Abstract
Background. Tick-borne encephalitis virus (TBEV) infections may be asymptomatic or cause severe symptoms in the central nervous system. A mutation in the chemokine receptor 5 gene has been associated with increased risk of TBE but explains only a limited number of cases. Investigations of further risk factors are needed.
Method. To investigate the importance of the innate immune response, we analyzed 128 TBE patients, 77 patients with aseptic meningoencephalitis (AME) and 135 healthy controls, for 3mutations: 2 in the Toll-like receptor 3 (TLR3) gene and 1 in the 2′-5′-oligoadenylate synthetase (OAS1) gene.
Results. Although no association was found between the mutation in the OAS1 gene and TBE, the genotype distribution ofrs3775291, a mutation in TLR3, differed significantly between TBE patients and controls; 61%, 32%, and 7% of the TBE patients were carriers of the wild-type, heterozygous, and mutant genotype of rs3775291, respectively. The corresponding percentages among healthy controls (n = 126) were 52%, 29%, and 19% (P = .02), and among AME patients (n = 75) were 47%, 32%, and 21% (P = .009). Additionally, the wild-type rs3775291 allele was more common among TBE patients than among healthy controls (allele frequency, .768 vs .663; P = .01).
Conclusion. A functional TLR3 is a risk factor for TBEV infection.
Tick-borne encephalitis virus (TBEV) is a flavivirus of the family Flaviviridae that is transmitted to humans by Ixodes ricinus and Ixodes persulcatus ticks [1]. TBEVs are divided into 3 subtypes: Far Eastern, Siberian, and European [2]. The European subtype is endemic in large parts of northern and central Europe, and although most cases of European subtype TBEV infections are asymptomatic [2], a small fraction of patients develop tick-borne encephalitis (TBE) with symptoms such as meningitis or encephalitis [3, 4]. The difference in disease outcome between individuals is most likely due to viral properties and variations in the immune response of the patient [5]. We have earlier shown that a mutation in the chemokine receptor 5 (CCR5) gene increases the risk of TBE after TBEV infection [6]. This mutation was shown, however, to be associated with only a few TBE patients, and further investigation on host genetic association to TBE is needed.
Studies of the West Nile virus (WNV), a flavivirus that shares many characteristics with TBEV, have suggested an association between mutations in genes modulating the innate immune response and increased risk of severe WNV disease. An important molecule modulating the immune response is the Toll-like receptor 3 (TLR3), a receptor recognizing double-stranded RNA (dsRNA). Stimulation of TLR3 leads to the production of type I interferons (IFNs) and inflammatory cytokines such as tumor necrosis factor α (TNFα) [7]. Wang et al [8] first showed that a knockout of the TLR3 gene decreased the risk of central nervous system (CNS) infiltration after WNV infection in mice, suggesting a protective role of a non-unctional TLR3 gene. However, this report was followed by data from Daffis et al [9], who suggested that TLR3 has a protective role against severe WNV disease in mice.
Another modulator of the innate immune response is 2′-5′-oligoadenylate synthetase (OAS) encoded by the OAS1 gene. OAS catalyzes synthesis of 2′-5′-linked oligoadenylates (2-5A) from ATP. The 2-5A molecule can bind and activate ribonuclease L (RNaseL), ultimately leading to degradation of host and viral RNAs [10]. Lim et al [11] investigated the role of OAS in human WNV disease and found that a missense mutation in OAS1 increased the risk of initial WNV infection. These findings are in agreement with data from mouse studies [8], wherein a nonfunctional OAS was associated with decreased ability to protect the animals from WNV disease.
The objective of this study was to investigate the importance of mutations in TLR3 and OAS1 gene among severe TBEV infections. Examination of 128 Lithuanian patients with encephalitis and meningitis caused by TBEV infections revealed the wild-type TLR3 gene as a risk factor to develop TBE. This is the first study investigating a possible association between mutations in TLR3 and infection with a flavivirus in humans.
MATERIALS AND METHODS
The study material from the Lithuanian study was identical to that used in earlier studies [6, 12], with a few exceptions. We analyzed prospectively collected data from 128 TBE patients as well as 77 patients with aseptic meningoencephalitis (AME) who were antibody-negative for TBEV. We also included as controls 135 healthy Lithuanians, matched geographically and by age, who were seronegative for TBEV. As described in a previous article [6], TBE patients were stratified by severity of symptoms, as mild, moderate, or severe, depending on whether the patients suffered from meningitis (mild) or encephalitis with mono- (moderate) or muli-focal (severe) symptoms. All the DNA samples were analyzed for 3 missense mutations: rs5743305 in TLR3, rs3775291 in TLR3 and rs10774671 in OAS1.
We analyzed TLR3 rs5743305 (in the promoter region), TLR3 rs3775291 (in exon 4, Leu412Phe), and OAS1 rs10774671 (located in a splicing site) by polymerase chain reaction (PCR) and pyrosequencing, essentially as described in a previous article [6]. For PCR, we used ≥1 μL DNA, 2.5 μL 10xPCR buffer II (Applied Biosystems), 2 μL 50 mmol/L MgCl (Applied biosystems), 1 μL 10 mmol/L GeneAmp dNTP mix with dTTP (Applied Biosystems), 1 μL 20 μmol/L Fw, 1 μL 20 μmol/L reverse primer (Table 1), and .2 μL AmpliTaq Gold DNA Polymerase per sample. The final volume was adjusted with ultraclean water to a final volume of 25 μL. The reaction was performed at 95°C for 5 min followed by 50 cycles of 15 s at 95°C, 30 s at 55–65°C depending on the amplified sequence (Table 1), and 30 s at 72°C, and finally 1 cycle of 5 min at 95°C. Samples were frozen at −20°C before further analysis.
Table 1.
Primers, annealing temperatures and dispension orders used for amplification and sequencing of the different mutations
| Mutation (gene) | PCR primer pair | Sequencing primer | PCR annealing temperature | Dispension order |
| rs5743305 | F: 5′-GTAGCCCTGAGCCCAGTAACTATA-3′ | 5′- TGAGCCCAGTAACTATAAAG-3′ | TCGTAGCTA | |
| (TLR3) | R: 5′-BIOTIN-CCTAAGAACATGCCACAAGCCACCA-3′ | (forward direction) | 60°C | |
| rs3775291 | F: 5′-TCATTAAGGCCCAGGTCAAG-3′ | 5′- TTATTCTTGGTTAGGTTGA-3′ | GAGTATGT | |
| (TLR3) | R: 5′-BIOTIN-TGGCTAAAATGTTTGGAGCA-3′ | (forward direction) | 55°C | |
| rs10774671 | F: 5′-CCGTAAATGCTCACTGAATCC-3′ | 5′-TCATGTGTCTCACCCTT-3′ | GAGTATGT | |
| (OAS1) | R: 5′-BIOTIN-TGCAGGTCCAGTCCTCTTCT-3′ | (forward direction) | 60°C |
NOTE. PCR, polymerase chain reaction.
Pyrosequencing was performed in a PSQ 96 MA Instrument (Biotage) as described in an earlier article [6]. Sequencing primers and dispension orders are shown in Table 1. The method distinguishes between homozygous wild-type (wt/wt), heterozygous, and homozygous mutant (mut/mut) genotypes.
We statistically analyzed data by χ2 (3 x 2 contingency analysis) and Fisher exact test (2 x 2 contingency analysis) using Prism 5 software for Mac OS X (GraphPad).
The study was approved by the local ethical committee (M140-08) and all the participants gave their informed consent to participate in the study.
RESULTS
In total, 340 individuals were genotyped for each of the 3 single nucleotide polymorphisms (SNPs) included in the study. Of these, 1000 analyses were successful and 20 (2.0%) failed. Of the successful analyses, 1.2% were for TLR3 rs5743305, 3.5% were for TLR3 rs3775291, and 1.2% were for OAS1 rs10774671. Statistical calculations included only samples that were successfully genotyped. The frequencies of the mutated allele for TLR3 rs5743305 and OAS1 rs10774671 were in agreement with the frequencies earlier reported for Caucasian populations in the NCBI database for SNPs (http://www.ncbi.nlm.nih.gov/SNP/), whereas the frequency of the mutated (mut) allele of TLR3 rs3775291 was either in agreement or slightly higher than expected.
The 2′-5′Oligoadenylate Synthetase Gene
For OAS1 rs1077471, the genotype distribution among TBE patients was 69/128 (54%) for wt/wt, 50/128 (39%) for heterozygous, and 9/128 (7%) for mut/mut genotypes (Table 2). The genotype distribution among TBE patients was in concordance with that among controls (n = 135; 54% wt/wt, 43% heterozygous, and 3% mut/mut) and among AME the patients (n = 73; 55% wt/wt, 34% heterozygous, and 11% mut/mut; P > .05), suggesting that the mutation in OAS1 had no effect on the risk of developing TBE in this population.
Table 2.
Genotype distribution of rs5743305, rs3775291 and rs10774671 among TBE patients, AME patients and controls
| Genotype |
||||||
| Population | wt/wt | wt/mut | mut/mut | Failed | Allele prevalence (wt/mut allele) | Note |
| TLR3 rs5743305 | ||||||
| Lithuanian TBE (n=128) | 47 (37%) | 63 (50%) | 16 (13%) | 2 (2%) | 0.623/0.377 | |
| Lithuanian AME (n=77) | 36 (47%) | 29 (38%) | 11 (14%) | 1 (1%) | 0.664/0.336 | |
| Lithuanian healthy controls (n=135) | 56 (42%) | 63 (47%) | 15 (11%) | 1 (1%) | 0.653/0.347 | |
| TLR3 rs3775291 | ||||||
| Lithuanian TBE (n=128) | 77 (61%) | 41 (32%) | 9 (7%) | 1 (1%) | 0.768/0.232 | * |
| Lithuanian AME (n=77) | 35 (47%) | 24 (32%) | 16 (21%) | 2 (3%) | 0.627/0.373 | * |
| Lithuanian healthy controls (n=135) | 65 (52%) | 37 (29%) | 24 (19%) | 9 (7%) | 0.663/0.337 | * |
| OAS1 rs10774671 | ||||||
| Lithuanian TBE (n=128) | 69 (54%) | 50 (39%) | 9 (7%) | 0 (0%) | 0.734/0.266 | |
| Lithuanian AME (n=77) | 40 (55%) | 25 (34%) | 8 (11%) | 4 (5%) | 0.719/0.280 | |
| (n=135) | 73 (54%) | 58 (43%) | 4 (3%) | 0 (0%) | 0.756/0.244 | |
NOTE. TBE, Tick-borne encephalitis; AME, aseptic meningoencephalitis; OR, odds ratio; CI, confidence interval.
*TBE vs controls: P=0.02, Chi-square, genotypic contingency analysis (3x2), 2-sided test. TBE vs AME: P=0.009. The wild type allele of rs3775291 is associated with increased risk of TBE compared to healthy controls (P=0.01, Fisher s exact test, 2-sided, OR: 1.68 95% CI 1.14-2.49).
Toll-Like Receptor 3
We found no difference in allele frequency between TBE patients and healthy Lithuanian controls regarding TLR3 rs5743305 polymorphism (Table 2). Among TBE patients, 47/126 (37%), 63/126 (50%), and 16/126 (13%) were carriers of the wt/wt, heterozygous, and mut/mut allele of TLR3 rs5743305, respectively. The corresponding percentages for healthy controls (n = 134) were 42%, 47%, and 11%, and for AME patients (n = 76) were 47%, 38%, and 14%, respectively. Hence no difference was observed in the allele distribution among patients with TBE or AME and the healthy controls (P > .05).
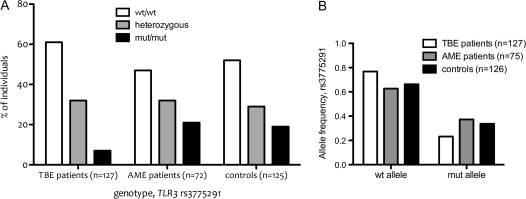
In contrast, the wt/wt genotype as well as the wt allele per se for TLR3 rs3775291 was found more frequently among TBE patients than controls and the AME patients (Figure 1A and 1B). A genotypic contingency analysis (3 x 2) for TLR3 rs3775291 was applied and revealed a statistically significant difference between the control and TBE patients (P = .02, χ2) as well as between the AME and TBE patients (P = .009). The wt allele was found to be a risk factor for severe TBEV infection when compared with controls (P = .01, Fisher exact test; odds ratio [OR], 1.68; 95% CI, 1.14–2.49) and was also more common among the TBE than among the AME patients (P = .003). No significant difference was observed between AME patients and controls regarding TLR3 rs3775291 genotype distribution (P = .8) or allele frequency (P = .52). When TBE patients were stratified for severity of disease, the allele frequency of TLR3 rs3775291 did not differ between the groups.
Figure 1.
(A) The genotype distribution of TLR3 rs3775291 differed significantly among the tick-borne encephalitis patients and controls and aseptic meningoencephalitis patients (P = .02 and P = .009, respectively, χ2 test). (B) The wild-type allele of TLR3 rs3775291 was significantly more common among tick-borne encephalitis patients than among controls and aseptic meningoencephalitis patients (P = .01 and P = .003, respectively).
DISCUSSION
TBEV is an increasing problem in Europe [13], and explanations of why certain individuals develop severe disease after infection are important for the understanding of TBEV pathogenesis and for development of new means for treatment. We have previously reported that a nonfunctional CCR5 protein is a risk factor for the development of TBE [6], but this mutation explains only a small fraction of cases. Further studies are needed.
In this study, we investigated the role of mutations in TLR3 and OAS1, genes that encode modulators of the innate immune response and are thus presumably associated with the pathogenesis of TBEV infections. The rationale for investigating these mutations was that earlier studies have shown an association between mutations in OAS1 and risk of infection with WNV and that lack of TLR3 in mice affects the pathogenesis of the same virus. Here we found no difference in genotype distribution between TBE patients and AME patients or between TBE patients and healthy controls regarding either OAS1 rs1077471 or the polymorphism rs5743305 in TLR3. However, a significantly higher frequency of the wt rs3775291 allele in TLR3 was found among TBE patients compared with AME patients (P = .009) or controls (P = .02). Also, the genotype distribution differed significantly between TBE patients and controls (P = .01) and between TBE and AME patients (P = .003), suggesting that the wt TLR3 rs3775291 allele is associated with increased risk of developing TBE. TLR3 rs3775291 is located in exon 4 and is a missense mutation (G > A, Leu412Phe) resulting in a functionally impaired receptor [14]. In vitro studies with HEK293 cells transfected with Phe-412 constructs and stable cell lines expressing Phe-412 have shown attenuated TLR3 signaling [15]. Furthermore, the same mutants reduce coxsackievirus-mediated TLR3 signaling and increase viral replication [15], suggesting that the TLR3 Leu412Phe mutation is associated with decreased TLR3 signaling and thus may have reduced the inflammatory response in the CNS in the TBEV infected patients. TLR3 is expressed on endosomes in the dendritic cells [7], astrocytes, microglia, oligodendrocytes [16], Schwann cells [17], and epithelial cells [18], and it recognizes dsRNA, a common intermediate product of the RNA virus replication cycle [19]. The TLR3-phenotype characteristics of the rs3775291 mutation have previously been described by Ranjith-Kumar et al [14] and Gorbea et al [15]. They reported reduction in the induction of NF-κB and type I interferon signaling and observed only homozygous phenylalanine to be associated with poor outcomes. The leucine 412 is located next to a glycosylated aspargine residue whose glycan interacts with dsRNA [20]. Substitution of leucine 412 for phenylalanine may hinder the interaction of the carbohydrate with dsRNA, or it could affect glycosylation and thus reduce the signaling activity of Phe-412. Another possibility proposed by Ranjith-Kumar et al [14] is that the Phe-412 variant is retained in the endoplasmic reticulum by the calnexin/calreticulin system, a proposal also supported by Gorbea et al [15].
A lack of TLR3 has previously been investigated for importance in WNV infection in mice but with contradictory results [8, 9]. Wang et al [8] have shown that mice lacking TLR3 are protected compared with mice with functional TLR3 receptors, when infected intraperitoneally with WNV. They found that mice with wt TLR3 had increased viral titers and increased leukocyte infiltration in the brain as compared with TLR negative mice. This was suggested to be because TLR3 signaling leads to TNFα release followed by decreased integrity of the blood-brain barrier (BBB), which then allows the passage of WNV more than when TLR3 is nonfunctional [8]. Following this observation, Daffis et al reported that TLR3 had a protective role against WNV infection [9]. They showed that lack of TLR3 was associated with enhanced viral replication in neuronal cell culture and increased WNV infection in CNS neurons after intracranial inoculation. Infection via footpad and intraperitoneal inoculation resulted in higher survival in wt than TLR-negative mice. The intracranial inoculation does not allow the passage over the BBB to be investigated, which may explain the contradictory results, as TLR3 may play different roles before and after the virus reaches the CNS. The discrepancy between results of intraperitoneal inoculation in the studies as well as between subcutaneous and intraperitoneal inoculation [8, 9] remains to be elucidated, and thus the role of TLR3 in flavivirus pathogenesis is still not known. The pathological findings in the brains of humans infected with TBEV are nonspecific, and lesions are located in the brain stem, cerebrum cerebella cortex, pons cerebellum, thalamus, and motor neurons [21]. Our observations indicate involvement of inflammation in the pathology of TBE. Hayasaka et al [21] concluded that CNS pathology alone is unlikely to be the sole determinant of mortality following TBEV infection; the immunopathological effects also contribute to the severity. They found that TNFα levels were significantly increased in mice that died following TBEV infection. TNFα is a proinflammatory cytokine that contribute beneficially, but inappropriate or excessive production can be harmful. Furthermore, Ruzek et al [22] reported a key role of CD8+T cells in the immunopathology of TBE, as demonstrated by prolonged survival of severe combined immunodeficiency (SCID) or CD8-/- mice following infection compared with immunocompetent mice or mice with transferred CD8+ T cells. They concluded that TBE is an immunopathological disease and that the inflammatory reaction significantly contributes to the fatal outcome of infection.
In accordance with the result by Wang et al on WNV [8], a study of phlebovirus in mice has shown that lack of TLR3 limits the severity of infection. Also, mice deficient in TLR3 have been shown to survive infection with influenza A virus in greater numbers than wt mice [23].
Our data agree with the results by Wang et al [8], suggesting that a functional TLR3 is a risk factor in flavivirus infections. This may be, as suggested by Wang et al [8], because of the effect on the BBB but also on the fact that flavivirus disease is at least partly immunologically driven [22]. It is thus possible that an impaired TLR3 response attenuates a too-strong immune response and thus inflammation and a more severe disease.
Hardarson et al [25] have previously reported that mice deficient in TLR3 are susceptible to encephalomyocarditis virus infection, resulting in significant mortality. The TLR3 deficient mice had impaired expression of inflammatory cytokines and chemokines in the heart and had higher viral load in the heart and liver. Similar data have recently been reported in coxsackievirus B infection in TLR3-deficient mice [25, 26]. In support for a role of TLR3 signaling deficiency in viral diseases associated with myocardia, Gorbea et al [15] screened TLR3 in patients diagnosed with enteroviral myocarditis/cardiomyopathy and found an association between certain polymorphisms and susceptibility, more precisely the Leu412Phe mutation.
In contrast to our data, TLR3 deficiency was previously associated with an increased risk of herpes simplex encephalitis (HSE). Zhang et al [27] reported 2 children with HSE who were found to be heterozygous carriers of a dominant-negative mutation at nucleotide 1660 (G > C, Pro554Ser) in exon 4 of TLR3, causing impaired IFN type I signaling. Moreover, Casrouge et al [28] reported 2 children with HSE with autosomal recessive deficiency in the intracellular protein UNC-93B, resulting in impaired cellular interferon responses. Casrouge et al [28] investigated TLR3 signaling in fibroblasts and PBMC cells from the patients and found an impaired response to polyriboinosinic polyribocytidylic acid (poly(I:C)) in fibroblasts but normal response of the patients' blood cells, which may indicate a strict TLR3 dependence of the poly(I:C) response in fibroblasts and a TLR3-independent pathway in PBMC. Altogether, this may indicate cell-specific, TLR3-specific signaling. The observations show that the inflammatory response in the CNS can be of benefit to a host infected with the neurotropic herpes simplex virus but is fraught with danger to a host infected with the blood-borne TBEV. The mechanisms responsible for the different importance in inflammatory responses between HSE and TBE is unknown but may be due to the route of entry to the CNS.
Furthermore, Ishizaki et al [29] found an association between the mutated allele of TLR3 rs3775291 and an increased risk of subacute sclerosing panencephalitis caused by measles virus. Human TLR3 alleles rs3775291 and rs5743305 have also been investigated for an association with liver disease manifestations in chronic hepatitis C infections [30], but no difference in genotype distribution was found between the different patient groups.
Both OAS1 [31] and TLR3 [32] are up regulated by type 1 IFN, and interferons in general are known to be important for the immune response against TBEV [33]. Hence, apart from further investigations of TLR, studies regarding mutations in IFNα and β or the IFN receptor would be of interest. Furthermore, even though no difference in allele distribution for the OAS1 rs1077471 was found, it cannot be ruled out that OAS plays an important role in the pathogenesis of TBE or initial TBEV infection, as has been observed for WNV.
In summary, we report an association between the wt TLR3 rs3775291 allele and increased risk of TBE, suggesting that a functional TLR3 is a risk factor for severe symptoms following TBEV infection. This is the first report of an association between a mutation in TLR3 and human flavivirus disease. Further studies on larger human populations as well as in an animal models are needed to elucidate the role of TLR3 in TBEV infections.
Funding
This work was supported by the County Council of Östergötland (LIO-79581 to E.K.; FORSS-80771 to L.S.).
Acknowledgments
We thank Carolin Jönsson for excellent technical assistance.
References
- 1.Lindenbach BP, Rice CM. Flaviviridae: The viruses their replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields Virology. 4th ed. Vol. 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. 1:991–1003. [Google Scholar]
- 2.Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antiviral Res. 2003;57:129–46. doi: 10.1016/s0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 3.Haglund M, Günther G. Tick-borne encephalitis—pathogenesis, clinical course long-term follow-up. Vaccine. 2003;21:S11–8. doi: 10.1016/s0264-410x(02)00811-3. [DOI] [PubMed] [Google Scholar]
- 4.Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21:S36–40. doi: 10.1016/s0264-410x(02)00819-8. [DOI] [PubMed] [Google Scholar]
- 5.Svensson L, Kindberg E. Human genetic factors involved in viral pathogenesis. In: Brasier AR, García-Sastre A, Lemon SM, editors. Cellular signaling innate immune responses to RNA virus infections. Washington, DC: ASM Press; 2009. pp. 177–93. [Google Scholar]
- 6.Kindberg E, Mickiene A, Ax C, et al. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008;197:266–9. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
- 7.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 9.Daffis S, Samuel MA, Suthar MS, Gale M, Jr., Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovanessian AG, Justesen J. The human 2′-5′oligoadenylate synthetase family: Unique interferon-inducible enzymes catalyzing 2′-5′ instead of 3′-5′ phosphodiester bond formation. Biochimie. 2007;89:779–88. doi: 10.1016/j.biochi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Lim JK, Lisco A, McDermott DH, et al. Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog. 2009;5:e1000321. doi: 10.1371/journal.ppat.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mickiene A, Laiskonis A, Günther G, Vene S, Lundkvist A, Lindquist L. Tickborne encephalitis in an area of high endemicity in Lithuania: Disease severity long-term prognosis. Clin Infect Dis. 2002;35:650–8. doi: 10.1086/342059. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus—a review of an emerging zoonosis. J Gen Virol. 2009;90:1781–94. doi: 10.1099/vir.0.011437-0. [DOI] [PubMed] [Google Scholar]
- 14.Ranjith-Kumar CT, Miller W, Sun J, et al. Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity expression in cultured cells. J Biol Chem. 2007;282:17696–705. doi: 10.1074/jbc.M700209200. [DOI] [PubMed] [Google Scholar]
- 15.Gorbea C, Makar KA, Pauschinger M, et al. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J Biol Chem. 2010;285:23208–23. doi: 10.1074/jbc.M109.047464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Park C, Cho IH, et al. Double-stranded RNA induces iNOS gene expression in Schwann cells, sensory neuronal death, and peripheral nerve demyelination. Glia. 2007;55:712–22. doi: 10.1002/glia.20493. [DOI] [PubMed] [Google Scholar]
- 18.Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, 9. J Invest Dermatol. 2007;127:331–41. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 19.Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Molecular Biology. 3rd ed. Vol. 1. Washington, DC: ASM Press; 2009. Principles of Virology. [Google Scholar]
- 20.Liu L, Botos I, Wang Y, et al. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–81. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayasaka D, Nagata N, Fujii Y, et al. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology. 2009;390:139–50. doi: 10.1016/j.virol.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Růzek D, Salát J, Palus M, et al. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology. 2009;384:1–6. doi: 10.1016/j.virol.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardarson HS, Baker JS, Yang Z, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251–8. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 25.Negishi H, Osawa T, Ogami K, et al. A critical link between Toll-like receptor 3 type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci U S A. 2008;105:20446–51. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richer MJ, Lavallée DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One. 2009;4:e4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 28.Casrouge A, Zhang SY, Eidenschenk C, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–12. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaki Y, Takemoto M, Kira R, et al. Association of Toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J Neurovirol. 2008;14:486–91. doi: 10.1080/13550280802298120. [DOI] [PubMed] [Google Scholar]
- 30.Askar E, Bregadze R, Mertens J, et al. TLR3 gene polymorphisms liver disease manifestations in chronic hepatitis C. J Med Virol. 2009;81:1204–11. doi: 10.1002/jmv.21491. [DOI] [PubMed] [Google Scholar]
- 31.Hovanessian AG. Interferon-induced double-stranded RNA-activated enzymes: A specific protein kinase and 2',5'-oligoadenylate synthetases. J Interferon Res. 1991;11:199–205. doi: 10.1089/jir.1991.11.199. [DOI] [PubMed] [Google Scholar]
- 32.Tissari J, Sirén J, Meri S, Julkunen I, Matikainen S. IFN-α enhances TLR3-mediated antiviral cytokine expression in human endothelial epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174:4289–94. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 33.Robertson SJ, Mitzel DN, Taylor RT, Best SM, Bloom ME. Tick-borne flaviviruses: Dissecting host immune responses and virus countermeasures. Immunol Res. 2009;43:172–186. doi: 10.1007/s12026-008-8065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]