Abstract
Background. Effectiveness of cotrimoxazole (CTX) compared with sulfadoxine-pyrimethamine (SP) intermittent-preventive-therapy (IPTp) for malaria in HIV-infected pregnant women is unknown. We examined effectiveness of CTX with or without SP-IPTp versus SP-IPTp at reducing malaria parasitemia and anemia.
Methods. From 2005 to 2009, we conducted a cross-sectional study of HIV-infected pregnant women at Thyolo Hospital, Malawi. Blood was tested for malaria parasitemia and anemia (hemoglobin<11g/dl). Data were collected on use of anti-malaria interventions and other risk factors. CTX prophylaxis policy for HIV-infected pregnant women was introduced in 2007, but implementation problems resulted in some women receiving both CTX and SP-IPTp.
Findings. We enrolled 1,142 women, of whom 1,121 had data on CTX and/or SP-IPTp intake. Of these, 49.7%, 29.8%, and 15.4% reported taking SP-IPTp only, CTX only and SP-IPTp plus CTX, respectively. Compared with women taking SP-IPTp, those taking SP-IPTp plus CTX and CTX were less likely to have malaria parasitemia (OR, [95%CI]: 0.09, [0.01-0.66] and 0.43, [0.19-0.97], respectively) or anemia (PR, [95% CI]: 0.67, [0.54-0.83] and 0.72, [0.61-0.83], respectively).
Conclusion. In HIV-infected pregnant women, daily CTX was associated with reduced malaria parasitemia and anemia compared with SP-IPTp. CTX plus SP-IPTp was associated with further reduction in malaria parasitemia but toxicity was not fully assessed.
Malaria in pregnancy is a preventable cause of significant maternal and perinatal morbidity and mortality in sub-Saharan Africa [1]. Human immunodeficiency virus (HIV) infection increases the risks of placental and peripheral malaria, high-density parasitemia, and febrile malaria illness among pregnant women [2–5]. HIV-infected pregnant women are at an increased risk of premature delivery, severe anemia, delivery of low-birth-weight infants, and maternal death as a result of frequent and severe malaria infections [5].
In countries where malaria is endemic, women receive sulfadoxine-pyrimethamine (SP) intermittent preventive therapy during pregnancy (IPTp) to prevent malaria and its complications [6]. The World Health Organization (WHO) currently recommends that HIV-infected pregnant women receiving daily trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis should not be given as SP-IPTp, to avoid adverse drug reactions associated with sulfa drug toxicity [7]. Countries such as Uganda and Malawi currently recommend daily TMP-SMX to all HIV-infected pregnant women to prevent opportunistic infections [8]. However, these recommendations are not based on empirical evidence [9, 10]. Although TMP-SMX has been shown to decrease malaria morbidity in children and HIV-infected adults [11–14], its efficacy, safety, and effectiveness to prevent malaria have not been evaluated in HIV-infected pregnant women [15]. No study has assessed the effects of daily TMP-SMX prophylaxis, compared with SP-IPTp, in HIV-infected pregnant women.
We analyzed data from a cross-sectional study that was conducted to investigate the effects of iron supplementation on maternal morbidities. We compared the prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking daily TMP-SMX, with or without SP-IPTp, with the prevalence in those taking SP-IPTp only.
METHODS
Study Site
The study was conducted from December 2005 through July 2009 in the antenatal clinic at Thyolo District Hospital in southern Malawi. The hospital provides free antenatal services and has a well-established program for prevention of mother-to-child transmission of HIV infection (PMTCT). Measurements of CD4 T cell count and WHO HIV clinical staging are performed on all women found to be HIV infected. HIV-infected pregnant women classified in WHO HIV stage 3 or 4 or those with a CD4 cell count <250 cells/μL received combination antiretroviral therapy (ART) containing stavudine (30–40 mg), lamivudine (150 mg), and nevirapine (200 mg) twice daily as a fixed-dose combination (Triomune) [16]. Those not eligible for ART received single-dose nevirapine for PMTCT, in accordance with the HIVNET 012 protocol. However, in 2007, these women started receiving 300 mg zidovudine twice daily. Before 2007, women with a CD4 cell count <500 cells/μL or those classified as WHO HIV stage 2, 3, or 4 were eligible for TMP-SMX prophylaxis [17]. In line with the Malawi Ministry of Health recommendations, TMP-SMX prophylaxis (480 mg twice daily) to all HIV-infected pregnant women for the prevention of opportunistic infections was introduced at the hospital in 2007. However, confusion about the exact implementation of this policy resulted in some women receiving SP-IPTp only and others receiving a combination of daily TMP-SMX and SP-IPTp.
Study Population, Enrollment, and Data Collection
We used data from a cross-sectional study investigating the effects of routine iron supplementation in HIV-infected pregnant women on maternal morbidities. The study included HIV-infected pregnant women aged ≥15 years and with gestation ≥34 weeks who were attending routine antenatal services at the hospital. Women with immediate life-threatening medical and obstetric conditions were excluded.
A study nurse administered a standardized questionnaire to collect data that included sociodemographic and medical information; intake of medications, such as ART, SP-IPTp, TMP-SMX, and iron supplements; and the use of bed nets. Physical medical examination was also performed, which included measurement of temperature, blood pressure, mid-upper arm circumference (MUAC), weight, and height. Thereafter, peripheral venous blood samples were collected for thick blood smear malaria microscopy and, later, for malaria DNA detection using real-time polymerase chain reaction (PCR). The study was approved by the Malawi College of Medicine Research and Ethics Committee and by the institutional review board of the University of North Carolina at Chapel Hill. All women provided informed consent before enrollment.
Laboratory Tests
Thick blood smears were stained with Field stains A and B [18]. Using light microscopy, 2 independent laboratory technicians examined the stained smears to detect asexual forms of Plasmodium falciparum malaria parasites. In case of discrepancy of results, an experienced microscopist reread the slides, as a tie-breaker. Malaria parasites were counted against 200 leukocytes. To estimate the parasite density per microliter of blood, the number of parasites counted was multiplied by 40 under the assumption of leukocyte count of 8000 cells/μL of blood. A blood film result was considered to be negative if no parasites were detected per 200 leukocytes.
To increase the detection of malaria infection, we also conducted real-time PCR for malaria parasite DNA at the University of North Carolina at Chapel Hill (UNC-CH). DNA was extracted from dried filter paper using an invitrogen PureLink Genomic DNA kit (Invitrogen) according to manufacturer's instructions. PCR amplification of the malaria parasite DNA was done in 2 steps using a pooling method as described in Taylor et al [19]. CD4 T cell count measurement was performed using Partec CyFlow counter (Partec GmbH). Hemoglobin concentration measurement was performed for whole blood samples with use of a Hemocue hemoglobinometer (HemoCue AB).
Definitions
Microscopic malaria infection was defined as presence of malaria parasites on microscopy; PCR-detected malaria infection was defined as positive result of PCR for malaria regardless of microscopy results. Anemia was defined as hemoglobin concentration <11g/dL. ART use was defined as any use of ART. Women were classified into 4 groups of antimalarial drug exposure based on reported information verified by antenatal records: (1) SP-IPTp plus TMP-SMX, (2) TMP-SMX only, (3) SP-IPTp only, and (4) none (neither SP-IPTp nor TMP-SMX).
Statistical Analysis
Data were analyzed using SAS software, version 9.1 (SAS Institute). In bivariate analyses, we used analysis of variance or Student's t test to compare differences among outcomes or exposure and continuous variables that were normally distributed variables. We used the Kruskal–Wallis test or Wilcoxon rank sum test for continuous variables that were not normally distributed variables, and the Pearson χ2 test for categorical variables.
We evaluated effect measure modification with use of the likelihood ratio test of the interaction term. We used directed acyclic graphs to identify potential confounders for the association of antimalarial drug exposure with malaria infection or maternal anemia [20, 21]. A backward elimination strategy was applied to select the best model using the criterion of ≥10% absolute change in estimate. Variables that were assessed for confounding and effect measure modification included age (<27 y or ≥27 years), gravidity (primigravidae, secondigravidae, or multigravidae), number of antenatal visits, CD4 cell count (<200, 200–499, or ≥500 cells/μL), body mass index, and socioeconomic status (poor, middle, or high). Socioeconomic status was obtained by calculating the wealth index with use of the method described in Gwatkin et al [22, 23].
Logistic regression was used in the analysis of the association between antimalarial drug exposure and malaria infection. We used log-binomial regression [24–26] and linear regression in the analysis of the association of antimalarial drug exposure with anemia and hemoglobin concentration, respectively. We used a Poisson regression model with robust variance estimator to confirm estimates from log-binomial regression [24, 27].
Because SP-IPTp has been the standard of care for malaria prevention during pregnancy, the SP-IPTp group was used as a referent.
RESULTS
Study Population
From December 2005 through July 2009, 1142 HIV-infected pregnant women were enrolled. Of the women with available data, 71 (6.2%) of 1141 were primigravidae and 1034 (90.8%) of 1139 were married. Participants had a median age of 27 years (range, 16–46 years) and median CD4 cell count of 423 cells/μL (range, 11–1528 cells/μL). Use of bed nets was reported by 675 (59.6%) of 1133 of the women, and 554 (48.5%) of 1142 of the women reported taking ART.
Antimalarial Drug Exposure
Data on the use of SP-IPTp and TMP-SMX were available for 1121 (98.2%) of 1142 of the enrolled women. Among these participants, 557 (49.7%) reported taking SP-IPTp only, 334 (29.8%) TMP-SMX only, and 173 (15.4%) TMP-SMXSP-IPTp plus TMP-SMX. Only 57 (5.1%) reported taking none of the drugs. The groups were similar in terms of HIV disease clinical stage, CD4 count, SES, and marital status (Table 1). However, women in the SP-IPTp-only group were significantly younger, less likely to use bed nets, more likely to be primigravidae, and less likely to report taking antiretroviral drugs compared with women in TMP-SMX or TMP-SMX plus SP-IPTp groups (Table 1).
Table 1.
Characteristics of HIV-infected pregnant women according to sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy and cotrimoxazoleTMP-SMX intake at Thyolo District Hospital antenatal clinic, Malawi (2005–2009)
| Drug Group |
|||||
| Characteristic | SP & TMP-SMX (n = 173) | TMP-SMX only (n = 334) | SP only (n = 557) | None (n = 57) | P Value |
| Age, median (range), y | 28 (18–40) | 27 (16–46) | 26 (16–45) | 27 (16–37) | <.0001§ |
| Gravidity, median (range) | 4 (1–9) | 4 (1–9) | 3 (1–11) | 3 (1–7) | .002§ |
| Primigravidae, n (%) | 8 (4.6) | 16 (4.8) | 44 (7.9) | 2 (3.5) | |
| Secundigravidae, n (%) | 25 (14.5) | 42 (12.6) | 112 (20.1) | 20 (35.1) | .0001γ |
| ANC visits, median (range) | 3 (1–5) | 3 (1–8) | 3 (1–8) | 2 (1–4) | .0001§ |
| <3 visits, n (%) | 40 (23.1) | 116 (34.8) | 236 (42.4) | 44 (77.2) | <.0001 γ |
| Any bed net use* | |||||
| Yes, n (%) | 110 (63.6) | 264 (79.5) | 271 (49.2) | 24 (42.1) | .001 γ |
| CD4 cell count (cells/μL) | |||||
| <200, n (%) | 19 (11.0) | 25 (7.5) | 65 (11.7) | 5 (8.8) | .05 γ |
| 200–499, n (%) | 100 (57.8) | 170 (50.9) | 296 (53.1) | 24 (42.1) | |
| ≥ 500, n (%) | 54 (31.2) | 139 (41.6) | 196 (35.2) | 28 (49.1) | |
| WHO HIV clinical stage | |||||
| 1 or 2, n (%) | 142 (83.0) | 298 (89.8) | 487 (87.4) | 52 (92.9) | .10 γ |
| 3 or 4, n (%) | 29 (17.0) | 34 (10.2) | 70 (12.6) | 4 (7.1) | |
| Antiretroviral drug use (%) | |||||
| Yes, n (%) | 142 (82.1) | 277 (82.9) | 128 (23.0) | 4 (7.0) | .0001 γ |
| Body mass index, mean (SD)** | 23.6 (2.5) | 23.7 (2.6) | 23.2 (2.4) | 23.4 (2.3) | .04π |
| MUAC in cm, median (range) ¥ | 25.4 (20.0–33.0) | 25.2 (20.0–35.0) | 24.1 (18.0–36.8) | 24.4 (20.6–30.8) | .001§ |
| Socioeconomic status, n (%) | |||||
| Poor | 59 (35.1) | 127 (38.5) | 230 (41.6) | 27 (48.2) | .52 γ |
| Middle | 69 (41.1) | 120 (36.4) | 203 (36.7) | 19 (33.9) | |
| High | 40 (23.8) | 83 (25.2) | 120 (21.7) | 10 (17.9) | |
| Married, n (%)† | 157 (90.8) | 302 (90.4) | 510 (91.7) | 47 (82.5) | .14 γ |
NOTE. Abbreviations: HIV, human immunodeficiency virus; SP, sulfadoxine-pyrimethamine; TMP-SMX, cotrimoxazole; OR, odds ratio; ANC, Antenatal clinic; WHO, World Health Organization; SD, standard deviation; MUAC, mid-upper arm circumference.
*Bed net use missing in groups: cotrimoxazole (TMP-SMX): n = 2; SP-IPTp: n = 6
**Body mass index missing in groups: TMP-SMX : n = 2; SP-IPTp: n = 2
Mid-upper arm circumference (MUAC) missing in groups: TMP-SMX & SP-IPTp: n = 1; TMP-SMX: n = 20; SP-IPTp : n = 2; None : n = 1
Marital status missing in group: None, n = 1.
Kruskal–Wallis test; γχ2 test; πanalysis of variance test.
Prevalence of Malaria parasitemia and Anemia
Blood smear microscopy and real-time PCR results were available for 1114 (97.5%) of 1142 and 1128 (98.8%) of 1142 of women, respectively. The prevalence of PCR-detected malaria infection was almost 2 times higher (10.0% [113 of 1128]) than that of microscopic malaria (5.5% [61 of 1114]). All PCR-positive malaria infections were due to P. falciparum among those that had information on parasite species (n = 70). However, 4.3% (3/70) had P. falciparum and P. malariae mixed infections, whereas 1 (1.4%) of 70 had P. falciparum and P. ovale. Data on hemoglobin concentration were available for 1140 (99.8%) of 1142 of the women. The prevalence of any anemia and moderate-to-severe anemia (hemoglobin concentration, <8 g/dL) were 45.1% (514 of 1140) and 1.6% (18 of 1140), respectively.
Associations Between TMP-SMX or SP-IPTp and Malaria parasitemia
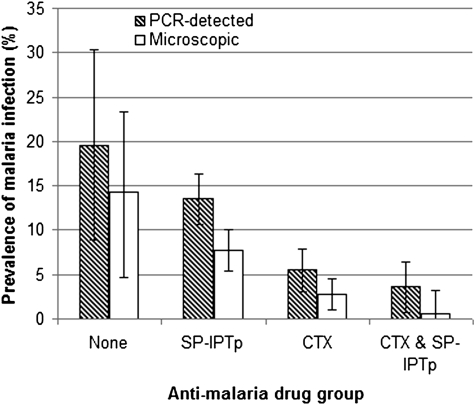
The prevalence of microscopic and PCR-detected malaria infection was significantly lower in women reporting taking TMP-SMX plus SP-IPTp (.6% and 3.6%) and TMP-SMX only (2.7% and 5.5%) than in those reporting taking SP-IPTp only (7.7% and 13.5%) (Figure 1). The prevalence of microscopic malaria infection and PCR-detected malaria infection tended to be higher in women who reported not taking TMP-SMX or SP-IPT (14.0% and 19.6%) than those reporting taking SP-IPTp only, but the difference was not statistically significant (Table 2).
Figure 1.
Prevalence of malaria with 95% confidence interval by antimalarial drug intake in HIV-infected pregnant women at Thyolo District Hospital, Malawi (2005–2009).
Table 2.
Factors associated with malaria infection among HIV-infected pregnant women from Thyolo district Hospital antenatal clinic in Malawi (2005–2009)
| Microscopic Malaria |
PCR-detected Malaria |
|||||
| Characteristic | n (% infected) | Crude OR (95% CI) | Adjusted OR (95% CI)¥ | n (% infected) | Crude OR (95% CI) | Adjusted OR (95% CI)¥ |
| Antimalarial drug | ||||||
| None | 57 (14.0) | 1.96 (.87–4.41) | 1.84 (.76–4.46) | 56 (19.6) | 1.56 (.77–3.15) | 1.39 (.66–2.94) |
| SP-IPTp | 532 (7.7) | 1.00 | 1.00 | 554 (13.5) | 1.00 | 1.00 |
| Cotrimoxazole | 333 (2.7) | .33 (.16–.69) | .43 (.19–.98) | 329 (5.5) | .37 (.22–.63) | .44 (.25–.78) |
| SP-IPTp & Cotrimoxazole | 171 (.6) | .07 (.01–.52) | .09 (.01–.66) | 168 (3.6) | .24 (.10–.55) | .24 (.10–.62) |
| Age, y | ||||||
| ≥ 27 | 584 (4.1) | 1.00 | 1.00 | 593 (8.1) | 1.00 | 1.00 |
| <27 | 529 (7.0) | 1.76 (1.04–2.98) | 1.27 (.68–2.38) | 536 (11.9) | 1.54 (1.04–2.28) | 1.16 (.73–1.85) |
| Gravidity | ||||||
| Multigravidae | 849 (4.7) | 1.00 | 1.00 | 861 (8.6) | 1.00 | 1.00 |
| Secundigravidae | 194 (8.8) | 1.94 (1.08–3.51) | 1.75 (.88–3.46) | 198 (13.6) | 1.68 (1.05–2.69) | 1.58 (.93–2.71) |
| Primigravidae | 70 (5.7) | 1.23 (.43–3.53) | 1.12 (.36–3.52) | 70 (15.7) | 1.98 (1.00–3.93) | 2.00 (.93–4.29) |
| ANC visits | ||||||
| ≤3 visits | 667 (5.0) | 1.00 | 1.00 | 675 (8.3) | 1.00 | 1.00 |
| >3 visits | 445 (6.3) | 1.29 (.7–2.17) | .85 (.48–1.53) | 451 (12.4) | 1.57 (1.06–2.31) | 1.20 (.78–1.84) |
| Any bed net use | ||||||
| Yes | 662 (3.02) | 1.00 | 1.00 | 665 (7.4) | 1.00 | 1.00 |
| No | 443 (8.6) | 3.01 (1.73–5.25) | 2.11 (1.16–3.83) | 454 (13.2) | 1.91 (1.29–2.85) | 1.31 (.85–2.02) |
| CD4 T cell count cells/μL | ||||||
| ≥ 500 | 416 (5.8) | 1.00 | 1.00 | 419 (10.3) | 1.00 | 1.00 |
| 200–499 | 581 (5.3) | .92 (.53–1.59) | 1.00 (.55–1.81) | 591 (10.3) | 1.01 (.67–1.52) | 1.01 (.65–1.57) |
| <200 | 117 (5.1) | .88 (.35–2.21) | 1.08 (.41–2.82) | 118 (7.6) | .72 (.34–1.53) | .82 (.38–1.77) |
| WHO HIV clinical stage | ||||||
| 3 or 4 | 134 (1.5) | 1.00 | * | 138 (6.5) | 1.00 | * |
| 1 or 2 | 975 (6.0) | 4.17 (1.00–17.29) | 985 (10.5) | 1.67 (.83–3.39) | ||
| Antiretroviral drug use | ||||||
| Yes | 546 (2.0) | 1.00 | * | 546 (5.3) | 1.00 | * |
| No | 568 (8.8) | 4.70 (2.42–9.12) | 582 (14.4) | 3.01 (1.94–4.67) | ||
| Season of enrollment | ||||||
| Dry | 607 (4.0) | 1.00 | ** | 613 (6.2) | 1.00 | ** |
| Rainy | 505 (7.3) | 1.92 (1.13–3.26) | 513 (14.2) | 2.55 (1.69–3.85) | ||
| BMI | ||||||
| >24.9 | 277 (4.7) | 1.00 | ** | 277 (7.6) | 1.00 | ** |
| 18.5–24.9 | 817 (5.9) | 831 (10.9) | 1.50 (.91–2.46) | |||
| <18.5 | 16 (.0) | 1.27 (.68–2.38) | 16 (6.2) | .81 (.10–6.46) | ||
| Socioeconomic status | ||||||
| High | 248 (2.4) | 1.00 | 1.00 | 247 (6.1) | 1.00 | 1.00 |
| Middle | 409 (6.1) | 2.63 (1.06–6.49) | 2.10 (.82–5.35) | 419 (9.6) | 1.63 (.88–3.02) | 2.16 (1.16–4.00) |
| Poor | 442 (6.8) | 2.94 (1.21–7.15) | 2.51 (1.00–6.30) | 448 (12.3) | 2.17 (1.20–3.92) | 1.50 (.79–2.83) |
NOTE. Abbreviations: PCR, polymerase chain reaction; OR, odds ratio; CI, confidence interval; SP-ITPp, sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy; ANC, Antenatal clinic; WHO, World Health Organization; HIV, human immunodeficiency virus; BMI, body mass index.
Adjusted for age, gravidity, number of antenatal visits, bed net use, CD4 cell count, and socioeconomic status.
*Variables not included in the model due to correlation with CD4 count.
After adjusting for age, gravidity, number of antenatal visits, CD4 cell count, bed net use and SES, the odds of microscopic malaria infection were significantly lower in women reporting taking TMP-SMX plus SP-IPTp (adjusted OR, .09; 95% CI, .01–.66) or TMP-SMX (adjusted OR, .43; 95% CI, .19–.97) than in women who reported taking SP-IPTp only (Table 2). Likewise, after adjusting for same variables above, the odds of PCR-detected malaria infection were significantly lower in women who reported taking TMP-SMX plus SP-IPTp (adjusted OR, .24; 95% CI, .10–.62) or TMP-SMX only (adjusted OR, .44; 95% CI, .25–.78) than women who reported taking SP-IPTp only (Table 2). The odds of microscopic malaria infection were higher in women who reported not taking TMP-SMX or SP-IPTp compared with those who reported taking SP-IPTp only (adjusted OR, 1.84; 95% CI, .87–4.41) but this was not statistically different (Table 2). The odds of microscopic malaria infection or PCR-detected malaria infection were not statistically different in women who reported taking TMP-SMX plus SP-IPTp (adjusted OR, .21; 95% CI, .03–1.67) compared with women who reported taking TMP-SMX only (adjusted OR, .56; 95% CI, .20–1.56). We also observed that the prevalence of microscopic and PCR-detected malaria infection were lower in women who had taken TMP-SMX for ≥30 d than in women who took TMP-SMX for <30 d (χ2 test, 1.2% versus 6.7% and 4.0% versus 9.6% P = .002 and P = .041, respectively).
Associations of TMP-SMX or SP-IPTp With Anemia and Hemoglobin Concentration
The prevalence of anemia was significantly lower in women who reported taking TMP-SMX plus SP-IPTp (34.7%) or TMP-SMX only (35.6%) than in women who reported taking SP-IPTp only (52.4%) (Table 3). After adjusting for age, gravidity, number of antenatal visits, CD4 count, and BMI, the prevalence of maternal anemia remained significantly lower in women who reported taking TMP-SMX plus SP-IPTp (adjusted prevalence ratio [APR], .67; 95% CI, .54–.83) or TMP-SMX only (APR, .72; 95% CI, .61–.83) than in women who reported taking SP-IPTp only.
Table 3.
Maternal anemia (hemoglobin concentration [Hb] < 11g/dL) and hemoglobin concentration by antimalarial drug group in HIV-infected pregnant women at Thyolo District Hospital, Malawi (2005–2009)
| Hb |
Anemia |
||||||
| Group | n | Mean Hb (SD), g/dL* | Difference in Hb means (95% CI), g/dL § | P value | % | PR (95% CI)§ | P value |
| Antimalarial drug | |||||||
| None | 57 | 10.64 (1.32) | -.27 (-.64 to .09) | 61.4 | 1.21 (.99–1.48) | ||
| SP-IPTp | 557 | 10.86 (1.35) | 1.00 | .14 | 52.4 | 1.00 | .06 |
| Cotrimoxazole | 334 | 11.37 (1.36)a | .46 (.27 to .64) | <.0001 | 35.6 | .72 (.61–.83) | .0001 |
| SP-IPTp & cotrimoxazole | 173 | 11.39 (1.33)b | .52 (.29 to .75) | <.0001 | 34.7 | .67 (.54–.83) | .0003 |
| Duration of cotrimoxazole | |||||||
| <30 d | 77 | 11.08 (1.11) | -.44 (-.80 to -.07) | .02 | 42.9 | 1.31 (.97–1.77) | .08 |
| 30–60 d | 124 | 11.24 (1.29) | -.25 (-.55 to .05) | .11 | 36.3 | 1.05 (.79–1.40) | .73 |
| >60 d | 282 | 11.49 (1.44) | 1.00. | 34.8 | 1.00 | ||
NOTE. Hb, hemoglobin concentration; SD, standard deviation; CI, confidence interval; PR, Prevalence ratio; SP-ITPp, sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy.
*Analysis of variance test for the means: antimalarial drug P ≤ .0001; duration of cotrimoxazole P = .04.
Adjusted for age, gravidity, number of antenatal visits, CD4 count, and body mass index.
at test between cotrimoxazole and SP-IPTp groups; P < .0001.
bt test between cotrimoxazole plus SP-IPTp and SP-IPTp groups; P < .0001.
Similarly, the mean hemoglobin concentration was significantly higher in women who reported taking TMP-SMX plus SP-IPTp (mean hemoglobin concentration [standard deviation (SD)], 11.4 [1.33] g/dL; P < .0001) or TMP-SMX only (mean hemoglobin concentration [SD], 11.4 [1.36] g/dL; P < .0001) than in women who reported taking SP-IPTp only (mean hemoglobin concentration [SD], 10.8 [1.35] g/dL) (Table 3). After adjusting for age, gravidity, number of antenatal visits, CD4 count, and BMI, the mean hemoglobin concentration remained significantly higher in women who reported taking TMP-SMX plus SP-IPTp (adjusted difference in hemoglobin concentration means, .52; 95% CI, .29–.75) or TMP-SMX only (adjusted difference in hemoglobin concentration means, .46; 95% CI, .27–.64). Compared with women who took TMP-SMX for >60 d, women who took TMP-SMX for <30 d had lower hemoglobin concentration (adjusted difference in hemoglobin concentration means, −.44; 95% CI, −.80 to −.07; P = .02) (Table 3). We found no significant association between duration of TMP-SMX intake and the prevalence of anemia (Table 3).
DISCUSSION
This study was conducted in a sub-Saharan Africa setting wherein approximately 1 in 5 pregnant women attending antenatal clinics is HIV-infected [28]. During the first years of the study, the Malawi national policy for prevention of malaria in HIV-infected pregnant women was the use of at least 3 doses of SP-IPTp and insecticide-treated bed nets (ITNs). Subsequently, the policy changed to the use of daily TMP-SMX in all HIV-infected pregnant women in addition to ITNs. Women who were on daily TMP-SMX prophylaxis were not supposed to receive SP-IPTp [17]. However, during the period of transition to this new policy, some women received both SP-IPTp and TMP-SMX.
Our study found that, after adjusting for important confounders, TMP-SMX with or without SP-IPTp was associated with reduced prevalence of microscopic and PCR-detected malaria infections and anemia compared with SP-IPTp alone in HIV-infected pregnant women. To our knowledge, this is the first report demonstrating the superior effectiveness of TMP-SMX against malaria compared with SP-IPTp in HIV-infected pregnant women. Our findings are similar to studies demonstrating the effectiveness of TMP-SMX in reducing malaria in children and HIV-infected adults [12–14]. However, a recent Ugandan study found no difference in the prevalence of placental malaria between HIV-infected pregnant who took TMP-SMX and HIV-uninfected pregnant women who took SP-IPTp [8]. This result is difficult to compare with our findings because HIV-infected and uninfected pregnant women used different drugs. Nevertheless, previous studies have shown that malaria infection is more frequent and SP-IPT is less efficacious in HIV-infected than in HIV-uninfected pregnant women [29–32]. In the Ugandan study, TMP-SMX reduced the risk of malaria in HIV-infected pregnant women to a level similar to SP-IPT in HIV-uninfected women [8], which indirectly suggests the superior efficacy of TMP-SMX in preventing malaria in HIV-infected pregnant women.
The antimalarial effects of TMP-SMX and SP depend on their ability to inhibit parasite dihydrofolate reductase (DHFR) and deoxyhypusine synthase (DHPS), thereby blocking parasite folate synthesis. The setting where this study was conducted has a high prevalence of triple Asn-108/Ile-51/Arg-59 DHFR and double Gly-437/Glu-540 DHPS mutations, which reduce the efficacy of these drugs and has rendered SP ineffective in children aged <5 y [33]. Why was TMP-SMX more effective than SP? First, this could be because of frequent dosing of TMP-SMX to the pregnant women compared with SP. TMP-SMX prophylaxis was taken daily which may have resulted in longer period of malaria protection compared with 2 or 3 doses of intermittent SP taken during pregnancy. Also, in pregnant women, treatment doses of SP (1500 mg, stat) given intermittently may not achieve adequate blood levels to prevent or clear malaria infection because of increased blood elimination of sulphadoxine and pyrimethamine [34]. Thus, the duration of effective prevention using SP could be limited because of longer period of malaria susceptibility compared with that of daily TMP-SMX. This explanation is partly supported by our observation of lower prevalence of malaria infection in women who took daily TMP-SMX for a longer duration compared to women who took TMP-SMX for a shorter duration. Some investigators have even suggested increasing the number of SP doses in HIV-infected pregnant women not on daily TMP-SMX [35]. Second, based on in vitro studies, it is possible that there is incomplete cross-resistance to pyrimethamine and trimethroprim [36], although other studies have found complete cross-resistance [37]. In vivo, some parasites that are resistant to pyrimethamine have been shown to be sensitive to trimethroprim [38].
Because TMP-SMX and SP are antifolates, intake of both drugs may increase the risk of anemia because of inhibition of folate synthesis [39]. TMP-SMX intake during pregnancy has been associated with increased risk of folate deficiency, maternal anemia, poor birth outcomes [40–43], and neural birth defects when taken in the first trimester [44–46]. However, we found that TMP-SMX with or without SP-IPTp was associated with reduced prevalence of maternal anemia and higher hemoglobin concentration, consistent with beneficial effects in birth outcomes as reported previously [47].
Our study had several limitations. First, changes in potential confounding factors such as ITN use, antenatal attendance, and ARV therapy may have occurred parallel to the change in antimalarial prevention policy, thereby explaining the observed difference between the treatment groups. We attempted to control for these factors in the multivariate analyses, but because the study was not randomized, there could still be residual confounding from unmeasured factors. Because of the cross-sectional design of our study, with only 1-point measurement of both the outcomes and exposure, no causal inferences can be made from our findings. Second, our study may have underestimated the true impact of TMP-SMX with or without SP-IPTp on malaria infection or anemia because participants were enrolled only in the third trimester of pregnancy. The study may have missed some malaria infections that occurred in the first or second trimester, during which pregnant women are at an increased risk of malaria infection [48]. Third, our results potentially may have been affected by information bias because antimalaria drug exposure was self-reported. Although antenatal records were used to verify prescription of medications, it was not possible to confirm compliance of drug intake because no drug levels were measured. Finally, our study did not evaluate the effects of TMP-SMX intake with or without SP-IPTp in different trimesters. This could have allowed better assessment of effects of TMP-SMX alone or in combination with SP-IPTp on fetal and maternal toxicity, especially if medication was taken during the first trimester, because simultaneous use of 2 sulfa-containing drugs is not recommended due to concerns about potential toxicity. Furthermore, our data were collected during the antenatal period only and did not follow up to delivery to assess the effects of TMP-SMX on birth outcomes such as placental malaria, low birth weight, and preterm birth deliveries. Nevertheless, the differences in malaria parasitemia among the groups were fairly large (Figure 1), suggesting real difference in the efficacy of these drugs.
Our study also demonstrated the challenges that resource-limited countries such as Malawi encounter when changing drug policies. In our retrospective study, some women received antimalarial drug regimens that were inconsistent with the newly introduced drug policy. This was common in settings where disease-specific programs such as HIV care services were not well integrated into the maternal health programs. This resulted in poor information exchange among staff providing care to the same client. Drug policy concerning TMP-SMX prophylaxis to prevent opportunistic infections was not adequately shared with or understood by antenatal clinic staff providing antenatal services. This experience shows that to effectively introduce a public health policy, proper planning and timely training of health personnel should always precede implementation, especially in settings where frequent policy changes do take place.
In conclusion, our study suggests that daily TMP-SMX is more effective at reducing malaria infections and anemia compared with SP-IPTp alone in HIV-infected pregnant women; however, the latter treatment was more effective than no antimalarial drug. This supports the policy of using daily TMP-SMX to prevent malaria in HIV-infected pregnant women instead of SP-IPTp. The fact that use of both TMP-SMX and SP-IPTp appeared to be even more efficacious than CTX alone warrants a randomized-controlled study assessing both the superior efficacy of the combination therapy and its safety.
Funding
This work was supported by the Fogarty International Center (5R01TW007305); and the National Institutes of Health (NIH/FIC 5 D43 TW006568-05).
Acknowledgments
We thank all the pregnant women who participated in this study; Dr Beatrice Mwagomba and the entire District Health Management Team and staff of Thyolo district hospital, for their support; members of staff from the Malawi-Liverpool Wellcome Clinical Research Programme and Médecins Sans Frontières-Luxembourg, including Dr Moses Massaquoi; Mrs Mphuka and her whole nursing team, for collecting all the data; Professor Steven Meshnick laboratory team at UNC-Chapel Hill, especially Dr Steve Taylor of Duke University, Stephanie Carrier, and Paul Trottman, for conducting trainings and technical support in performing PCR assays; and Visopo Harawa, James Palapandu, and Yohanne Makuta, for performing malaria slide microscopy.
References
- 1.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Perrault SD, Hajek J, Zhong K, et al. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am J Trop Med Hyg. 2009;80:119–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead RL. Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Trop Med Int Health. 1999;4:5–12. doi: 10.1046/j.1365-3156.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 4.van Eijk AM, Ayisi JG, ter Kuile FO, et al. HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. AIDS. 2003;17:595–603. doi: 10.1097/00002030-200303070-00015. [DOI] [PubMed] [Google Scholar]
- 5.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:41–54. [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: Recommendations for a public health approach. Geneva, Switzerland: World health Organization; 2006. [Google Scholar]
- 7.World Health Organization. Geneva, Switzerland: World health Organization;; 2006. Guidelines for the treatment of malaria. 1st ed. [Google Scholar]
- 8.Newman PM, Wanzira H, Tumwine G, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshnick SR, Mwapasa V, Rogerson SJ. Protecting pregnant women from malaria in areas of high HIV infection prevalence. J Infect Dis. 2006;194:273–5. doi: 10.1086/505087. [DOI] [PubMed] [Google Scholar]
- 10.Briand V, Badaut C, Cot M. Placental malaria, maternal HIV infection and infant morbidity. Ann Trop Paediatr. 2009;29:71–83. doi: 10.1179/146532809X440699. [DOI] [PubMed] [Google Scholar]
- 11.Hamel MJ, Greene C, Chiller T, et al. Does cotrimoxazole prophylaxis for the prevention of HIV-associated opportunistic infections select for resistant pathogens in Kenyan adults? Am J Trop Med Hyg. 2008;79:320–30. [PubMed] [Google Scholar]
- 12.Kamya MR, Gasasira AF, Achan J, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–66. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 13.Mermin J, Ekwaru JP, Liechty CA, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: A prospective cohort study. Lancet. 2006;367:1256–61. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 14.Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–9. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Malaria and HIV interactions and their implications for public health policy. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 16.Government of Malawi Ministry of Health. Treatment of AIDS: Guidelines for the use of antiretroviral therapy in Malawi. 2nd ed. Lilongwe, Malawi: Malawi Ministry of Health; 2006. [Google Scholar]
- 17.Government of Malawi Ministry of Health. National guidelines for the diagnosis, treatment and prevention of malaria in Malawi. Lilongwe, Malawi: Malawi Ministry of Health; 2007. [Google Scholar]
- 18.Gilles HM. Diagnostic methods in malaria. In: Bruce-Chwatt LJ, Gilles HM, Warrell DA, editors. Essential malariology. 3rd ed. London, UK: Edward Arnold; 1993. pp. 78–98. [Google Scholar]
- 19.Taylor SM, Juliano JJ, Trottman PA, et al. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol. 2010;48:512–9. doi: 10.1128/JCM.01800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 21.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 22.Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic differences in health, nutrition, and population: Malawi 1992, 2000. Washington, DC: World Bank; 2000. Report No.: 39450. [PubMed] [Google Scholar]
- 23.Vyas S, Kumaranayake L. Constructing socio-economic status indices: How to use principal components analysis. Health Policy Plan. 2006;21:459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 24.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 26.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:9. doi: 10.1186/1471-2288-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutinho LM, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica. 2008;42:992–8. [PubMed] [Google Scholar]
- 28.Bello GA, Chipeta J, Aberle-Grasse J. Assessment of trends in biological and behavioural surveillance data: Is there any evidence of declining HIV prevalence or incidence in Malawi? Sex Transm Infect. 2006;82:i9–13. doi: 10.1136/sti.2005.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filler SJ, Kazembe P, Thigpen M, et al. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. J Infect Dis. 2006;194:286–93. doi: 10.1086/505080. [DOI] [PubMed] [Google Scholar]
- 30.Parise ME, Ayisi JG, Nahlen BL, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–22. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 31.Hamer DH, Mwanakasale V, Macleod WB, et al. Two-dose versus monthly intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine in HIV-seropositive pregnant Zambian women. J Infect Dis. 2007;196:1585–94. doi: 10.1086/522142. [DOI] [PubMed] [Google Scholar]
- 32.Gill CJ, Macleod WB, Mwanakasale V, et al. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. J Infect Dis. 2007;196:1577–84. doi: 10.1086/522137. [DOI] [PubMed] [Google Scholar]
- 33.Bwijo B, Kaneko A, Takechi M, et al. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 2003;85:363–73. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 34.Green MD, van Eijk AM, ter Kuile FO, et al. Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in Western Kenya. J Infect Dis. 2007;196:1403–8. doi: 10.1086/522632. [DOI] [PubMed] [Google Scholar]
- 35.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: A systematic review. JAMA. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 36.Petersen E. In vitro susceptibility of Plasmodium falciparum malaria to pyrimethamine, sulfadoxine, trimethoprim and sulfamethoxazole, singly and in combination. Trans R Soc Trop Med Hyg. 1987;81:238–41. doi: 10.1016/0035-9203(87)90226-4. [DOI] [PubMed] [Google Scholar]
- 37.Iyer JK, Milhous WK, Cortese JF, Kublin JG, Plowe CV. Plasmodium falciparum cross-resistance between trimethoprim and pyrimethamine. Lancet. 2001;358:1066–7. doi: 10.1016/S0140-6736(01)06201-8. [DOI] [PubMed] [Google Scholar]
- 38.Martin DC, Arnold JD. Treatment of acute falciparum malaria with sulfalene and trimethoprim. JAMA. 1968;203:476–80. [PubMed] [Google Scholar]
- 39.Peters PJ, Thigpen MC, Parise ME, Newman RD. Safety and toxicity of sulfadoxine/pyrimethamine: Iimplications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007;30:481–501. doi: 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- 40.Siega-Riz AM, Savitz DA, Zeisel SH, Thorp JM, Herring A. Second trimester folate status and preterm birth. Am J Obstet Gynecol. 2004;191:1851–7. doi: 10.1016/j.ajog.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 41.Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr Bull. 2008;29:S101–15. doi: 10.1177/15648265080292S114. [DOI] [PubMed] [Google Scholar]
- 42.Folate and vitamin B12 deficiencies: Pproceedings of a WHO technical consultation held 18–21 October, 2005, in Geneva, Switzerland. Introduction. Food Nutr Bull. 2008;29:S3–4. [PubMed] [Google Scholar]
- 43.Heimpel H, Raghavachar A. Hematological side effects of co-trimoxazole. Infection. 1987;15:S248–53. doi: 10.1007/BF01643198. [DOI] [PubMed] [Google Scholar]
- 44.Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–14. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 45.Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153:961–8. doi: 10.1093/aje/153.10.961. [DOI] [PubMed] [Google Scholar]
- 46.Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. The teratogenic risk of trimethoprim-sulfonamides: A population based case-control study. Reprod Toxicol. 2001;15:637–46. doi: 10.1016/s0890-6238(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 47.Walter J, Mwiya M, Scott N, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. J Infect Dis. 2006;194:1510–8. doi: 10.1086/508996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen-Dinh P, Steketee RW, Greenberg AE, Wirima JJ, Mulenda O, Williams SB. Rapid spontaneous postpartum clearance of Plasmodium falciparum parasitaemia in African women. Lancet. 1988;2:751–2. doi: 10.1016/s0140-6736(88)90229-2. [DOI] [PubMed] [Google Scholar]