Abstract
Background. Vibrio parahaemolyticus causes acute gastroenteritis and inflammations in humans. A variety of pathogenic bacteria can stimulate mitogen-activated protein kinases (MAPKs) in host cells. Phosphorylation of MAPKs leads to production of interleukin (IL)— 8 and subsequently causes inflammations. Thus, MAPK cascades were strong candidates for the main signaling pathway of V. parahaemolyticus-induced acute inflammation
Methods. To determine whether the signaling pathway on V. parahaemolyticus infection induces inflammation, we analyzed the secretion level of IL-8 and phosphorylation of MAPKs by use of intestinal epithelial Caco-2 cells.
Results. V. parahaemolyticus infection of Caco-2 cells activated extracellular signal-regulated kinase (ERK) 1/2 and p38 MAPK signal pathways, leading to IL-8 secretion, whereas MAPK inhibitors, UO126 or SB203580, suppressed IL-8 secretion. A strain carrying a deletion of VP1680, a type three secretion system 1 (T3SS1) effector protein, failed to activate phosphorylation of ERK1/2 and p38 MAPK and secretion of IL-8. ERK1/2 pathway inhibitor, UO126, failed IL-8 promoter activity, whereas p38 MAPK inhibitor, SB203580, decreased the stabilization of IL-8 messenger RNA following V. parahaemolyticus infection.
Conclusions. We showed that V. parahaemolyticus infection of Caco-2 cells results in the secretion of IL-8, and that VP1680 plays a pivotal role in manipulating host cell signaling and is responsible for triggering IL-8 secretion.
Vibrio parahaemolyticus, a human pathogenic Vibrio species, is a gram-negative halophilic bacterium that naturally occurs in marine and estuarine environments [1]. This organism causes food-borne acute gastroenteritis, often associated with the consumption of raw or undercooked seafood [2, 3]. Clinical symptoms of V. parahaemolyticus infections include watery diarrhea, abdominal cramps, nausea, vomiting, headaches, fever, and chills [4, 5]. Strong associations have been found between gastroenteritis and the thermostable direct hemolysin (TDH) and/or TDH-related hemolysin (TRH), which are encoded by the tdh and trh genes, respectively [6–8]. In contrast, enterotoxic studies have disclosed that a tdh-deletion mutant in a trh-negative strain maintained partial fluid-accumulating activity [9]. Furthermore, TDH and TRH are not associated with V. parahaemolyticus disruption of epithelial cell tight junctions [10]. These studies suggest that other unknown factors contribute to the pathogenesis of V. parahaemolyticus.
Analysis of the genome sequence of V. parahaemolyticus strain RIMD2210633 revealed 2 sets of the type III secretion system (T3SS), one set on chromosome 1 (T3SS1) and one set on chromosome 2 (T3SS2) [11]. During infection, T3SSs are able to inject virulence factors (also called effectors) from the bacterial cell directly to the host cell, where they interfere with normal cellular functions [12, 13]. In V. parahaemolyticus infection, the cellular dysfunction caused by T3SS-containing pathogens is remarkable. T3SS2 is found in only Kanagawa phenomenon (KP)-positive strains and produces enterotoxicity that can be assayed using the rabbit ileal loop model. T3SS1 has been found in all isolated strains and is related to the cytotoxicity observed in HeLa cells [14]. Activation of T3SS1 of V. parahaemolyticus causes parallel autophagy, cell rounding, and cell lysis in host cells that eventually lead to the proinflammatory death of infected cells [15]. Nevertheless, the mechanism of T3SS1-induced cell death-dependent inflammatory responses in host cells remains poorly understood.
Mitogen-activated protein kinases (MAPKs) are a group of serine/threonine protein kinases that regulate the transcription of inflammatory cytokines, including interleukin (IL)— 8, in response to various extracellular stimuli through a cascade of protein phosphorylation, leading to the activation of transcription factors [16]. A variety of bacteria can stimulate MAPKs in host cells upon infection [17]. Because V. parahaemolyticus causes acute gastroenteritis and inflammations in humans [18], MAPK cascades have been presented as candidates for the main signaling pathway of V. parahaemolyticus-induced acute inflammations. However, it is not known whether V. parahaemolyticus infection actually induces MAPK activation and inflammatory cytokine production.
MAPKs include 3 groups of family members: extracellular signal-regulated kinases (ERK) 1 and 2 (also known as p44/p42 MAPK), stress-activated protein kinases/c-Jun N-terminal kinase (SAPK/JNK) and p38 MAPK. Three MAPK pathways contribute to IL-8 gene expression. All of them are activated by phosphorylation through MAPK kinases (MKK) and have been shown to regulate activator protein-1 (AP-1) activity [19].
This study was designed to investigate the ability of V. parahaemolyticus inflammation to induce IL-8 secretion via MAPK signaling. We showed that VP1680, an effector protein of V. parahaemolyticus secreted by T3SS1, induces IL-8 production in host cells through MAPKs (ERK1/2 and p38 MAPK). This article described the mechanism underlying V. parahaemolyticus-induced inflammations.
MATERIALS AND METHODS
Bacterial Strains and Culture Conditions
V. parahaemolyticus strain RIMD2210633 (KP-positive, serotype O3:K6) was used as the standard strain [11]. The bacteria were cultured at 37°C with shaking in Luria-Bertani (LB) medium supplemented with 3% NaCl.
Deletion Mutants and Complementation of Deleted Genes in Mutant Strains of V. parahaemolyticus
Deletion mutant strains (ΔtdhAS, ΔT3SS1, ΔT3SS2, ΔVP1680, ΔVP1686, and ΔVPA0450) were previously described [9, 14, 20]. Complementation of deleted genes was performed as previously described [20]. Polymerase chain reaction (PCR) products were cloned into pSA19CP-MCS, and the plasmid construct was introduced into deletion mutant strains by electroporation.
Cell Culture
Caco-2 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Gibco BRL), and 100 μg/mL gentamicin (Sigma-Aldrich). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. The cells were seeded in 6-well culture dishes at a density of 2 × 105 cells/well. Caco-2 cells that had been cultured for 4 to 5 days were used for subsequent experiments.
Infection Protocol
At least 4 hours before infection, the culture medium was replaced with fresh DMEM (without supplements). After cultivation, bacteria were harvested by centrifugation and resuspended in phosphate buffered saline (PBS) (pH 7.4). The concentration was adjusted with PBS. Bacteria were added to each well (2 × 104 colony-forming units/dish). Infections were allowed to proceed at 37°C in 5% CO2.
Measurement of IL-8 Secretion Levels
The level of IL-8 released into the culture medium was assessed using an enzyme-linked immunosorbent assay (ELISA) kit (Pierce), in accordance with the manufacturer's instructions.
Preparation of Cell Lysates
Following the infection period, the medium was removed and the cells were washed once with cold PBS. The cells were solubilized using RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 5 μg/mL aprotinin, 5 μg/mL leupeptin, 1% Triton X - 100, 1% sodium deoxycholate, and .1% sodium dodecyl sulfate [SDS]). Cell lysates were homogenized using a needle and syringe then centrifuged at 15,000 rpm for 10 minutes at 4°C. Total protein was determined using a BCA Protein Assay Kit (Pierce) and stored at −80°C.
Isolation of Soluble, Cytoplasmic, and Nuclear Extracts
Following bacterial infection, the cells were washed once with cold PBS and cells were lysed in Nuclei Lysis Buffer (10 mM Tris-HCl, pH 7.6, 10 mM NaCl, 3 mM MgCl2, .5% NP-40). Cell lysates were centrifuged at 2000 × g for 4 minutes at 4°C. Thereafter, the supernatants (cytoplasmic extracts) were transferred to a fresh tube, and the nuclear pellets were resuspended in RIPA buffer (nuclear extracts).
Western Blotting
The cell lysates were mixed with sample buffer, boiled for 5 minutes, and separated through an SDS - polyacrylamide gel electrophoresis (PAGE). Following electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with primary antibody and incubated with horseradish peroxidase-conjugated secondary antibody. The blots were visualized with the enhanced chemiluminescence (ECL) Western Blotting Kit (GE Healthcare Bio-Sciences). Rabbit-anti NF-κBp65 and Lamin A antibodies were obtained from Santa Cruz Biotechnology. ERK1/2, p38 MAPK, ATF-2, CREB, c-Fos, c-Jun, B-actin, phospho-ERK1/2, phosphor-p38 MAPK, phosphor-ATF-2, and phosphor-CREB antibodies were purchased from Cell Signaling Technology.
Construction of IL-8 Luciferase Reporter Gene Constructs
The 5′-flanking region spanning from -133 to +44 bp of the IL-8 gene was created by PCR using the forward primer (5′-AGTGTGATGACTCAGGTTTGCC-3′), reverse primer (5′-AGCTTGTGTGCTCTGCTGTCTC-3′), and Caco-2 genomic DNA. PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen). Each DNA insert was digested with KpnI and XhoI and cloned into pGL3 (digested with the same enzymes). This plasmid (pGL3 -133) was used as a standard construct.
Site-directed mutagenesis of the IL-8 promoter was performed with pGL3-133 plasmid and the PCR primers. The primers used for point mutation of the AP-1 site (TGACTCA to TATCTCA; mutation is underlined) were 5′-AGTGTGATATCTCAGGTTTGCC-3′ (forward) and 5′-GGCAAACCTGAGATATCACACT-3′ (reverse). For C/EBP (CAGTTGCAAATCGT to AGCTTGCAAATCGT), the point mutation primers were 5’-GGATGGGCCATAGCTTGCAAATCGTGG-3′ (forward) and 5′-CCACGATTTGCAAGCTATGGCCCATCC-3′ (reverse). For NF-κB (GGAATTTCCT to TAACTTTCCT), the point mutation primers were 5′-GTTGCAAATCGTTAACTTTCCTCTGACATAATG-3′ (forward) and 5′-CATTATGTCAGAGGAAAGTTAACGATTTGCAAC-3′ (reverse). These plasmid constructs were confirmed by sequencing.
Transfection and Luciferase Reporter Gene Assay
Caco-2 (1 × 105) cells transfected with the indicated constructs were seeded on 24-well plates and cultured for 36 hours in DMEM. Constructs (.4 μg) were mixed with .1 μg β-galactosidase (β-gal) expression vector, pCMV-β, in 25 μL of DMEM. The solution was mixed with .8 μL of Lipofectamine 2000 Reagent diluted in 25 μL of DMEM and incubated at room temperature for 20 minutes. After incubation, the cell culture medium was replaced with 200 μL of fresh DMEM (without supplements) and 50 μL of the constructs in DMEM solution were cotransfected into Caco-2 cells. After a 6-hour transfection, the medium was replaced with fresh DMEM. The next day, the cells were infected by bacteria for 6 hours, followed by the addition of gentamicin (100 μg/mL) to each well to avoid excess infection. After incubating for 12 hours, the cells were washed with 200 μL of ice-cold PBS and lysed by adding 100 μL of lysis buffer, supplied with the Luciferase Assay Kit (Promega). The lysates were assayed for luciferase activity and β-gal activity. Transfection efficiency for the luciferase activity was normalized against β-gal activity.
Immunostaining
Caco-2 cells plated on glass cover slips in 6-well plates were infected by bacteria for 6 hours. After infection, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 10 minutes and washed 3 times with PBS. Cells permeabilized with PBS containing .1% Triton X - 100 for 7 minutes were treated with 3% BSA blocking buffer for 60 minutes. They were then incubated with primary antibody overnight at 4°C and washed 3 times with PBS. Secondary antibody conjugated with Alexa Fluor 568 (Molecular Probes) was incubated at room temperature for 60 minutes and stained with 500 nM DAPI (Molecular Probes) for 5 minutes at room temperature. NF-κBp65 (Santa Cruz Biotechnology) antibody was used at a dilution of 1/500, and secondary antibody at a dilution of 1/200.
RNA Extraction and Complementary DNA (cDNA) Synthesis
Total RNA was extracted from infected Caco-2 cells using TRIzol reagent (Invitrogen). In this process, RNA was treated with RNase-free DNase I (TaKaRa) to prevent carryover of genomic DNA. cDNA synthesis was prepared from 1 μg of the total RNA using the PrimeScript RT-Reagent Kit (TaKaRa), in accordance with the manufacturer's instructions.
Quantitative Real-Time Reverse Transcription PCR
Quantitative real-time reverse-transcription PCR (real-time RT-PCR) was performed in the LightCycler Real-Time PCR System (Roche Applied Science) with SYBER Premix Ex Taq (TaKaRa). Thermocycling was performed in a final volume of 20 μL containing 2 μL of cDNA, .2 μM of each primer, and 10 μL of SYBER Premix Ex Taq. After the reaction, the specificity of PCR products was confirmed with melt curve analysis and agarose gel electrophoresis.
The expression levels of IL-8 were normalized with the 18S ribosomal RNA housekeeping gene. Human-specific primers for IL-8 were 5′-CGGAAGGAACCATCTCACTG-3′ (forward) and 5′-AGCACTCCTTGGCAAAACTG-3′ (reverse). 18S primers were 5′-AAACGGCTACCACATCCAAG-3′ (forward) and 5′-GGCCTCGAAAGAGTCCTGTA-3′ (reverse).
RESULTS
V. parahaemolyticus Induces VP1680-Dependent IL-8 Secretion in Caco-2 cells Through T3SS1
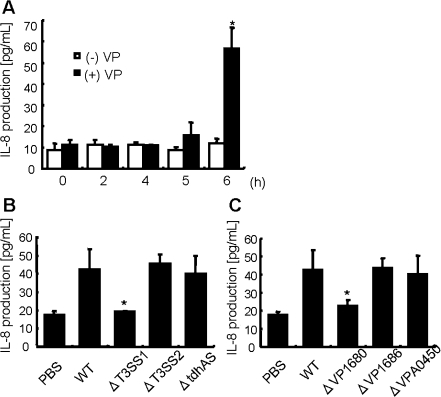
To develop a better understanding of the immunologic and inflammatory responses in host cells, we analyzed secretion levels of the inflammatory-related chemokine, IL-8, and the contribution of TDH or T3SSs to IL-8 secretion. Infection with V. parahaemolyticus increased the secretion of IL-8 from the host cells in a time-dependent manner (Figure 1A). T3SS1 (ΔvcrD1), T3SS2 (ΔvcrD2), and TDH (tdhA and tdhS) deletion mutants were used to determine which genes influence IL-8 secretion levels. Infection with the T3SS1 deletion strain did not increase IL-8 release. In contrast, infection with the T3SS2 deletion mutant or the TDH deletion strain increased Caco-2 cells IL-8 secretions (Figure 1B). Moreover, deletion of VP1680, a T3SS1-dependent effector [20], reduced the IL-8 release from Caco-2 cells (Figure 1C). These data clearly indicated that VP1680 was the main effector triggering IL-8 secretion in Caco-2 cells.
Figure 1.
V. parahaemolyticus induces IL-8 production in Caco-2 cells. A, Caco-2 cells were infected with V. parahaemolyticus (black bar) or left uninfected (white bar) for the indicated times. Statistical significance: *, P < .05 compared with (-)VP-0 hour. B, Caco-2 cells were infected with wild-type V. parahaemolyticus (WT) or mutant strains ΔtdhAS, ΔT3SS1 (ΔvcrD1), ΔT3SS2 (ΔvcrD2), ΔVP1680, ΔVP1686, or ΔVPA0450 for 6 hours (B, C). IL-8 production in the culture medium was measured by enzyme-linked immunosorbent assay (ELISA). The results are shown as the mean ± SD calculated from 3 independent experiments. Statistical significance: *, P < .05 compared with WT.
Implication of MAPKs in V. parahaemolyticus-Induced IL-8 Production
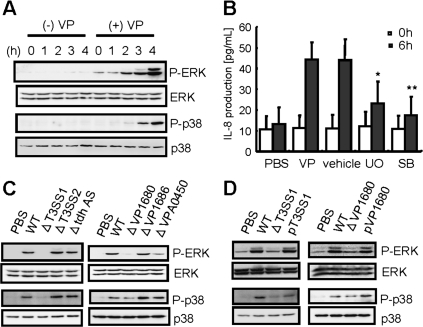
There have been many reports that IL-8 production and secretion are regulated by the activity of MAPKs [21, 22]. Mainly, MAPKs separate to 3 pathways, ERK1/2, p38, and JNK. To determine the role of IL-8 production in host cells, we first investigated whether phosphorylation of MAPKs was activated by V. parahaemolyticus infection. ERK1/2 and p38 MAPK were gradually phosphorylated during 3-hour infections of V. parahaemolyticus (Figure 2A). In contrast, JNK, which regulates transcription through its effects on c-Jun and other transcription factors, was not evidently phosphorylated by infection (data not shown). Pre-treatment of host cells with UO126, an inhibitor of the MEK/ERK pathway, or SB203580, an inhibitor of p38 MAPK, significantly reduced IL-8 secretion (Figure 2B). These data suggested that ERK1/2 and p38 MAPK activities contribute to IL-8 secretion after V. parahaemolyticus infection. As shown in Figure 1B–C, VP1680 was expected to be a very strong candidate for V. parahaemolyticus triggering IL-8 production. The specificity of MAPKs phosphorylation in response to VP1680 function was tested by infecting Caco-2 cells with WT V. parahaemolyticus, T3SS1, T3SS2, tdhAS, and strains deleted for T3SS1-dependent effectors (VP1680, VP1686, and VPA0450) for 3 hours (Figure 2C). The T3SS1 and VP1680 deletion mutants suppressed the phosphorylation of ERK1/2 and p38 MAPK stimulated by V. parahaemolyticus infection, which was restored by complementation with wild type T3SS1 and VP 1680 (Figure 2D). The results suggested that VP1680-induced production of IL-8 occurs proximal to, or at the point of, the activation of the ERK1/2 and p38 MAPK signal pathways.
Figure 2.
Activation of MAPK is essential for the production of IL-8 induced by V. parahaemolyticus. A, Caco-2 cells were infected with V. parahaemolyticus for the indicated times. After infection, the cells were lysed and normalized for protein content. Activation of MAPKs (ERK1/2 and p38) was analyzed by Western blotting with anti-phospho-p44/42 (P-ERK1/2) and anti-phospho-p38 (P-p38) specific antibodies. The blots were stripped and re-probed with anti-p44/42 (ERK1/2) and anti-p38 (p38) antibodies. B, Caco-2 cells were treated with the MAPK inhibitor UO126 (10 μM) or SB203580 (30 μM) for 60 minutes prior to infection. IL-8 production was measured by enzyme-linked immunosorbent assay (ELISA). C, D, Caco-2 cells were infected with wild type V. parahaemolyticus (WT); mutant strains ΔtdhAS, ΔT3SS1 (ΔvcrD1), ΔT3SS2 (ΔvcrD2), ΔVP1680, ΔVP1686, ΔVPA0450, pT3SS1 (expressing plasmid encoded vcrD1 into ΔvcrD1); or pVP1680 (expressing plasmid-encoded VP1680 into ΔVP1680). Three hours after infection, the cells were lysed and normalized for protein content. Activation of ERK1/2 and p38 MAPK was analyzed by Western blotting. Data are means ± SD of 3 independent experiments with assays in duplicate. Statistical significance: *, P < .05; **, P < .01 compared with 6 hour-VP.
ERK1/2 Plays an Important Role in V. parahaemolyticus-Induced IL-8 Transcription
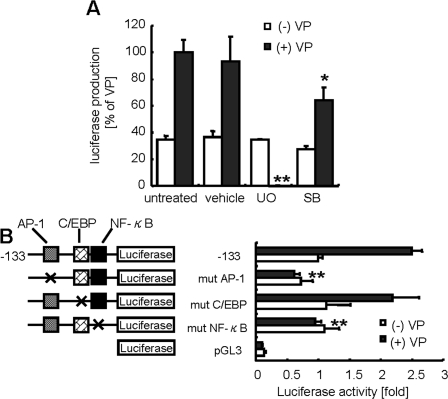
A study of the effect of MAPK inhibitors on the transcription of IL-8 promoter-luciferase constructs transfected into Caco-2 cells revealed that UO126 led to an apparent reduction of luciferase activity. Treatment with SB203580 also produced a trend of decreasing luciferase activity, but the effect was much lower than with UO126 (Figure 3A). The data imply that activation of both p38 MAPK and ERK1/2 is essential for IL-8 production, and that ERK1/2 in particular may play a major role in IL-8 transcription triggered by V. parahaemolyticus infection. The MAPK inhibitors, UO126 or SB203580, did not affect the cell viabilities (data not shown).
Figure 3.
The ERK1/2 pathway plays an important role in V. parahaemolyticus infection-triggered AP-1 and NF-κB mediated IL-8 transcription. Activation of IL-8 transcription was estimated by luciferase reporter assay. Caco-2 cells were transfected with an IL-8 promoter (-133 to +44 bp within the 5’ flanking region) construct. A, After a 6- hour infection, gentamicin (100 μg/mL) was added to avoid excess infection. The cells were incubated for 12 hours, and cell extracts were prepared for determination of luciferase activity. Statistical significance: *, P < .05; **, P < .01 compared with untreated (+)VP. B, Mutant (mut) vectors containing AP-1, C/EBP1, or NF-κB binding sites were transfected into Caco-2 cells, which were infected with V. parahaemolyticus (black bar) or left uninfected (white bar) for 6 hours. The next day, the cells were lysed and luciferase activity was measured. The results are presented as the relative increase in activation (n-fold) of control cells (transfected with standard construct). Luciferase activity was normalized against β-galactosidase activity. The results are presented as the relative increase in active control cells (n-%). Data are means ± SD of 3 independent experiments with assays in duplicate. Statistical significance: *, P < .05; **, P < .01 compared with untreated -133 (+)VP.
V. parahaemolyticus Regulates IL-8 Secretion Through AP-1 and NF-κB Sites in the IL-8 Promoter
The distinction in IL-8 suppression produced by UO126 and SB203580 treatments suggests differential regulation of IL-8 secretion by ERK1/2 and p38 MAPK. IL-8 production is regulated by 2 pathways: ERK1/2 regulates trans-activational levels and p38 MAPK controls messenger RNA (mRNA) stability [21, 22]. Thus, the contributions of ERK1/2 and p38 MAPK to IL-8 secretions induced by V. parahaemolyticus infections needed to be clarified. Production of IL-8 can occur through transcriptional and posttranscriptional mechanisms. The sequence spanning nucleotides -133 to -1 in the 5’ flanking region of the IL-8 gene is necessary and essential for transcriptional regulation [23, 24]. This sequence contains binding sites for AP-1, CCAAT/enhancer binding protein (C/EBP) and NF-κB. To define which sites in the IL-8 promoter are responsible for IL-8 transcription, Caco-2 cells were transfected with IL-8 reporter plasmids containing site-specific point mutations in the AP-1, C/EBP, or NF-κB binding site. V. parahaemolyticus-induced luciferase activity of the wild-type promoter was elevated ∼2.5-fold, compared with non-infected cells. This effect was reduced to uninfected levels by mutation of AP-1 or NF-κB regions, suggesting that the V. parahaemolyticus infection was mediated in part through the AP-1 and NF-κB regions (Figure 3B).
V. parahaemolyticus-Induced Expression of c-Fos and c-Jun is Dependent on ERK1/2 Activation
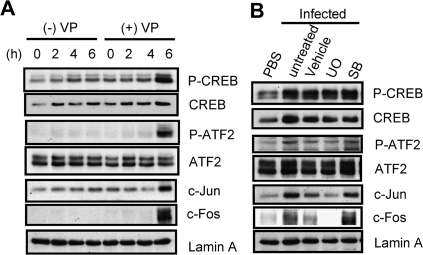
AP-1 is a dimeric complex that contains members of the Jun (c-Jun, JunB, JunD), Fos (c-Fos, Fra-1, Fra-2, FosB), and ATF/CREB families [25]. Although Fos and Jun involve many members, c-Fos and c-Jun were the main elements in the bacterial infections that induced IL-8 secretions [26–28]. Thus, we focused on the c-Jun and c-Fos. To evaluate the involvement of ATF-2, CREB, c-Fos, and c-Jun in IL-8 production, we examined the relation between transcription factors and MAPKs with nuclear extracts isolated from infected Caco-2 cells. The expression of c-Fos protein is rapidly and transiently induced by a variety of stimuli [29]. Fos proteins dimerize with Jun to form AP-1 [30]. Phosphorylation of ATF-2 and CREB were clearly detected after a 6-hour infection, along with distinct expression of c-Fos and c-Jun (Figure 4A). c-Fos and c-Jun protein expression induced by V. parahaemolyticus infection were reduced by UO126 pretreatment. However, the phosphorylation levels of ATF-2 and CREB were not reduced by the MAPK inhibitors UO126 or SB203580 (Figure 4B). The results suggest that c-Fos and c-Jun regulation are required for V. parahaemolyticus-induced IL-8 production, which is regulated by activation of ERK1/2. These results are consistent with the study of the effect of MAPK inhibitors on the transcription of IL-8 promoter-luciferase (Figure 3A). Thus, transcriptional regulation is mainly controlled by the ERK1/2 pathway.
Figure 4.
V. parahaemolyticus infection regulates c-Fos and c-Jun expression via the ERK1/2 pathway. A, Caco-2 cells were infected with V. parahaemolyticus (+VP) or left uninfected (- VP) for the indicated times. B, Caco-2 cells were treated with inhibitors UO126 (10 μM) or SB203580 (30 μM) for 60 minutes prior to infection. After a 6-hour infection, nuclear extracts were isolated from the infected cells and transcription factor activity and expression level were assayed by Western blotting. A blot representative of those obtained from 3 independent experiments is shown.
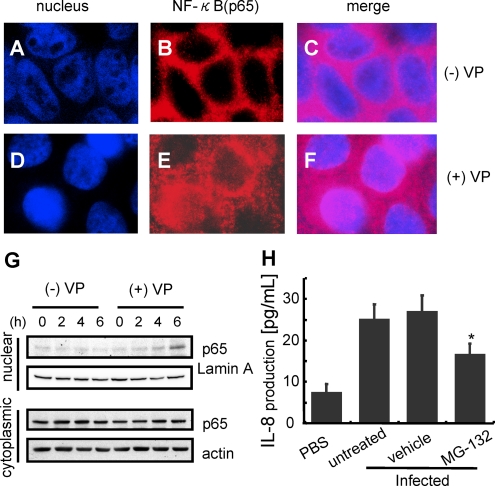
V. parahaemolyticus-Induced Activation and Translocation of NF-κB to the Nucleus
In the luciferase reporter gene assay, activation of the IL-8 promoter was required for activation of the transcription factor NF-κB by V. parahaemolyticus infection (Figure 3B). To examine the ability of V. parahaemolyticus to stimulate IL-8 induction through an NF-κB-mediated pathway, infected Caco-2 cells were analyzed for translocation of NF-κB to nucleus and its association with IL-8 release. Immunostaining with anti-NF-κBp65 showed V. parahaemolyticus-induced translocation of NF-κBp65 to the nucleus, whereas uninfected cells did not cause NF-κBp65 translocation (Figure 5A–G). A similar result was acquired by Western blotting with nuclear extracts. Treatment with MG132, a proteasome inhibitor that blocks the degradation of IκBα and nuclear translocation of unphosphorylated p65, led to the reduction in V. parahaemolyticus-induced IL-8 production by Caco-2 cells (Figure 5H). These data indicate that the NF-κB pathway also activates V. parahaemolyticus-induced IL-8 expression.
Figure 5.
V. parahaemolyticus induced activation and translocation of NF-κB. Caco-2 cells were uninfected (A - C) or infected with V. parahaemolyticus (D - F). The cells were fixed with 4% paraformaldehyde for 10 minutes and treated with .1% Triton X - 100 for 7 minutes. The fixed cells were incubated with anti-NF-κB antibody (1/500) in PBS containing 3% BSA. After treatment with the respective primary antibody, the cells were incubated with secondary antibody conjugated with Alexa fluor 568 (1/200) in PBS containing 3% BSA (B, E). Nuclei were stained with 500 nM DAPI followed by incubation with secondary antibody (A, D). G, Caco-2 cells were infected with V. parahaemolyticus (+VP) or uninfected (-VP) for the indicated times. H, After infection, nuclear extracts and cytoplasmic extracts were isolated from the infected cells, and the translocation level of NF-κB was shown by Western blotting. MG132 (25 μM), an NF-kB inhibitor, was added to the culture medium for 60 minutes prior to infection. IL-8 secretion into the medium was measured by enzyme-linked immunosorbent assay (ELISA). The results are shown as the mean ± SD calculated from 3 independent experiments. Statistical significance: *, P < .05 compared with untreated.
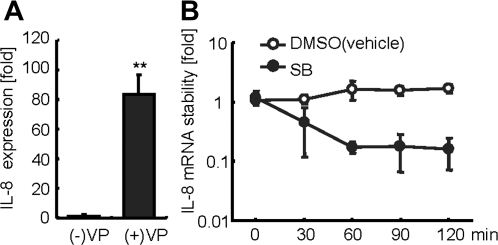
V. parahaemolyticus-Induced IL-8 mRNA is Stabilized by p38 MAPK Phosphorylation
V. parahaemolyticus-induced p38 MAPK activation was required for IL-8 production (Figure 2B). However, the contribution of p38 MAPK on V. parahaemolyticus-induced IL-8 transcription was relatively low (Figure 3A). We hypothesized that activated p38 MAPK enhances IL-8 mRNA stabilization, accelerating production of IL-8 protein. Analysis of the expression level of IL-8 mRNA induced by V. parahaemolyticus infection was accomplished using quantitative RT-PCR. Elevation of IL-8 mRNA was observed in Caco-2 cells following a 6-hour infection with V. parahaemolyticus (Figure 6A). The effect of p38 MAPK on IL-8 stability was determined by infecting cells for 6 hours to accumulate IL-8 mRNA. The cells were subsequently treated with gentamicin to block excess infection, actinomycin D to block transcription, and SB203580 to block p38 activity. Treatment with SB203580 caused a rapid decay of IL-8 mRNA. In contrast, IL-8 mRNA in control cells was more stable, compared with the mRNA of cells treated by the p38 MAPK inhibitor (Figure 6B). These data suggest that the p38 MAPK may play a part in IL-8 mRNA stabilization triggered by V. parahaemolyticus infection.
Figure 6.
.P-p38 MAPK stabilizes IL-8 messenger RNA (mRNA). A, Caco-2 cells were infected with V. parahaemolyticus for 6 hours to allow transcription of IL-8 mRNA. B, Infected Caco-2 cells were then treated with gentamicin (100 μg/mL), actinomycin D (5 μg/mL), and p38 inhibitor SB203580 (30 μM) or DMSO for the indicated times. Following inhibitor treatment, RNA was extracted and reverse transcribed by LightCycler polymerase chain reaction (PCR) to examine IL-8 mRNA stability. IL-8 mRNA was normalized with the 18S ribosomal RNA housekeeping gene. The results are presented as the relative decrease in stabilization of 18S ribosomal RNA. Data shown are the means ± SD of 3 independent experiments with assays in duplicate.
DISCUSSION
V. parahaemolyticus virulence has historically been associated with TDH or T3SSs [7–14]; however, their contributions to the inflammatory response are not well characterized. The paradigm of T3SS1-dependent cell death recently revealed that V. parahaemolyticus infection results in autophagy, cell rounding, and cell lysis, which eventually leads to the proinflammatory death of infected host cells [15]. These studies predicted that T3SS1-dependent cell death can be strongly related to V. parahaemolyticus-induced inflammation.
IL-8 is well known for its leukocyte chemotactic properties and associated role in inflammatory and infectious diseases [31]. To our knowledge, this is the first report that VP1680, a T3SS1-dependent virulence factor, is the main effector of V. parahaemolyticus responsible for triggering IL-8 secretion. Earlier reports using infections by Salmonella enterica serovar Typhimurium [32], enterohemorrhagic Escherichia coli (EHEC) [33], and enteropathogenic E. coli (EPEC) [34, 35] models provided increasing evidence that MAPK activation is important for IL-8 secretion. Thus, it was conceivable that MAPK signaling pathways also play a central role in the induction of IL-8 secretion induced after V. parahaemolyticus infection. Trosky et al [36] in 2004 reported that VopA (also known as VopP), a T3SS2 dependent effector, inhibits activation of the ERK pathway. T3SS1 appears to have the opposite effect on ERK signaling pathway against T3SS2. However, we did not observe any differences in the activities of MAPK signaling pathways between infection of wild-type V. parahaemolyticus and T3SS2 deletion mutants. We speculate that the inhibitory effect of T3SS2 on the ERK signaling pathway may be relatively lower than the activating ability of T3SS1 under our experimental conditions. Zhou et al [37] in 2008 reported that the expression level of T3SS1 is dramatically altered in each different culture condition. It is possible that activation of T3SS2 would differ depending on culture conditions, because the specific factors that exert an effect on expression level have not yet been identified.
Finally, we focused on determining how VP1680 modulates IL-8 secretion via MAPK. We found that the ERK1/2 pathway activates the transcription of IL-8, and that the p38 MAPK pathway stabilizes IL-8 mRNA, following V. parahaemolyticus infection. The ERK1/2 and p38 pathways are known to be activated by other bacterial infections, such as Yersinia enterocolitica [38]. It is interesting that ERK1/2 and p38 MAPK simultaneously stimulate different routes of IL-8 production via V. parahaemolyticus infection and virulence factor VP1680. It will be interesting to discern how VP1680 activates the 2 pathways in host cells at the same time, which will help elucidate the mechanism of the inflammation response in host cells.
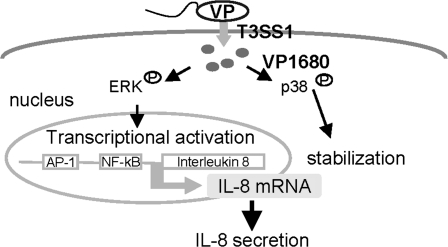
Finally, the stimulation of IL-8 induced by V. parahaemolyticus infection is summarized in Figure 7. Understanding how VP1680 manipulates host cell signaling and transcription in cells could serve as a paradigm for how Vibrio species modulate the innate immune system.
Figure 7.
Model depicting stimulation of IL-8 production by V. parahaemolyticus infection.
Acknowledgments
Financial support: This work was supported by a Grant-in-Aid for the 21st Century Center of Excellence (COE) Program, Human Nutritional Science on Stress Control, University of Tokushima, and Practical Application Research from the Japan Science and Technology Agency.
References
- 1.Daniels NA, MacKinnon L, Bishop R, et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis. 2000;181:1661–6. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 2.DePaola A, Kaysner CA, Bowers J, Cook DW. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998) Appl Environ Microbiol. 2000;66:4649–54. doi: 10.1128/aem.66.11.4649-4654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin JB, DePaola A, Bopp CA, et al. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med. 2005;353:1463–70. doi: 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 4.Barker WH, Jr., Gangarosa EJ. Food poisoning due to Vibrio parahaemolyticus. Annu Rev Med. 1974;25:75–81. doi: 10.1146/annurev.me.25.020174.000451. [DOI] [PubMed] [Google Scholar]
- 5.Levine WC, Griffin PM. Vibrio infections on the Gulf Coast: results of first year of regional surveillance. Gulf Coast Vibrio Working Group. J Infect Dis. 1993;167:479–83. doi: 10.1093/infdis/167.2.479. [DOI] [PubMed] [Google Scholar]
- 6.Nishibuchi M, Kaper JB. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect Immun. 1995;63:2093–9. doi: 10.1128/iai.63.6.2093-2099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda T, Ni YX, Miwatani T. Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect Immun. 1988;56:961–5. doi: 10.1128/iai.56.4.961-965.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda T, Abad-Lapuebla MA, Ni YX, Yamamoto K, Miwatani T. Characterization of a new thermostable direct haemolysin produced by a Kanagawa-phenomenon-negative clinical isolate of Vibrio parahaemolyticus. J Gen Microbiol. 1991;137:253–9. doi: 10.1099/00221287-137-2-253. [DOI] [PubMed] [Google Scholar]
- 9.Park KS, Ono T, Rokuda M, Jang MH, Iida T, Honda T. Cytotoxicity and enterotoxicity of the thermostable direct hemolysin-deletion mutants of Vibrio parahaemolyticus. Microbiol Immunol. 2004;48:313–8. doi: 10.1111/j.1348-0421.2004.tb03512.x. [DOI] [PubMed] [Google Scholar]
- 10.Lynch T, Livingstone S, Buenaventura E, et al. Vibrio parahaemolyticus disruption of epithelial cell tight junctions occurs independently of toxin production. Infect Immun. 2005;73:1275–83. doi: 10.1128/IAI.73.3.1275-1283.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makino K, Oshima K, Kurokawa K, et al. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet. 2003;361:743–9. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–25. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 13.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin Microbiol Rev. 2007;20:535–49. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KS, Ono T, Rokuda M, et al. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun. 2004;72:6659–65. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdette DL, Yarbrough ML, Orvedahl A, Gilpin CJ, Orth K. Vibrio parahaemolyticus orchestrates a multifaceted host cell infection by induction of autophagy, cell rounding, and then cell lysis. Proc Natl Acad Sci USA. 2008;105:12497–502. doi: 10.1073/pnas.0802773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–6. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 18.Qadri F, Alam MS, Nishibuchi M, et al. Adaptive and inflammatory immune responses in patients infected with strains of Vibrio parahaemolyticus. J Infect Dis. 2003;187:1085–96. doi: 10.1086/368257. [DOI] [PubMed] [Google Scholar]
- 19.Ono T, Park KS, Ueta M, Iida T, Honda T. Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect Immun. 2006;74:1032–42. doi: 10.1128/IAI.74.2.1032-1042.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 21.Holtmann H, Winzen R, Holland P, et al. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–53. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 23.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–33. [PubMed] [Google Scholar]
- 24.Harant H, de Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJ. Synergistic activation of interleukin-8 gene transcription by all-trans-retinoic acid and tumor necrosis factor-alpha involves the transcription factor NF-kappaB. J Biol Chem. 1996;271:26954–61. doi: 10.1074/jbc.271.43.26954. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 2005;35:2648–57. doi: 10.1002/eji.200526321. [DOI] [PubMed] [Google Scholar]
- 27.Choi IJ, Fujimoto S, Yamauchi K, Graham DY, Yamaoka Y. Helicobacter pylori environmental interactions: effect of acidic conditions on H. pylori-induced gastric mucosal interleukin-8 production. Cell Microbiol. 2007;9:2457–69. doi: 10.1111/j.1462-5822.2007.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, Park HR, Oh YK, et al. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med. 2007;85:1393–404. doi: 10.1007/s00109-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 29.Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A. Ets-related protein Elk-1 is homologous to the c-Fos regulatory factor p62TCF. Nature. 1991;354:531–4. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- 30.Han TH, Prywes R. Regulatory role of MEF2D in serum induction of the c-Jun promoter. Mol Cell Biol. 1995;15:2907–15. doi: 10.1128/mcb.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–64. [PubMed] [Google Scholar]
- 32.Hobbie S, Chen LM, Davis RJ, Galán JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella Typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–9. [PubMed] [Google Scholar]
- 33.Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect Immun. 2002;70:2304–10. doi: 10.1128/IAI.70.5.2304-2310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun. 2001;69:1298–305. doi: 10.1128/IAI.69.3.1298-1305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Grado M, Rosenberger CM, Gauthier A, Vallance BA, Finlay BB. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect Immun. 2001;69:6217–24. doi: 10.1128/IAI.69.10.6217-6224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trosky JE, Mukherjee S, Burdette DL, et al. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J Biol Chem. 2004;279:51953–7. doi: 10.1074/jbc.M407001200. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Shah DH, Konkel ME, Call DR. Type III secretion system 1 genes in Vibrio parahaemolyticus are positively regulated by ExsA and negatively regulated by ExsD. Mol Microbiol. 2008;69:747–64. doi: 10.1111/j.1365-2958.2008.06326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassl GA, Kracht M, Wiedemann A, et al. Activation of NF-kappaB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell Microbiol. 2003;5:957–71. doi: 10.1046/j.1462-5822.2003.00339.x. [DOI] [PubMed] [Google Scholar]