of HPV infection among HIV seroconverters (20 of 26 women) was significantly higher than that among those who remained HIV negative (22 of 44 women) [26]. In the same cohort, an increase in infection with multiple HPV types was observed shortly after HIV seroconversion [27]. Another group compared the density of HPV type-specific infections among 92 HIV seroconverters and 252 HIV-negative women in Zimbabwe and found that HPV infection density increased significantly within the first 6 months following HIV acquisition (1.96 types per incident event) compared with HIV-negative women (1.44 types per incident event; P < .001) [28]. Not only does our study confirm these observations in older South African women enrolled from the general population, but our large sample size and study design strongly support the hypothesis that HIV seroconversion influences HPV-related diseases at the early stage of HIV infection.
Previous studies have shown strong correlations between markers of systemic immune impairment, such as peripheral CD4+ T cell count and plasma HIV RNA levels, and increased risk of HPV infection [1, 9]. The current study shows that the increase in HPV detection occurs relatively early after HIV acquisition and before systemic immune impairment becomes apparent. Longitudinal analysis indicates that the increased detection of HPV in the year after seroconversion is due to both an increased risk of newly detected HPV (AHR, 4.02; 95% CI, 2.26–7.13) and a decreased likelihood of HPV clearance (AHR, 0.25; 95% CI, 0.06–1.03). Although we did not collect detailed sexual behavior data at all follow-up visits or information on the participants’ sexual partners, it seems unlikely that this rapid increase in HPV infection prevalence from 23.6% (which is already elevated compared with the general population [11.9%]), to 49.1% within 1 year can be explained solely by high-risk behaviors. It is not possible to distinguish reactivation of previous HPV infections from new acquisition of HPV infections. However, given the characteristics of the study population (older women who had a relatively high risk of prior exposure to HPV based on their sexual behavior characteristics at enrollment, such as an early sexual debut age and having multiple sexual partners), it is likely that most of these newly detected HPV infections are due to reactivation. Moreover, if newly detected HPV was acquired at the same time as the HIV infection, the increase in HPV should be detected at the seroconversion visit, which it was not.
We hypothesize that increases in HPV detection within 1 year of HIV seroconversion is caused by the cascade of biological changes that occur soon after HIV infection. One potential mechanism is mucosal immune dysfunction, which is now known to occur early. At the time of acute HIV infection, there is a rapid, dramatic, and persistent depletion of mucosa-associated lymphoid tissue–based memory CD4+ T cells observed in HIV-infected individuals in the gut and genital mucosa [29–32]. The substantial increase in HPV detection that we observed after seroconversion is consistent with this new model of rapidly induced immune dysfunction following HIV infection. Although the changes in mucosal immunity after acquisition of HIV may be of no clinical significance [31], the current study provides evidence of a clinical effect in promoting HPV infection. After HIV infection, depletion of CD4+ T cells in the cervical mucosa could allow latent HPV infection to be activated. At the same time, mucosal immune dysfunction may reduce HPV clearance, resulting in a higher HPV infection prevalence.
There are several limitations to the current analysis that should be considered. First, because of interactions between cryotherapy and HPV infections [23], women who underwent cryotherapy were excluded from this analysis. Since women undergoing cryotherapy were more likely to be HPV infected, the HPV infection prevalence in the overall study population is slightly higher than that presented here. This affects all cohorts equally and should not bias the measures of association. Second, because detailed sexual behavior data were not collected at every visit, we can only adjust for baseline and 6-month sexual behavior. Thus, we cannot rule out the possibility of sudden changes in sexual behavior after seroconversion. Third, we did not collect data on CD4+ T cell counts or viral loads for the HIV-infected participants. As the usual time course of systemic CD4+ T cell changes after seroconversion is well established, it is unlikely that the incident HIV infection cohort would contain an unusually large proportion of women with rapid declines in systemic CD4 cell counts. Our study was conducted in the years before antiretroviral therapy became available in these communities, and it is unlikely that any of the study participants were receiving therapy. Finally, because we only followed up women at 1-year intervals after the first 6 months, we cannot identify the exact time of HIV acquisition or examine the effects during the acute phase after HIV acquisition. Since increases in HPV infection were only detected at the visit following the seroconversion visit, we hypothesize that this rapid rise is secondary to an accumulation of early immunologic effects of HIV infection.
When we compared the distribution of HPV types by HIV status, we found that HIV-infected participants were more likely to have HPV types 18, 33, 35, and 66 and were more likely to have multiple types than HIV-negative participants. This is consistent with a meta-analysis that reported an underrepresentation of HPV type 16, but an elevated representation of HPV types 18, 33, 51, 52, and 58, as well as infection with multiple types among HIV-positive women relative to HIV-negative women [33].
In conclusion, we found a rapid increase in HPV infection among women who acquired HIV during a large, population-based cervical cancer screening trial in South Africa. The increase of HPV detection occurred during the first year after HIV seroconversion, suggesting that mucosal immune dysfunction at an early stage of HIV infection is relevant to enhancing HPV infection. This increase in both HPV infection and low-grade cervical disease early in the course of HIV infection indicates that cervical cancer screening programs should be provided for HIV-infected women at all stages of infection. Consideration should be given to combining cervical cancer screening programs with HIV screening and treatment programs.
Funding
This work was supported by the Bill and Melinda Gates Foundation (grant to the Alliance for Cervical Cancer Prevention); the Cancer Association of South Africa; and the Department of National Health, South Africa.
References
- 1.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17:545–54. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 2.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–6. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh DK, Anastos K, Hoover DR, et al. Human papillomavirus infection cervical cytology in HIV-infected and HIV-uninfected Rwandan women. J Infect Dis. 2009;199:1851–61. doi: 10.1086/599123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC., Jr. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337:1343–9. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 5.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–36. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 6.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 7.Sun XW, Ellerbrock TV, Lungu O, Chiasson MA, Bush TJ, Wright TC., Jr. Human papillomavirus infection in human immunodeficiency virus-seropositive women. Obstet Gynecol. 1995;85:680–6. doi: 10.1016/0029-7844(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 8.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 9.Wright TC. Immunosuppression and the cervix: human immunovirus (HIV) In: Jordan JA, editor. The cervix. 2nd ed. Oxford, UK: Blackwell Publishing Ltd. 2006. pp. 504–17. [Google Scholar]
- 10.Palefsky J. Human papillomavirus infection in HIV-infected persons. Top HIV Med. 2007;15:130–3. [PubMed] [Google Scholar]
- 11.Henrard DR, Daar E, Farzadegan H, et al. Virologic and immunologic characterization of symptomatic and asymptomatic primary HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:305–10. [PubMed] [Google Scholar]
- 12.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–9. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 13.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 17.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–76. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 18.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 19.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider T, Ullrich R, Zeitz M. The immunologic aspects of human immunodeficiency virus infection in the gastrointestinal tract. Semin Gastrointest Dis. 1996;7:19–29. [PubMed] [Google Scholar]
- 21.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 22.Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC., Jr. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer. 2010;102(20):1557–67. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 23.Taylor S, Wang C, Wright TC, Denny L, Tsai WY, Kuhn L. Reduced acquisition and reactivation of human papillomavirus infections among older women treated with cryotherapy: results from a randomized trial in South Africa. BMC Med. 2010;8:40. doi: 10.1186/1741-7015-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA. 1994;271:1866–9. [PubMed] [Google Scholar]
- 25.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Marais D, Carrara H, Kay P, Ramjee G, Allan B, Williamson AL. The impact of the use of COL-1492, a nonoxynol-9 vaginal gel, on the presence of cervical human papillomavirus in female sex workers. Virus Res. 2006;121:220–2. doi: 10.1016/j.virusres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Marais DJ, Carrara H, Ramjee G, Kay P, Williamson AL. HIV-1 seroconversion promotes rapid changes in cervical human papillomavirus (HPV) prevalence and HPV-16 antibodies in female sex workers. J Med Virol. 2009;81:203–10. doi: 10.1002/jmv.21343. [DOI] [PubMed] [Google Scholar]
- 28.Nowak RG, Gravitt PE, Gange SJ, et al. 25th International Papillomavirus Conference. Malmo, Sweden: Blackwell; 2009. Risk of HPV immediately after HIV acquisition among Zimbabwean women. [Google Scholar]
- 29.Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nat Immunol. 2005;6:430–2. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 30.Veazey RS, Lackner AA. HIV swiftly guts the immune system. Nat Med. 2005;11:469–70. doi: 10.1038/nm0505-469. [DOI] [PubMed] [Google Scholar]
- 31.Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008;10:36–46. [PubMed] [Google Scholar]
- 32.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–61. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford GM, Goncalves MA, Franceschi S. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20:2337–44. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]