Abstract
Background. Current evidence supporting the effectiveness of influenza vaccine in preventing hospitalizations in older adults is insufficient.
Methods. During 3 influenza seasons, 2006–2009, community-dwelling adults aged ≥50 y hospitalized with respiratory symptoms were prospectively enrolled in this study. We tested nose and throat samples for influenza virus by reverse transcriptase–polymerase chain reaction. We estimated vaccine effectiveness by comparing vaccination status between influenza-positive cases and influenza-negative controls using logistic regression models with propensity score adjustment.
Results. Overall, 450 (59%) of 763 eligible patients were enrolled; 417 (93%) of enrolled patients had adequate respiratory samples, had known influenza vaccination status, and were community-dwelling. The proportions of influenza-positive patients were 8%, 20%, and 6% in the 3 successive seasons. Of 39 influenza-positive participants, 14 (36%) were vaccinated compared with 250 (66%) of 378 influenza-negative controls. Propensity score–adjusted vaccine effectiveness for the 3 seasons combined was 61.2% (95% confidence interval, 17.5%–81.8%).
Conclusion. Overall, in this moderately well-vaccinated population of older adults, laboratory-confirmed influenza virus accounted for 9.3% (95% confidence interval, 6.6%–12.1%) of all respiratory hospitalizations during 3 influenza seasons, and influenza vaccination prevented 61.2% of such hospitalizations.
US public health officials [1] recommend yearly influenza vaccination for all adults aged ≥50 y primarily because of the estimated 300,000 hospitalizations and 23,000 deaths due to influenza in this age group annually [2, 3]. However, the assessment of the actual impact of influenza vaccination on hospitalizations and deaths has been challenging, and some have questioned whether vaccination has any impact on these outcomes at all [4]. To our knowledge, no randomized clinical trials of influenza vaccine have evaluated laboratory-confirmed influenza hospitalization in older adults. Because influenza vaccination is the standard of care for older adults in the United States, enrolling a placebo control group is problematic. Observational studies using large administrative databases have been limited by the use of nonspecific end points to identify influenza-associated hospitalizations. These limitations were highlighted in the 2010 Cochrane review of 68 observational studies and clinical trials of influenza vaccine effectiveness in elderly adults, which concluded that “available evidence is of poor quality and provides no guidance regarding the safety, efficacy or effectiveness of influenza vaccines for people aged 65 years or older” [5].
Given these concerns, we prospectively enrolled hospitalized patients, aged ≥50 y with respiratory symptoms or fever, and we tested them for influenza using highly sensitive reverse transcriptase–polymerase chain reaction (RT-PCR) methods. Among enrolled patients, influenza vaccination status was compared in those with and without laboratory-confirmed influenza to determine vaccine effectiveness for the prevention of hospitalizations. This prospective observational design offered several advantages over prior retrospective observational studies. First, influenza-positive cases were compared with those of influenza-negative controls who were hospitalized for other respiratory illnesses, making controls comparable to cases in their propensity to be hospitalized. Second, our case definition was highly sensitive and specific. All participants had molecular testing for influenza. All cases were laboratory-confirmed as positive, and all controls were confirmed negative using similar molecular techniques. Third, influenza confirmation was blinded to vaccination status. Finally, we included both self-reported and medical record–confirmed vaccinations, because many adults obtain influenza vaccine outside of their usual medical homes [6].
METHODS
Study Design
During 3 influenza seasons, we enrolled adults aged ≥50 y hospitalized with respiratory symptoms or nonlocalizing fever in Davidson County, TN (Nashville). During the 2006–2007 influenza season, enrollment occurred at 1 academic and 1 community hospital [7]; during the 2007–2008 influenza season at 1 academic hospital; and during the 2008–2009 influenza season at 1 academic and 3 community hospitals. The number of participating hospitals was based on yearly availability of research staff and funding. Each hospital individually contributed between 10% and 20% of the market share for pneumonia and influenza hospitalizations in the Davidson County catchment area based on discharge data from all Tennessee hospitals.
Recruitment occurred from November through April, beginning 2 d/week and increasing to 4–5 days per week when influenza virus was identified for 2 consecutive weeks in the academic hospital laboratory. Adults aged ≥50 years residing in the surveillance county, who were admitted during a 24-h surveillance period for each enrollment day, were eligible for enrollment if they reported having any respiratory symptoms (cough, nasal congestion/coryza, dyspnea, or wheezing) or nonlocalizing fever. We used broad eligibility criteria to capture not only influenza-like illness but also complications of influenza such as pneumonia, congestive heart failure, and chronic obstructive pulmonary disease exacerbations. Participants were enrolled without regard to the performance or the results of physician-ordered influenza testing. To increase the generalizability of the study, we made no exclusions based on underlying medical conditions. Institutional Review Board approval was obtained from all participating hospitals.
Demographic and Clinical Information
Questionnaires and medical record data collection instruments were developed to capture age, sex, race, high-risk medical conditions as defined by the Centers for Disease Control and Prevention (CDC) [8], current smoking (self-reported smoking in the previous 6 months), use of specific medications (home oxygen, corticosteroids, or immunosuppressants), influenza vaccination status, clinical symptoms, intensive care unit admission, endotracheal intubation, length of hospital stay, and status at discharge. During the 2008–2009 influenza season, patients or their surrogates completed the Barthel Index, a brief questionnaire about patient functional status centered around 10 activities of daily living, rated from unable to perform task through needing assistance to fully independent, with a score of 100 representing full independence in all activities [9]. We collected discharge diagnoses through medical record review and categorized as any listed diagnosis of pneumonia and influenza (International Classification of Diseases, Ninth edition [ICD-9] codes: 480–487), any listed diagnosis of respiratory and circulatory conditions (ICD-9 codes: 390–519), or other conditions [2].
Verification of Influenza Vaccination of Adults
We asked patients if they had received an influenza vaccine for the current influenza season, and we attempted verification of the vaccination status by contacting the vaccine provider. Provider confirmation of vaccination was considered the gold standard. However, influenza vaccinations from nontraditional providers such as grocery chains and pharmacies were not uniformly verifiable, and self-reported vaccination was accepted in these cases. Vaccination status categories included “unvaccinated” (verified or self-reported as not vaccinated) and “vaccinated” (verified vaccination or self-reported as vaccinated in the absence of confirmatory data). To allow time for a complete immunologic response, patients vaccinated <14 d prior to hospitalization were considered unvaccinated [10].
Laboratory Methods
Nasal- and throat-swab specimens were obtained, placed in lysis buffer, and tested for influenza virus by real-time RT-PCR using primers and probes designed by the CDC (kindly provided by Stephen Lindstrom). Identical primers and probes were previously tested in our laboratory and found to detect virus in symptomatic older patients for up to 14 d of illness [11]. To determine the quality of the specimens obtained, samples were also tested for β-actin (Applied Biosystems) during the 2006–2008 seasons and with RNase P during the 2008–2009 season. If β-actin or RNase P were absent in 3 consecutive tests, we categorized the negative results of RT-PCR testing as indeterminate and excluded them from the analysis. All laboratory testing was completed by staff blinded to participant and vaccine exposure.
Identification of Cases and Controls
Influenza-positive cases were defined as participants with positive results of RT-PCR on duplicate testing. Influenza-negative controls were defined as participants who tested negative for influenza by initial RT-PCR testing and whose samples had evidence of β-actin or RNase P. We excluded from analyses any patient with indeterminate laboratory results, with unknown vaccination status, or who was admitted from a nursing home. Nursing home patients were excluded because the goal of the study was to assess vaccine effectiveness in community-dwelling older adults. For patients with more than 1 admission, only the first influenza-positive admission or the first admission, if none were influenza positive, was included.
Definitions and Covariates
Influenza seasons were defined by the total number of weeks that included all influenza positive specimens from enrolled patients each year. Covariates obtained by self-report or chart review included age in years, sex, race (black or other), current smoking (in the previous 6 months), home oxygen use, underlying medical conditions (diabetes mellitus, chronic heart or kidney disease, cardiovascular disease, asthma, chronic obstructive pulmonary disease, and asplenia (functional or anatomic), immunosuppression (HIV, corticosteroid use, or cancer), timing of admission relative to the onset of influenza season, and the specific influenza season. All covariates were considered as potential confounding variables.
Analyses
Characteristics of eligible enrolled and nonenrolled patients, vaccinated and nonvaccinated patients, and influenza positive cases and influenza negative controls were compared with the use of the Pearson χ2 test for categorical covariates and the Wilcoxon ranked sum test for continuous variables. Vaccine effectiveness estimates were calculated using the formula (1− odds ratio [OR]) × 100% [12]. Adjusted ORs for individual years were calculated to control for potential confounders using logistic regression with the cubic spline function of the propensity score. Propensity score [13] adjustment was used because of the large number of covariates relative to the number of influenza cases and was defined as the inverse logit transformation of the linear predictor derived from the logistic regression model with vaccine status as the outcome. Covariates included all those listed above. Propensity-score models were performed for each year and the overall vaccine effectiveness was calculated using logistic regression with additional covariates indicating individual year. These models showed good discrimination between vaccinated and unvaccinated participants with area under the curve (AUC) values for year 1, 2, and 3 of .682, .704, and .790, respectively. Although self-reported influenza vaccination is considered reliable in adults [14–16], the analysis was repeated excluding all patients for whom we were unable to confirm receipt of influenza vaccine. In addition, sensitivity analyses were performed with trimming of nonoverlapping propensity scores [17] as well as changing the vaccination immune-response period from 2 weeks to 1 week. All analyses were performed using R 2.8.1 (r-project.org).
RESULTS
Patient Characteristics
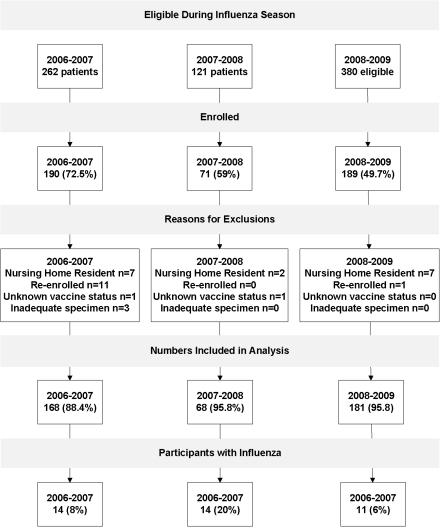
During the 3 influenza seasons, 450 (59%) of 763 eligible patients were enrolled, resulting in 438 unique persons. Of these participants, 417 (93%) had adequate respiratory specimens, had known influenza vaccination status, and were community dwelling; and 264 (63%) reported vaccination at least 2 weeks prior to illness onset (Figure 1). Enrolled patients were younger, more often female, and had cough, fever, wheezing, sore throat, earache, congestive heart failure symptoms, or loss of appetite more commonly than nonenrolled participants (Table 1). Reasons for nonenrollment included patient refusal (21%), surrogate refusal (37%), physician refusal (2%), no surrogate available (20%), patient unavailable or discharged before approached (18%), and no interpreter available (2%).
Figure 1.
Flow chart of participant enrollment and inclusion in analysis.
Table 1.
Baseline Characteristics of Eligible Patients* Compared with Enrolled Patients.
| Eligible, Nonenrolled Patients (N=312) | Enrolled Patients (N=438) | P Value | |
| Median age, years (Lower Quartile, Upper Quartile) | 74.3 (63.3, 83.0) | 67.0 (58.0,78.0) | <.001b |
| Sex, female (%) | 44% | 53% | .012a |
| Symptoms | |||
| Cough | 59% | 76% | <.001a |
| Fever | 32% | 45% | <.001a |
| Dyspnea | 84% | 83% | .63a |
| Loss of appetite | 7% | 40% | <.001a |
| Earache | 1% | 4% | .03a |
| Sore throat | 2% | 11% | <.001a |
| Wheezing | 24% | 50% | <.001a |
| Acute mental status changes | 5% | 3% | .20a |
| CHF | 13% | 22% | .005a |
NOTE. IQR, interquartile range; CHF, congestive heart failure.
*Patients with repeat eligibility and enrollment were included only once.
Pearson χ2 test;
Wilcoxon ranked sum test.
The proportions of patients with RT-PCR-confirmed influenza during the 3 successive influenza seasons were 8.3% (95% confidence interval [CI], 4.1%–12.5%), 20.6% (95% CI, 11.0%–30.2%), and 6.1% (95% CI, 2.6%–9.6%), respectively. Influenza-positive cases were similar to influenza-negative controls, except that they were younger, had a higher prevalence of women, had higher smoking rates, and had lower vaccination rates (Table 2). Of those with confirmed influenza, 87% (35 of 39 patients) had an underlying high-risk condition, 12.8% (5 of 39 patients) were treated with antivirals, 8% (3 of 39 patients) were admitted to an intensive care unit, and none died during hospitalization.
Table 2.
Patient characteristics and demographics of hospitalized older adults by RT-PCR-confirmed influenza
| Influenza Positive (n=39) | Influenza Negative (n=378) | P Value | |
| Race Other Black |
64% 36% |
69% 31% |
0.501 |
| Median Age, years (Lower Quartile, Upper Quartile) | 60.0 (54.6, 68.0) |
68.0 (58.7, 78.0) |
0.0022 |
| Age Group 50–64 ≥65 |
72% 28% |
41% 59% |
<0.0011 |
| Gender (Female) | 69% | 52% | 0.041 |
| Home Oxygen use | 13% | 26% | 0.061 |
| High Risk Medical Conditions Chronic pulmonary disease Chronic cardiovascular disease Immunosuppression Diabetes mellitus Kidney or liver disease Asplenia |
72% 46% 36% 33% 21% 3% |
65% 60% 44% 37% 23% 7% |
0.381 0.091 0.311 0.651 0.701 0.321 |
| Current Smoking | 44% | 24% | 0.0061 |
| Vaccinated | 36% | 66% | <0.0011 |
| ICU admission | 8% | 10% | 0.601 |
| Death | 0% | 2% | 0.361 |
| Length of Stay (Days) | 4 | 4 | 0.562 |
| Duration of illness prior to enrollment | 5 | 5 | 0.252 |
| Year 2006–2007 2007–2008 2008–2009 |
36% 36% 28% |
41% 14% 45% |
0.0021 |
NOTE.
Pearson Chi Square test; 2 Wilcoxon Ranked Sum Test
Immunosuppression: HIV, chemotherapy, cancer, corticosteroid use.
Among influenza-positive patients, only 28.2% had a discharge diagnosis of pneumonia or influenza (ICD-9 codes: 480–487), whereas 69.2% had discharge diagnostic codes indicating other respiratory or circulatory disorders (ICD-9 codes: 390–519, excluding codes 480–487). Among influenza-negative controls, the discharge diagnoses were nearly identical, with 28.3% having pneumonia or influenza discharge codes, and 67.2% having other respiratory and circulatory diagnostic codes. During the 2008–2009 influenza season, the only season in which functional status was assessed, 100% (11 of 11 patients) of influenza-positive and 98% (167 of 170 patients) of influenza-negative patients completed the functional assessment. The mean Barthel Index score of 91.7 and the median score of 100 (interquartile range [IQR]: 10) were identical in both the vaccinated and unvaccinated patients (P = .94). Overall, 67% of the enrolled influenza-negative patients were vaccinated. Among influenza-negative controls, vaccinated patients were more likely to be older or white, and they had a higher prevalence of chronic cardiovascular or pulmonary disease (Table 3).
Table 3.
Demographics of vaccinated and unvaccinated control patients
| Vaccinated (n=250) | Not Vaccinated (n=128) | P Value | |
| Race Other Black |
76% 24% |
57% 43% |
<0.0011 |
| Median Age, years (Lower Quartile, Upper Quartile) | 70.0 (60.0, 78.8) |
64.0 (57.0, 74.5) |
0.0032 |
| Age Group 50–64 ≥65 |
36% 64% |
51% 49% |
0.0041 |
| Gender (Male) | 50% | 45% | 0.431 |
| Home Oxygen Use | 30% | 20% | 0.051 |
| High Risk Medical Conditions Chronic pulmonary disease Chronic cardiovascular disease Immunosuppression Diabetes mellitus Kidney or liver disease Asplenia |
69% 65% 46% 34% 24% 9% |
56% 50% 41% 44% 21% 2% |
0.011 0.0041 0.281 0.051 0.471 0.021 |
| Smoking | 22% | 27% | 0.321 |
| ICU admission | 11% | 9% | 0.431 |
| Death | 2% | 3% | 0.331 |
| Length of Stay (Days) | 4 | 4 | 0.212 |
| Year 2006–2007 2007–2008 2008–2009 |
36% 13% 55% |
51% 17% 32% |
0.0011 |
NOTE.
Pearson Chi Square test; 2 Wilcoxon Ranked Sum Test
Immunosuppression: HIV, chemotherapy, cancer, corticosteroid use.
Circulating Influenza Strains
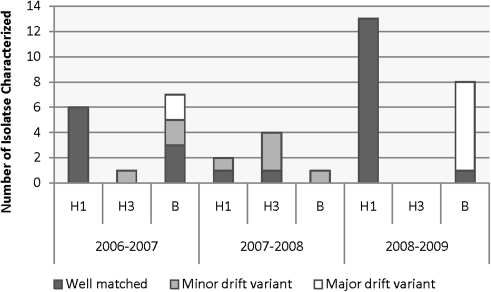
At the conclusion of each season, influenza-positive RT-PCR samples were sent to the CDC for further antigenic characterization and subtyping. During the 2006–2007 influenza season, 6 samples were characterized as H1N1, 1 as H3N2, and 7 as B influenza viruses, with 9 (64%) of 14 results representing good matches to the vaccine strain, 3 (21%) minor antigenic variants, and 2 (14%) poor matches. During the 2007–2008 influenza season, 4 H3N2, 2 H1N1, and 1 B influenza isolates were characterized with 2 (29%) considered good matches and 5 (71%) minor mismatches. During the 2008–2009 influenza season, 11 H1N1 and 8 B influenza viruses were characterized and all 11 H1N1 isolates were considered good matches, but only 1 of 8 B isolates (12.5%) was considered a good match. These strain results are shown graphically in Figure 2.
Figure 2.
Characterization of circulating strains in comparison with the individual seasonal vaccine strains. The y-axis delineates the number of isolates characterized.
Vaccine Effectiveness
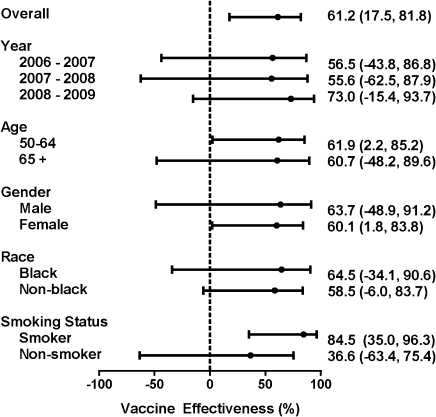
Overall, 14 (36%) of 39 influenza-positive cases and 250 (66%) of 378 influenza-negative controls were vaccinated, yielding an unadjusted vaccine effectiveness of 71.3% (95% CI, 42.9%–85.6%). For each of the 3 successive study years, unadjusted annual estimates were 59.4% (95% CI, −26.7% to 87.0%), 61.8% (95% CI, −29.4% to 88.7%), and 81.8% (95% CI, 34.8%,–94.9%), respectively. With propensity-score adjustment, overall vaccine effectiveness for the 3 influenza seasons was 61.2% (95% CI, 17.5%–81.8%) (Figure 3). Vaccine effectiveness was not significantly different in groups stratified by year, age, gender, race, and smoking status.
Figure 3.
Propensity score–adjusted vaccine effectiveness ([1 - Odds Ratio]*100%) overall and stratified by year for hospitalization, age, sex, race, and smoking status in adults aged ≥50 y, Davidson County, Tennessee
*Adjusted for age in y, sex, race, smoking status, home oxygen use, underlying medical conditions, immunosuppression, timing of admission relative to onset of influenza season, and specific influenza season (2006–2007, 2007–2008, or 2008–2009).
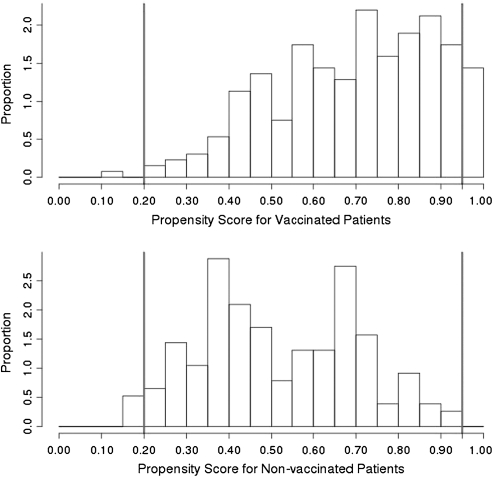
Three sensitivity analyses were performed to test the robustness of our findings. Among those classified as vaccinated, we were unable to verify self-reported vaccination in 6 (42.9%) of 14 cases and 77 (30.8%) of 250 controls. Exclusion of these 83 patients yielded an overall adjusted vaccine effectiveness of 72.0% (95% CI, 28.8%–89.0%). Trimming of nonoverlapping propensity scores by restricting the cohort to propensity scores between .2 and .95 resulted in dropping 21 controls and 3 cases (Figure 4). In the trimmed population, 14 (38.9%) of 36 cases and 230 (64.4%) of 357 controls were vaccinated, yielding an overall adjusted vaccine effectiveness (VE) of 60.2% (95% CI, 14.4%–81.5%). Finally, because most adults mount an immune response to vaccination within 1 week, [10] an additional analysis that required only 1 week for vaccination to be considered valid yielded an adjusted VE of 61.1% (95% CI, 17.2%–81.7%).
Figure 4.
Histogram plot of distribution of propensity score.
DISCUSSION
Our study yielded 2 important findings relevant to adults aged 50 years and older. First, laboratory-confirmed influenza was associated with nearly 10% of overall respiratory hospitalizations during 3 successive influenza seasons. Second, influenza vaccine had an estimated overall effectiveness of 61% for the prevention of influenza-associated hospitalizations.
The bulk of information on the burden of influenza in older adults has come from modeling studies that have used administrative databases to estimate influenza-associated hospitalizations. Thompson et al used national hospital discharge data collected over 22 seasons (1979–2001) to estimate average annual age-specific hospitalization rates [2]. According to their estimates, influenza-associated hospitalizations increased remarkably with age from nearly 1 per 1000 persons aged 50–64 years to 17 per 1000 persons aged 85 years and older. Mullooly et al [18] found a similarly high rate of estimated influenza-associated hospitalizations in older adults using data from 3 health maintenance organizations over 4 influenza seasons. Estimated hospitalizations among unvaccinated high-risk persons aged 50–64 years were 2 per 1000 and among unvaccinated persons 65 years and older, without and with high-risk conditions, were 3 per 1000 and 11 per 1000, respectively.
Few studies have examined the prevalence of influenza among older hospitalized adults using modern molecular diagnostic techniques. Falsey et al [19] prospectively enrolled adults admitted for acute respiratory symptoms during 4 winter respiratory virus seasons (1999–2003) and used RT-PCR to assess the burden of hospitalizations due to both influenza and respiratory syncytial virus (RSV). Similar to our study, these investigators included both admissions for pneumonia and influenza and for other conditions such as chronic obstructive pulmonary disease and congestive heart failure exacerbations. Although they focused exclusively on adults aged 65 years and older, 11.6% (170 of 1471 patients) of their hospitalizations were due to influenza, remarkably similar to the 9% reported in our study. Both studies highlight the difficulties of performing vaccine effectiveness studies using nonspecific endpoints, such as acute respiratory hospitalizations, because a minority of hospitalizations in both studies were due to influenza.
The second major finding of our study was that the overall estimated vaccine effectiveness for the prevention of laboratory-confirmed influenza-associated hospitalizations was 61%, despite that 2 of the 3 seasons had considerable mismatch between the vaccine and circulating strains. Although the individual yearly estimates were nearly identical to the overall rate, the numbers were too small to detect meaningful between-year differences.
Although this was not a randomized control trial, our data are important for several reasons. First, the recent Cochrane review [5] identified only 1 high-quality randomized clinical trial of influenza vaccine efficacy in older adults. In that trial, vaccine efficacy was reported to be 58% for the prevention of serologically confirmed influenza in healthy ambulatory adults aged ≥60 y [20] and 57% (95% CI, −36% to 87%) in those ≥70 y of age [21]. Because that trial was relatively small and performed in healthy elderly persons, it did not provide data on vaccine effectiveness in frail elderly persons, or estimate effectiveness against influenza-associated hospitalizations or deaths, endpoints that have been the subject of recent controversy [22]. The remainder of the 68 Cochran-reviewed studies of vaccine effectiveness evaluated nursing home patients, did not evaluate hospitalizations, or did not use laboratory-confirmed influenza hospitalizations as an end point. As noted previously, influenza admissions constitute only about 10% of acute respiratory hospitalizations during influenza seasons, introducing major misclassification of outcome into these other assessments.
A recent study by Herrera et al [23], not included in the Cochrane analysis, evaluated influenza vaccine effectiveness for the prevention of hospitalization in adults 50–64 years of age using laboratory-confirmed influenza and community controls. Vaccine effectiveness was 35.6% (95% CI, 0%–63.2%) and 90.5% (95% CI, 68.1%–97.2%) for those with and without high-risk conditions, respectively. Methodologically, our study differed from the Herrera study in that we used hospitalized rather than community-based controls. Although the use of hospitalized controls has traditionally been considered a weaker design option than community-based controls, it may allow better control for factors associated with need for hospitalization. In addition, using influenza-negative hospitalized controls has been shown to be an efficient design for vaccine-effectiveness evaluations [24] and has been used effectively in pediatric [25] and adult outpatient settings [26] to measure influenza-vaccine effectiveness. A second recent study, not included in the Cochrane review, by Baxter et al [27] estimated influenza vaccine effectiveness against influenza-associated pneumonia and influenza hospitalizations. This study used models to estimate influenza-associated hospitalizations from nonspecific hospitalizations coupled with viral surveillance data and effectively accounted for frailty bias attributed to prior modeling studies. Average influenza vaccine effectiveness over the eleven study years was 28% (95% CI, 8.5%–30.0%) for those ages 50 to 64 years and 48% (95% CI, 12.4%–26.0%) for those 65 y and older.
One might expect that vaccination would be better at preventing serious outcomes (hospitalizations, deaths) than more mild disease. However, patients with more serious influenza-associated events are generally a select group of older persons with other comorbidities. Indeed, nearly all our enrolled patients had at least 1 high-risk condition and may have been less likely to mount a brisk immune response after vaccination. Despite this, our vaccine effectiveness estimates are remarkably similar to those reported in the only RCT conducted in healthy older adults [20].
We chose a hospital-based case control design to assure that cases and controls were similar with respect to underlying risk factors for hospitalization. Although we enrolled only about 50% of eligible cases, exclusions were based mainly on patient or surrogate refusals and were unlikely to be influenced by influenza status, which was unknown at the time of enrollment. Those with influenza were actually remarkably similar to controls who were hospitalized with other acute respiratory illnesses. Although functional status was measured in only 1 study year, the distribution of functional status scores, using the Barthel Index, was similar in cases and controls and suggested that the enrolled patients were functioning well. (Table 2) This likely limits the generalizability of our findings to the more highly functioning hospitalized adults. Although differences were noted between vaccinated and unvaccinated (Table 3) participants, these differences were modest and were controlled for by propensity score adjustment. Three sensitivity analyses were conducted and yielded consistent results; 1 that excluded patients without confirmation of vaccination, the second that trimmed propensity scores to exclude those with very low or high propensity for vaccination, and the third that considered vaccinations after only 1 rather than 2 weeks prior to hospitalization. In addition, results were consistent when patients were stratified by year, age, sex, race, and smoking status, although these subgroups were small in number. Because of the limited number of cases, further detailed analyses such as estimation of effectiveness based on quantitative PCR, low risk medical conditions, age, and sex could not be performed.
Each year there are an estimated 300,000 hospitalizations attributable to influenza in the United States, most of which are in older adults [2]. Based on our vaccine effectiveness estimates, these numbers would more than double in the absence of the current vaccination program. Better coverage with current vaccines and development of more effective influenza vaccines could reduce these numbers further. This study strongly supports the current United States policy of recommending annual influenza vaccination for older adults.
Funding
This work was supported by the Vaccine and Treatment Evaluation Units of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant N01 AI25462); the Centers for Disease Control and Prevention (grant 1U181P000184-01); the National Center for Research Resources at the National Institutes of Health (grant 5 K12 RR017697-05 to H.K.T.); the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant 1K23AI074863-01 to H.K.T.); and the Vanderbilt Clinical and Translational Science Award (grant 1 UL1 RR024975).
The funders did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript.
Acknowledgments
We acknowledge Dr Paul Harris and Carlos Orozco, who designed and implemented the data entry and management system; Vanderbilt study nurses and coordinators: Ann Clay and Dayna Wyatt; Vanderbilt laboratory personnel, Amy Podsaid, who performed the RT-PCR; and the following investigators: Dr Paul McNabb, Dr Lisa Laya, and Dr Steve VanHook. We also wish to acknowledge and thank the Virus Surveillance and Diagnostics Branch, Influenza Division, NCIRD, CDC for their work in performing the antigenic characterization and subtyping of the influenza virus isolates. Finally, we thank all the adults who generously participated in this study.
References
- 1.Fiore AE, Shay DK, Broder K, et al. Prevention control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 59:1057–62. [PubMed] [Google Scholar]
- 4.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Syst Rev. 2010:CD004876. doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Greene SK, Shi P, Dutta-Linn MM, et al. Accuracy of data on influenza vaccination status at four Vaccine Safety Datalink sites. Am J Prev Med. 2009;37:552–5. doi: 10.1016/j.amepre.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: Impact of vaccination of school children. Vaccine. 2009;27:1923–7. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiore AE, Shay DK, Haber P, et al. Prevention control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56:1–54. [PubMed] [Google Scholar]
- 9.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 10.Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–9. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 11.Talbot HK, Williams JV, Zhu Y, Poehling KA, Griffin MR, Edwards KM. Failure of routine diagnostic methods to detect influenza in hospitalized older adults. Infect Control Hosp Epidemiol. 2010;31:683–8. doi: 10.1086/653202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orenstein WA, Bernier RH, Dondero TJ, et al. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–68. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 14.Mac Donald R, Baken L, Nelson A, Nichol KL. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med. 1999;16:173–7. doi: 10.1016/s0749-3797(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 15.Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect. 2007;135:139–43. doi: 10.1017/S0950268806006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skull SA, Andrews RM, Byrnes GB, et al. Validity of self-reported influenza and pneumococcal vaccination status among a cohort of hospitalized elderly inpatients. Vaccine. 2007;25:4775–83. doi: 10.1016/j.vaccine.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–9. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullooly JP, Bridges CB, Thompson WW, et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–55. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 20.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 21.Thijs C, Beyer WE, Govaert PM, Sprenger MJ, Dinant GJ, Knottnerus A. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis. 2008;8:460–1. doi: 10.1016/S1473-3099(08)70161-0. author reply 463–5. [DOI] [PubMed] [Google Scholar]
- 22.Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine. 2009;27:6300–4. doi: 10.1016/j.vaccine.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Herrera GA, Iwane MK, Cortese M, et al. Influenza vaccine effectiveness among 50–64-year-old persons during a season of poor antigenic match between vaccine and circulating influenza virus strains: Colorado, United States, 2003–2004. Vaccine. 2007;25:154–60. doi: 10.1016/j.vaccine.2006.05.129. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein EW, De Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007; 36:623–31. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg KW, Szilagyi PG, Fairbrother G, et al. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008;122:911–9. doi: 10.1542/peds.2007-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissling E, Valenciano M, Falcao J, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: Lessons learnt from a pilot multi-centric case-control study in Europe, 2008–9. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- 27.Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010;28:7267–72. doi: 10.1016/j.vaccine.2010.08.088. [DOI] [PubMed] [Google Scholar]