Abstract
A double-blinded, controlled study of vaccination of untreated patients with chronic human immunodeficiency virus type 1 (HIV-1) infection with 3 doses of autologous monocyte-derived dendritic cells (MD-DCs) pulsed with heat inactivated autologous HIV-1 was performed. Therapeutic vaccinations were feasible, safe, and well tolerated. At week 24 after first vaccination (primary end point), a modest significant decrease in plasma viral load was observed in vaccine recipients, compared with control subjects (P = .03). In addition, the change in plasma viral load after vaccination tended to be inversely associated with the increase in HIV-specific T cell responses in vaccinated patients but tended to be directly correlated with HIV-specific T cell responses in control subjects.
Clinical trial.gov NCT00402142
At least 8 dendritic cell (DC) immunotherapy clinical trials for human immunodeficiency virus type 1 (HIV-1) infection in humans have been published [1–8]. Most of them found that DC immunotherapy elicits some degree of immunological response. In the present double-blinded, controlled, clinical trial, we vaccinated untreated patients with HIV-1 infection with monocyte-derived dendritic cells (MD-DCs) pulsed with heat-inactivated autologous virus.
PATIENTS AND METHODS
Chronically HIV-1 infected patients with baseline CD4+ T lymphocyte counts >450 cells/mm3, nadir CD4+ T cell counts >350 cells/mm3, and plasma viral loads >10,000 HIV-1 RNA copies/mL were enrolled. Patients had not received combination antiretroviral therapy (cART) for at least the 2 years before enrollment. The coprimary end points were safety, change in plasma viral load at week 24, and proportion of patients with a decrease in plasma viral load of at least .5 log10 copies/mL at week 24. Secondary end points were plasma viral load response at weeks 16 and 48, proportion of patients who started therapy (cART) based on a CD4+ T cell count <300 cells/mm3 in 2 determinations separated by at least 1 month, and changes in CD4+ T cell count and HIV-1–specific immune responses.
Untreated chronically HIV-infected patients were randomized in a double-blind protocol to receive 3 doses of vaccine subcutaneously separated by 2 week intervals (weeks 0, 2, and 4) with at least 8 × 106 MD-DCs pulsed with heat-inactivated autologous virus (109 RNA copies/dose; DC-HIV group) or with nonpulsed 8 × 106 MD-DCs (DC-control group), according to the same schedule. Patients were followed up for 48 weeks after the first dose of vaccine (week 0). All the immunologic and virologic determinations were performed in a blinded fashion. The study was explained to all patients in detail, and all gave written informed consent. The study was approved by the institutional ethical review board and by the Spanish Regulatory Authorities.
One week before administration of each dose of vaccine, plasma monocyte samples were obtained and cultured for 7 days to develop MD-DCs. Virus for pulsing was isolated from subjects and expanded by culture in heterologous peripheral blood monocyte cells at a median of 10 months (interquartile range [IQR], 3–12 months) before the first vaccination. The virus isolates containing culture supernatants were collected and heat-inactivated twice at 56°C for 30 min, concentrated by ultrafiltration (300KDa Vivaspin 20; Sartorious) to a final volume of 1 mL, and stored frozen at −80°C until use for pulsing MD-DC. The final products achieved an HIV-1 concentration >109 copies/mL, a high infectivity reduction after heat-treatment (median, 5.5 log10 copies/mL), and were free from adventitious agents.
Serum neutralizing activity for CXCR4 and CCR5 viruses was measured using a cell-based infectivity assay with recombinant R5 (JR-FL) or X4 (pNL4.3) infectious HIV-1 clones harboring the Renilla luciferase reporter gene, as described elsewhere [9]. Enzyme-linked immunospot (ELISPOT) assays were performed to measure the number of interferon (IFN)–γ-producing peripheral blood mononuclear cells (PBMCs) directed against HIV-1 sequences. In brief, assays were performed with cryopreserved PBMC using 22 pools of peptides, consisting of 15-mers overlapping by 11, grouped in pools of 10–12 peptides each, covering the whole HIV-1 gag, nef, and env-gp41 sequences (kindly provided by ORVACS), as described elsewhere [10]. Lymphoproliferative responses were performed as described elsewhere [11].
The HIV RNA values were log10 transformed before analysis. Measurements were censored after ART initiation in patients who had to start ART according to preestablished criteria. The continuous variables were compared between groups with use of the nonparametric Mann-Whitney U test. The slopes of change in CD4 cells count before vaccination with 3 measurements at 3-month intervals and after vaccination were compared using a mixed linear regression model with random slopes for the time before and after the first vaccination visit and with unstructured matrix. Categorical variables were compared between groups with use of the Fisher exact test. Changes in plasma HIV-1 RNA level and the frequency of total HIV specific T cell responses over the 48 weeks of the trial were analyzed using an area-under-the-curve (AUC) measurement. Spearman rank-order correlations were calculated to assess the correlation between continuous variables.
RESULTS
Characteristics of Study Patients and Adverse Effects of Vaccinations
Twenty-four patients were enrolled. The median age was 40 years (IQR, 34–46 years), and most of the patients (83%) were men. In 67% of patients, the risk factor of HIV infection was men who had sex with men. The median baseline of CD4+ T cell count was 647 cells/mm3 (IQR, 532–776 cells/mm3), and the median plasma viral load was 4.48 log10 copies/mL (IQR, 4.26–4.86 copies/ml. Baseline characteristics of the patients in each arm were well balanced. Two patients in the DC-HIV-1 group were excluded from the analysis (one because of problems with the preparation of the viral stock and the other because of an unexpected decrease in CD4 T cell count; the latter patient was removed before receiving any vaccine).
Three patients in the DC-HIV-1 group and 3 in the DC-control group started cART (at weeks 16, 16, and 36 in the DC-HIV-1 group and at weeks 24, 36, and 36 in the DC-control group) according to protocol-specified criteria. Overall, the vaccine was well tolerated, with asymptomatic enlargement of local lymph nodes seen after vaccination in 3 patients (2 in the DC-HIV-1 group 6–12 h after injections at weeks 0, 2, and 4, and 1 in the DC-control group after injection at week 0) and 1 episode of influenza-like symptoms (in 1 patient in the DC-HIV group at week 0). The enlargement of lymph nodes regressed after 48–72 h without intervention in each instance. Seven patients in the DC-HIV-1 group reported at least 1 adverse event during the follow-up period, compared with 9 patients in the DC-control group. Except for the lymph node enlargement and the episode of influenza-like symptoms, the adverse events were classified as unrelated to vaccination. The DC injections were not associated with any clinical or serologic evidence of autoimmunity (data not shown).
Changes in Viral Load and CD4+ T cell Count after DC Vaccination
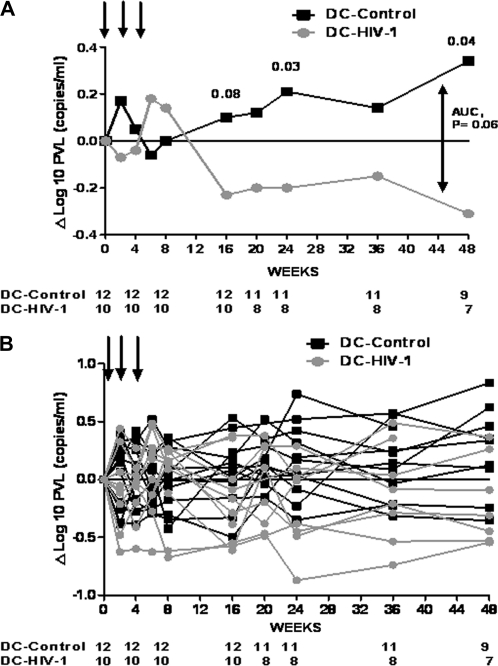
At weeks 16, 24, and 48, there was a median decrease in plasma viral load of .23, .20, and .31 log10 RNA copies/mL in DC-HIV-1 recipients, compared with an increase of .10, .21, and .34 log10 RNA copies/mL in DC-control recipients (P = .08, P = .03, and P = .04, respectively) (Figure 1). The results of the analysis did not change at week 24, when a sensitive analysis using last observation carried forward for the missing viral loads was performed (median change in plasma viral load ) −.20 vs .14 log10 RNA copies/ml in DC-HIV-1 vs DC-control; P = .03). In the patients in the DC-HIV-1 groups at weeks 16, 24, and 48, there was a decrease in plasma viral load of ≥.5 log copies/mL in 3 of 10 (patients 13, 24, and 25), 1 of 8 (patient 24) and 2 of 6 patients (patients 13 and 24), respectively, in the DC-control group, and 0 of 12, 0 of 11, and 0 of 9 patients showed a decrease in plasma viral load of ≥.5 log copies/mL (P = .08, P = .42, and P = .17, respectively). Over a 48-week period, the AUC analysis showed a trend for a plasma viral load difference between the groups (median, .14 [IQR, −.21 to .45] vs −.15 [IQR,−.47 to .27] in DC-control and DC-HIV, respectively; P = .06).
Figure 1.
Change in plasma viral load from baseline during the study period. A, Median values. B, Individual values. Arrows represent vaccinations. Numbers at bottom represent patients at risk. P values of Mann-Whitney U test are shown at weeks 16, 24, and 48. P value of area under the curve (AUC) is also shown.
Absolute values of CD4 and CD8 T cells did not change significantly during or after vaccinations. The median change in CD4+ T –cell count at weeks 16, 24, and 48 was 0, 26, and −76 cells/mm3 in the the DC-HIV-1 group and 14, −80, and −54 cells/mm3 in the DC-control group (P = .31, P = .13, and P = 1.00, respectively). To assess whether the decrease observed in CD4 T cell count after vaccination was higher than would be expected in the absence of vaccination, we compared the slope of change in CD4+ T cell count before and after the vaccinations. We found no statistically significant differences between the slopes (In DC-HIV-1 patients, −8.90 vs −4.92 [P = .16]; in DC-control patients, −6.42 vs −4.92 [P = .88]).
Changes in HIV-1–Specific Responses after DC Vaccination
Neutralizing activity (NA) of serum was analyzed at weeks 0, 8, and 24. The NA titers did not change significantly in DC-HIV-1 patients (median 50% inhibitory concentration [IC50], 1:41, 1:48, and 1:85 at weeks 0, 8, and 24, respectively) or in DC-control recipients (median IC50, 1:37, 1:17, and 1:34 at weeks 0, 8, and 24, respectively [P = .50, P = .78, P = .75, for the comparison between groups at each time point, respectively]).
During vaccinations at weeks 2 and 4, there was a median change in the frequency of total HIV-specific T cell responses (defined as the sum of positive individual responses per patient) of -1249 and -202 SFC/106 PBMC in the DC-HIV-1 vs 35 and -17 SFC/106 PBMC in the DC-control group (P = .13 and P= .96, respectively). After vaccinations, the median change in the frequency of total HIV-specific T cell responses in the DC-HIV-1 group, compared with that found in the DC-control group, was 632 vs -68 SFC/106 PBMCs (P = .82), 772 vs -969 SFC/106 PBMCs (P = .01), 332 vs -33 SFC/106 PBMCs (P = .97), and 1370 vs 766 SFC/106 PBMCs (P = .68) at weeks 16, 20, 24, and 48, repsectively. Over a 48-week period, the AUC analysis of the frequency of total HIV-specific T cell responses showed a trend for a difference between the groups (median, 720 [IQR , 281–1262] vs 1293 [IQR, 892–2120] in the DC-control and DC-HIV, respectively; P = .10). The increase in HIV-specific T cell responses observed after vaccination tended to be inversely associated with the decrease in plasma viral load in vaccinated patients (ρ = -.54; P = .10), although the change in HIV-specific T cell responses tended to be directly correlated with changes in plasma viral load in DC-control recipients (ρ = .53; P = .13) (Figure 2).
Figrue 2.
Correlation between the median change in the HIV-1-specific T cell responses with the median change in plasma viral load in patients in the DC-HIV-1 group and DC-control recipients during the study period. Each dot represents a time point.
No positive HIV-1–specific CD4 lymphoproliferative responses to p24 antigen were observed in any patient.
DISCUSSION
The clinical trials published to date suggest that DC immunotherapy in HIV-1 infection is able to elicit HIV-specific immunological responses [1–8]. However, only 2 of these studies reported virological responses to vaccination. A preliminary noncontrolled, nonrandomized clinical trial reported by Lu et al [2] was conducted in a population of cART-naive patients with chronic HIV-1 infection and used autologous DCs pulsed with whole aldrithiol-2 (AT-2)–inactivated autologous virus [2]. Those authors found that, after administration of 3 vaccine doses, plasma viral load decreased by 90% for at least 1 year in 8 of 18 patients. This decrease in plasma viral load was associated with strong, sustained HIV-1–specific cellular responses. Our group performed an open, randomized (2:1), clinical trial in patients who received ART with use of heat- inactivated autologous virus, finding partial and transient control of viral replication after interruption of ART [3]. In the present study, we found that our autologous DC vaccine HIV-1 elicited only weak HIV-1–specific T cell responses.
In the present study in patients who did not receive ART, we found that this strategy was feasible, safe, and well tolerated. We also found a modest virological response that was maintained for at least 48 weeks in the vaccinated group, as suggested by the AUC analysis. This change in plasma viral load in the vaccinated patients, compared with the control group, was small (range, .30–.60 log10 copies/mL) but was maintained from week 16 to week 48. The small decrease in plasma viral load in vaccinated patients tended to be inversely correlated with a modest increase in HIV-1–specific T cell responses. In contrast, as other authors have reported previously, in the control subjects, an observed increase in HIV-1–specific T cells responses tended to correlate directly with an increase in plasma viral load [11].
Although we repeated the same schedule of vaccinatins and used a comparable dose of immunogen manufactured with a similar procedure in HIV-1–infected patients with the same clinical characteristics, the results of our clinical trial differ from those reported by Lu et al [2]. The reasons for this difference are not clear. Our trial was double blind and randomized, whereas the trial by Lu et al [2] was open and not controlled. Another alternative explanation could be the inactivation method of the virus used for pulsing MD-DCs. AT-2 inactivation preserves the conformational and functional integrity of virion surface proteins, including envelope glycoproteins, which may improve virus uptake by MD-DCs, allowing inactivated virus to enter DCs through a receptor-mediated mechanism [12, 13] and eliciting a potent HLA-1–restricted CTL response [14, 15]. Because to date, AT-2–inactivated virus has not been used in studies approved by European regulatory authorities, we used heat inactivated virus. Inactivation of the virus by heating might have a marked effect on the native structure of viral proteins, jeopardizing the virus uptake, turnover, transfer, and presentation in human dendritic cells.
DCV2/MANON07-ORVACS study group:
HOSPITAL CARLOS III DE MADRID (HCIII): José Miguel Benito, Maria de la O López Vázquez de la Torre, Celia Ballesteros Blanco.
HIVACAT-HOSPITAL CLÍNIC DE BARCELONA (HCB): Teresa Gallart, Felipe García, Nuria Climent, Cristina Gil, Montserrat Plana, Agathe León, Llucia Alós Hernández, Miguel Caballero Borrego, Alba Díaz, Francisco Lomeña, José M Gatell.
HIVACAT-HOSPITAL GERMANS TRIAS I PUJOL. BADALONA (HGTiP): Joan Romeu Fontanillas, Margarita Bofill Soliguer, Christian Brander, Nuria Izquierdo, Judith Dalmau, Javier Martinez-Picado, Bonaventura Bonaventura Clotet.
HOSPITAL GREGORIO MARAÑÓN DE MADRID (HGUGM): Louis Chonco, Nickola Wever, Marjorie Pion, María Jesús Serramía, Miguel Relloso, Paula Ortega, Javier de la Mata, Rafael Gómez, María Ángeles Muñoz-Fernández.
HOSPITAL UNIVERSITARIO REINA SOFÍA DE CÓRDOBA (HRS): José Peña Martínez, Rafael González Fernández, Mario Frias Casas, Barbara Manzanares Martín y Laura Castro.
INSTITUTO DE SALUD CARLOS III. MADRID (ISCIII): Nuria González Fernandez, José Alcamí, Ma Teresa Perez Olmeda, Javier Garcia Perez, Luis Miguel Bedoya del Olmo.
Funding
This study was partially supported by grants: FIS PS09/01297, FIPSE 36750/08, SAF2008-04395, SAF 05/05566, FIPSE 36536/05, FIS 040503, FIS 070291, contract FIS 03/0072, Mutua Madrileña del Automóvil, STREP (UE) Life sciences, genomics and biotechnology for health LSH2005_2.3.0.10, PROFIT (FIT 090100-2005-9), MARATÓ TV3, RIS**, ORVACS***.
Dr Felipe García was recipient of a Research Grant from IDIBAPS****, Barcelona, Spain.
Dr. M. Plana was supported by contract FIS 03/0072 from the Fundació Privada Clínic per a la Recerca Biomédica in collaboration with the Spanish Health Department
Dr. Lifson is supported with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
*FIPSE is a non-profit Foundation including: Spanish Ministry of Health, Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme and Roche).
**Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria (FIS).
***ORVACS: Objectif recherche vaccin sida.
****IDIBAPS: Institut d'Investigacions Biomádiques August Pi I Sunyer The authors do not have a commercial or other association that might pose a conflict of interest.
APPENDIX.
METHODS
Preparation of Inactivated Autologous Viral Stocks for Pulsing Autologous MD-DC
For each subject, one lot of autologous inactivated HIV-1 stock was prepared according to clinical grade good manufacturing practice (cGMP). Briefly, for each infected patient a primary virus isolate was obtained and propagated by means of co-culture of 25 ×x 106 CD4 enriched PBMCs from both the HIV-1 infected subject along with 25 × x 106 CD3-pre-activated CD4-enriched PBMCs obtained from HIV-seronegative unrelated healthy volunteer, during 21 days in X-Vivo 20 media, 10% of AB human serum and IL-2. The virus containing culture supernatants were collected and heat-inactivated twice at 56°C for 30 minutes and concentrated by ultrafiltration (300KDa Vivaspin 20, Sartorious) to a final volume of 1 mL, which was divided into five .2 mL aliquots (contained a median (IQR) Viral Load of 9.1 (7.9–9.77) Log10 HIV-1 RNA copies/mL.) and stored frozen at −80°C until its use for pulsing MDDC. The HIV-1 infectivity reduction after heat-inactivation was assessed by viral titration of ten fold dilutions in PBMC cell cultures for 11 days, by using a modified procedure previously described elsewhere (1). The productive infection was determined by ELISA HIV Ag p24. A high infectivity reduction after heat-treatment (median of 5.5 log10). Adventitious agents were analyzed by cell culture in PBMCs, MRC-5 and VERO cell lines, microbiology cultures, gram and mycoplasma tests. A 515 bp fragment of the protease coding region of the virus present in the patient plasma at the moment of autologous HIV-I isolation sampling and the virus contained in the immunogen were analyzed, No significant differences between were found between both viruses.
Ex vivo Generation of MD-DCs, Their Pulsing With Inactivated HIV-1 and Maturation Under cGMP Conditions, and Immunizations
PBMC were isolated, within 1 hr of the blood extraction, from a 150-mL sample of EDTA-treated venous blood by means of standard Ficoll gradient centrifugation. After washing (4x) in cGMP PBS (Lonza, Walkersville, Maryland, USA), PBMC were resuspended (3 millions/mL) in MD-DC culture medium, which consisted of serum-free X-VIVO15 culture medium (Lonza) supplemented with 1% of heat-inactivated autologous serum (HI-AS), 50 microg/mL of phamaceutical Gentamicin (B/Braun Medical), 2.5 microg/mL of phamaceutical Fungizone (Bristol-MyersSquibb), 1 μM of phamaceutical zidovudine (Retrovir from GlaxoSmithKline), and then, settled down in sterile apyrogenic culture flasks (Corning, NY, USA). The reason for adding zidovudine was to avoid endogenous HIV-1 replication. After 2 h. of incubation (at 37°C in a humidified atmosphere of 5%-C02 in air), non-adherent cells were discarded, and adherent cells (≥95% CD14+) washed (3x) in pre-warmed (at 37 °C) cGMP PBS to eliminate residual contaminating lymphocytes, and then differentiated into immature MDDC in a 5-day culture with a total volume of 18 mL of the above MD-DC medium containing GMP recombinant human (rh) GM-CSF (1000 IU/mL) and IL-4 (1000 IU/mL) (both cytokines from CellGenix, Freiburg, Germany); these cytokines at the indicated concentration were also added at day 2. On day 5 of culture, MD-DCs were collected, washed (3x) with cGMP PBS and then resuspended (≥8 ×x 106) in a final volume of 3 mL of MD-DC medium (with GM-CSF and IL-4 (1000 IU/mL each) and pulsed with heat-inactivated autologous HIV-1 (.2 mL with >108 copies of HIV-1 RNA) for 2-3 hr in a cell incubator. Thereafter, 22 mL of MD-DC medium (also containing GM-CSF and IL-4, 1000 IU/mL each) were added and the cell culture prolonged for 45-46 hr, in the presence of the maturation cocktail, which consisted of GMP rh IL-1 β (300 IU/mL), IL-6 (1000 IU/mL) and TNF-α (1,000 IU/mL) (all cytokines from CellGenix). Afterward, MD-DCs were washed once and resuspended in .5 ml of pharmaceutical saline solution containing 1% of pharmaceutical human Albumin (from Grifols, Spain) and immediately injected subcutaneously in the upper-inner part of both arms (.25 ml each injection). Before, injection a small aliquot of MD-DCs, was used to assess the yield, viability and immunophenotype of MD-DC generated and to perform, Mycoplasma detection and Gram staining Before pulsing, microbiological culture was performed with uniform negative results. The amount (≥8 ×x 106 MD-DCs), purity (≥90%), viability (≥85%), and the maturation status (CD80 and CD83 markers ≥40%), as assessed by flow cytometry, were consistent throughout all the immunizations and fulfilled the predetermined specifications approved by the Spanish Agency of Medications for the current trial with the autologous DC-based vaccine loaded with autologous inactivated HIV-1, considered as an Investigational New Drug. Immunophenotyping of MD-DCs by flow cytometry was done as already reported (2)
Plasma Viral Load
Plasma viral load was analyzed by Versant HIV-1 RNA 3.0 Assay (b-DNA, Siemens Healthcare Diagnostics).
Neutralizing Activity Assay
Serum neutralizing activity for CXCR4 and CCR5 viruses was measured by using a cell-based infectivity assay with recombinant R5 (JR-FL) or X4 (pNL4.3) infectious HIV-1 clones harboring the Renilla luciferase reporter gene. Viral stocks were generated by transfection in 293 T-cells and titrated in the U87CD4 cell line. In neutralization assays a viral dose equivalent to 30.000 RLU/well was incubated for 1 h with serial 4-fold dilutions of sera before infection of either the U87CD4CXCR4 or the U87CD4CCR5 cells. Virus infectivity was determined 48 hours post-inoculation by measuring luciferase activity in cell lysates. HIV neutralization was calculated for each dilution. Using the formula: % inhibition = [(luciferase + Serum)/(luciferase – Serum)] × 100 Sigmoid curves were constructed and IC50 neutralizaiton titers (1/dilution which confers 50% inhibition) were calculated by non-linear regression using GraphPad v3.0 software.
ELISPOT Assay
ELISPOT assays were performed to measure the numbers of IFN-γ producing PBMC directed against HIV-1 sequences. Briefly, assays were performed with cryopreserved PBMC using 22 pools of peptides, consisting of 15-mers overlapping by 11, grouped in pools of 10–12 peptides each, covering the whole HIV-1 gag, nef and env-gp41 sequences (kindly provided by ORVACS). Negative control responses were obtained with unstimulated cells. Positive controls included cells stimulated with phytohemaglutinin A (PHA, Sigma) and a CEF pool containing 32 HLA-class I restricted peptides from CMV, EBV and Flu virus (NIH AIDS Research and Reference Research Program). All time-points for an individual patient were tested in a single assay. The ELISPOT assay was done in 96-well PVDF microtiter plates coated overnight with a mAb specific for human IFN-γ (mAb B-B1, Diaclone, BioNova, Spain). PBMC resuspended in RPMI plus 10% FCS were plated in the presence of different peptide pools (2 microg/ml, final concentration) and incubated overnight at 37°C, 5% CO2. Plates were developed using biotinylated anti-human IFN-γ, streptavidin conjugated to alkaline phosphatase (Amersham Biosciences, UK) and chromogenic substrate BCIP/NBT (Sigma). Spot forming cells (SFC) were counted using an AID ELISPOT reader (Autoimmun Diagnostica GmHb, Germany). Results were expressed as the number of spots-forming cells (SFC) per million of PBMC after substracting the background. A threshold for positive responses on the IFNgamma ELISPOT was set for 50 SFC/106 cells after substracting SFC present in negative control which should be at least <50 SFC/106 PBMC for being considered. Positive responses by ELISPOT were only considered when results were >50 FC/106 PBMC and at least 2 times the background. According to Kinloch-de Loes et al (3), with this methodology the 85% at least of the responding cells are CD8 T cells, but as all studies were conducted with unfractionated PBMC, responses are described as HIV-specific T cells.
Lymphoproliferative Responses
PBMC were washed twice and resuspended at 2 × 106/mL in serum-free medium X-VIVO 10 (BioWhittaker, Maryland). Cultures were plated in triplicate at 2 × 105/well in 7 day assays, in 96 round-bottomed microplates (TPP, Europe). Cells were cultured in the absence or presence of 10μg/ml Pokeweed mitogen (PWM) (Sigma) as a polyclonal stimulus or 5 μg/mL of recombinant HIV-1 proteins gp160, and p24 (Protein Sciences, Meriden, CT). Incorporation of tritium-labeled thymidine during the last 18h of 7-day culture was evaluated using a betaplate scintillation counter (LKB Wallac, Finland). Results were expressed as mean counts per minute (cpm). The stimulation index (SI) was calculated for each sample as: cpm for cells with stimulus/cpm for cells without stimulus. Positive antigen-specific responses were defined as more than 3000 cpm and SI greater than 3.
References
- 1.Kundu SK, Engleman E, Benike C, et al. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998;14:551–60. doi: 10.1089/aid.1998.14.551. [DOI] [PubMed] [Google Scholar]
- 2.Lu W, Arraes L, Ferreira W, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 3.García F, Lejeune M, Climent N, et al. Therapeutic immunization with dendritic cells loaded with inactivated autologous HIV-1 in chronic HIV-1 infected patients. J Infect Dis. 2005;195:1680–5. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 4.Ide F, Nakamura T, Tomizawa M, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78:711–8. doi: 10.1002/jmv.20612. [DOI] [PubMed] [Google Scholar]
- 5.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–92. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi RT, O'Neill D, Bosch RJ, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27:6088–94. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloverpris H, Karlsson I, Bonde J, et al. Induction of novel CD8+ T-cell responses during chronic untreated HIV-1 infection by immunization with subdominant cytotoxic T-lymphocyte epitopes. AIDS. 2009;23:1329–40. doi: 10.1097/QAD.0b013e32832d9b00. [DOI] [PubMed] [Google Scholar]
- 8.Routy JP, Boulassel MR, Yassine-Diab B, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–7. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenyo EM, Heath A, Dispinseri S, et al. International network for comparison of HIV neutralization assays: the NeutNet report. LoS One. 2009;4:e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez A, van der Lubbe N, Sanchez-Palomino S, et al. Phenotypic and functional characteristics of HIV-specific CD8 T cells and gag sequence variability after autologous dendritic cells based therapeutic vaccine. Vaccine. 2009;27:6166–78. doi: 10.1016/j.vaccine.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Plana M, Garcia F, Oxenius A, et al. Relevance of HIV-1-specific CD4+ T helper cell responses during structured treatment interruption in patients with a nadir CD4 T cells above 400/mm3. J Acquir Immune Defic Syndr. 2004;36:791–9. doi: 10.1097/00126334-200407010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Frank I, Piatak M, Jr., Stoessel H, et al. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J Virol. 2002;76:2936–51. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood. 2004;103:2648–54. doi: 10.1182/blood-2003-07-2532. [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Andrieu JM. In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells. J Virol. 2001;75:8949–56. doi: 10.1128/JVI.75.19.8949-8956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buseyne F, Le GS, Boccaccio C, et al. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat Med. 2001;7:344–9. doi: 10.1038/85493. [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1128/aac.37.5.1095. Japour AJ, Mayers DL, Johnson VA, et al. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother 1993;37:1095-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1371/journal.pone.0009436. Rodriguez-Garcia M, Climent N, Oliva H, et al. Increased alpha-defensins 1-3 production by dendritic cells in HIV-infected individuals is associated with slower disease progression. PLoS One 2010;5:e9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1086/432002. Kinloch-De LS, Hoen B, Smith DE, et al. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis 2005;192:607-17. [DOI] [PubMed] [Google Scholar]