Abstract
Altered alternative splicing and accumulation of brain microtubule-associated protein tau are found in several tauopathies and are believed to lead to these neurodegenerative diseases. We found that in addition to promote tau exon 10 inclusion, splicing factor SC35 also promoted tau expression in HEK-293T cells. The activity of SC35 in promotion of tau expression was limited to exon 10 containing tau isoforms. SC35 did not affect tau transcription, but stabilized tau mRNA by binding to the SC35-like element of exon 10. These results provide novel insight into the regulation of tau expression and a molecular mechanism of tauopathies.
Keywords: tau, Alzheimer's disease, SC35, mRNA stabilization
1. Introduction
The microtubule-associated protein tau interacts with tubulin to promote the assembly and stabilization of microtubules [1]. Neurodegenerative tauopathies, including Alzheimer disease (AD), are histopathologically characterized by the formation of neurofibrillary tangles (NFTs), which are composed of abnormally hyperphosphorylated forms of tau, in the brain [2-4]. It has been proposed that several different molecular mechanisms are involved in the development of NFTs [5].
Increased tau level has been observed in the brains of individuals with AD and Down syndrome [6,7]. High expression of tau in cultured cells results in cell toxicity and accumulation of the mitochondria in the perinuclear region [8]. Suppression of endogenous soluble tau or expressed human tau reduces the rate and degree of cognitive decline in transgenic mouse models of AD [9,10]. Therefore, reducing tau accumulation could be a therapeutic approach for neurodegenerative tauopathies.
Six isoforms of tau protein expressed in adult human brain are derived from a single tau gene by alternative splicing of its pre-mRNA [11,12]. The presence or absence of the second microtubule-binding repeat, which results from alternative splicing of tau exon 10, divides tau isoforms into two groups, 3R-tau (containing three microtubule-binding repeats) and 4R-tau (containing 4 such repeats) [12,13]. Normal adult human brain expresses approximately equal levels of 3R-tau and 4R-tau [14-16]. Disrupted 3R/4R-tau ratios have been found in several tauopathies [17-19]. Therefore, equal levels of 3R-tau and 4R-tau might be critical for maintaining optimal neuronal physiology [18].
The SR (serine/arginine rich) proteins are important in regulating alternative splicing [20]. They also play additional roles in different aspects of RNA processes, including transcription, RNA stability, mRNA transport, and mRNA translation [21]. SC35 was one of the prototypical SR proteins identified by monoclonal antibodies directed against purified spliceosomes [22,23]. Recently, it has been reported that SC35 may be essential for genomic stability [24] and that SC35 has an active role in transcriptional elongation [25].
To determine the role of SC35 in RNA processing and expression of tau, we investigated the effect of SC35 on tau mRNA and protein expressions in cultured cells. We found that SC35 stabilized tau mRNA by binding to the exon 10 and promoted tau expression in a manner dependent on its exon 10.
2. Materials and methods
2.1 Plasmids and Antibodies
pEGFP/tau23, pEGFP/tau24, pEGFP/tau37, pEGFP/tau46, pEGFP/tau39, and pEGFP/tau40 were subcloned from pKR172/taus (a gift from M. Goedert of Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom) into the BglII and EcoRI sites of pEGFP-C1 vector (Clonetech, Palo Alto, CA). pCI/tau39 and pCI/tau40 were subcloned from pEGFP/tau39 and pEGFP/tau40 into the XhoI/XbalI sites of PCI-neo vector (Clontech, Palo Alto, CA). pCEP4/SC35-HA was a gift from Dr. Tarn of the Institute of Biomedical Sciences, Academia Sinica, Taiwan. Polyclonal anti-tau (R134d) was described previously [26]. The monoclonal anti-HA and anti-β-actin were bought from Sigma (St. Loius, MO). Peroxidase-conjugated anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
2.2 Cell Culture and Transfection
HEK-293T cells were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37 °C. The cells were transfected by using FuGENE 6 (Roche Diagnotics, Indianapolis, IN) according to manufacture's protocol.
2.3 Expression and purification of HA-SC35
pCEP4-SC35 was transfected into HEK-293T Cells using FuGENE 6 for 48 h, and then the cells were lysed in 0.5 ml of IP lysis/wash buffer (Pierce Crosslink Immunoprecipitation Kit, Thermo Scientific, Rochford, IL, USA). The cell lysates were mixed with anti-HA cross-linked to protein G-Sepharose beads and incubated at 4°C overnight. After extensive washing, SC35 was eluted in 3 successive 100 μl fractions with elusion buffer provided in the kit and neutralized with 1M Tris.
2.4 In vitro transcription
RiboMAX Large Scale RNA Production System (Promega, Madison, WI, USA) was used for in vitro transcription of pCI/tau39 or pCI/tau40. pCI/tau39 or pCI/tau40 was linearized by digestion with BamHI and SalI, and the DNA template was purified by phenol extraction and ethanol precipitation. The linearized DNA was added into reaction mixture according to the manufacturer's protocol. The reaction mixture was incubated at 37°C for 2 h, and then the DNA template was removed by 1μg/μl RQ1 RNase-free DNase (Promega). The in vitro transcripted mRNA was analyzed by 1.5% agarose gel.
2.5 Electrophoretic Mobility Shift Assay (EMSA)
RNA primer of tau exon 10 containing SC35-like enhancer (5′GUGCAGAUAAUUAAUAAGAAGCUGGAUCUU3′) was labeled with [γ-32P]ATP (4500 Ci/mmol) using T4 polynucleotide kinase (New England Biolabs) and subsequently purified with MicroSpin G-25 column (Amersham Biosciences). To perform the EMSA assay, the recombinant protein in the buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM EDTA and 1 mM dithiothreitol) was mixed with 32P-labeled tau RNA primer in a total volume of 10 μl. The reaction mixture was incubated at 37°C for 40 min and analyzed with a 6% non-denaturing polyacrylamide gel, which was pre-run at 100 V for 10 min. Electrophoresis was carried out in TBE buffer (89 mM Tris borate, 2 mM EDTA) at 100 V for 60 min. The gel was dried and autoradiographed with a PhosphorImager (BAS- 1500, Fujifilm). The RNA substrates used in all experiments were at 2.4 nM, and the amounts of proteins were indicated in the figure legends.
2.6 Preparation of nuclear and cytoplasmic extracts
Nuclear and cytoplasmic extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo scientific, Rockford, IL) according to manufacture's protocol. Briefly, cells were suspended in ice-cold CER I and incubated on ice for 10 min, and then ice-cold CER II was added and incubated on ice for another 1 min. After centrifuging for 5 min at ∼16,000 × g, the supernatant was collected as cytoplasmic extract. The pellet containing nuclei was suspended in ice cold NER and placed on ice. After vortexing for 15 seconds every 10 minutes for a total of 40 min, the 16,000 × g supernatant was collected as nuclear extract.
2.6 RNA immunoprecipitation (RNA-IP)
The RNA-IP experiment was performed as described [27-29]. Briefly, HEK-293T cells co-transfected with pCEP4/SC35 and pCI/SI9-LI10 were incubated in PBS containing 1% formaldehyde at room temperature for 10 min, and then glycine was added to 125 mM to block free aldehyde groups. The cells were lyzed with lysis buffer (16.7mM Tris, pH 8.1, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, protease inhibitors cocktail, 50 U/ml SUPERase•in), and sonicated three times for 10 s each (resulting in an average fragment size of 0.5-1 kb). The supernatant was incubated with anti-HA to immunoprecipitate SC35. After successive washing, the precipitates were eluted with 1% SDS, 0.1 M NaHCO3, 50 U/ml SUPERase•in, and decrosslinked by adding NaCl to 200 mM and incubating at 65°C for about 2 h. The RNA was purified from the immunoprecipites with RNeasy mini kit (Qiagen GmbH, Germany) after protein and DNA were digested by Proteinase K at 42°C for 45 min and by RQ1 Rnase-free Dnase (Promega) at 37°C for 15 min, respectively. After reverse-transcription, PCR reactions were carried out with Prime-STARTM HS DNA Polymerase (Takara Bio Inc., Otsu, Shiga, Japan) with primers Forward: 5′ccgcctgcagacagcccccgtgcccatgccagac3′, Reverse: 5′cttccacctggccacctcctggtttatgatggatg3′. An initial denature for 5 min at 98°C was followed by 30 cycles with 98°C for 10 sec — 55°C for 15 sec — at 72°C for 30 sec and a final extension for 10 min at 72°C. PCR products were separated on a 1.5% agarose gel and visualized by ethidium bromide staining.
3. Results
3.1 SC35 increases the expression of tau
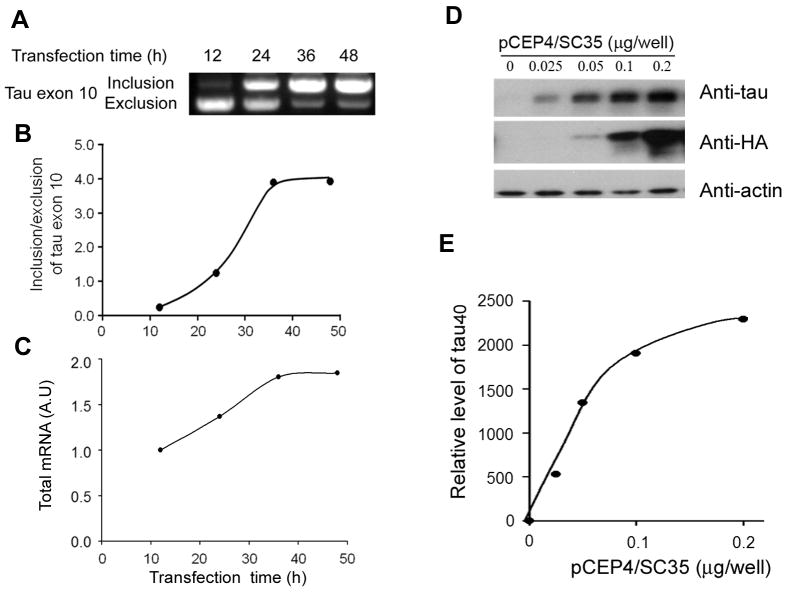
We recently demonstrated that the splicing factor SC35 acts on a SC35-like enhancer at 5′ exon 10 and promotes tau exon 10 inclusion ([7] and unpublished data). As expected, overexpression of SC35 in HEK-293T cells time-dependently enhanced the inclusion of tau exon 10 in mini-tau gene, pCI/SI9-SI10, which was consisted of exons 9, 10, and 11 and part of introns 9 and 10, (Fig. 1A, B). We also observed unexpectedly a time-dependent increase in the total tau mRNA with SC35 over-expression (Fig. 1A, C), suggesting that SC35 may regulate tau expression besides tau splicing. To investigate this possibility, we co-transfected pCI/tau40 together with various amounts of pCEP4/SC35 into HEK-293T cells and then measured the levels of tau and SC35 after 48 hr transfection. We observed a dose-dependent expression of SC35, as determined by antibody against HA that was tagged to SC35 (Fig. 1D). The expression level of tau40 also increased along with the increasing dose of pCEP4/SC35 (Fig. D,E), indicating that SC35 enhanced the expression of tau40 in HEK-293T cells.
Figure 1.
Overexpression of SC35 promotes tau exon 10 inclusion and expression of tau40. A, Tau mini-gene pCI/SI9-SI10 was co-transfected with pCEP4/SC35 into HEK-293T cells for various times, and then the splicing products of tau exon 10 were measured by RT-PCR. The tau exon 10 inclusion/exclusion ratio (B) and the total PCR products (mRNA) (C) are plotted against the transfection time. D,E, pEGFP/tau40 was co-transfected with different amount of pCEP4/SC35 into HEK-293FT cells. After a 48h transfection, the level of tau40, SC35, and actin were determined by Western blots developed with specific anti-tau (R134d), anti-HA and anti-actin antibodies, respectively.
3.2 SC35 promotes tau expression in a manner dependent on exon 10
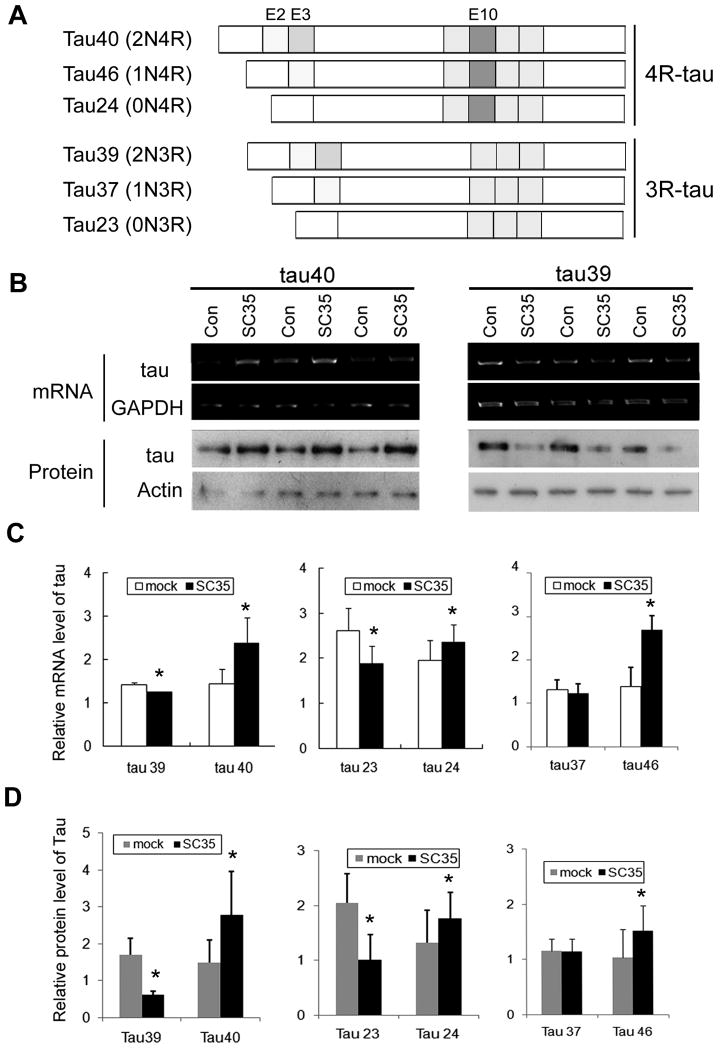
To determine whether SC35 regulates the expression of other tau isoforms (Fig. 2A), we co-transfected into HEK-293T cells with pCEP4/SC35 and pEGFP/tau40, pEGFP/tau39, pEGFP/tau23, pEGFP/tau24, pEGFP/tau37 or pEGFP/tau46, respectively. After 48 hour transfection, both the protein and the mRNA levels of each tau isoform were measured by using Western blots and RT-PCR, respectively. Interestingly, we found that the mRNA level and the protein level of tau isoforms containing the second microtubule-binding repeat encoded by exon 10 (i.e., 4R-taus, including tau24, tau46 and tau40) were increased significantly (Fig. 2). To the contrary, the expression of 3R-taus, including tau23, tau37, and tau39, was either decreased (tau23, tau39) or unchanged (tau37). These data suggest that SC35-induced increase in tau level probably resulted from an increase in the expression of tau and that tau exon 10 was required for the SC35's activity to promote tau expression.
Figure 2.
Overexpression of SC35 differentially regulates the expressions of mRNA and protein of six tau isoforms. A, Diagram of six human tau isoforms. B, pEGFP/tau40 (left panel) or pEGFP/tau39 (right panel) was co-transfected with pCEP4/SC35 or empty vector (Con) into HEK-293T cells. The total RNA was subjected to RT-PCR for measurement of mRNA level of tau39 and tau40 (upper panels). The protein levels of tau39 and tau40 were examined by Western blots developed with anti-tau (R134d) (lower panels). C, The mRNA levels of six isoforms of tau were quantitated by densitometry and normalized by mRNA level of GAPDH. The data are presented as means±S.D. *, p<0.05 versus control transfection. D, The protein levels of six isoforms of tau were quantitated by densitometry and normalized by the level of actin. The data are presented as means±S.D. *, p<0.05 versus control transfection.
3.3 SC35 stabilizes tau mRNA
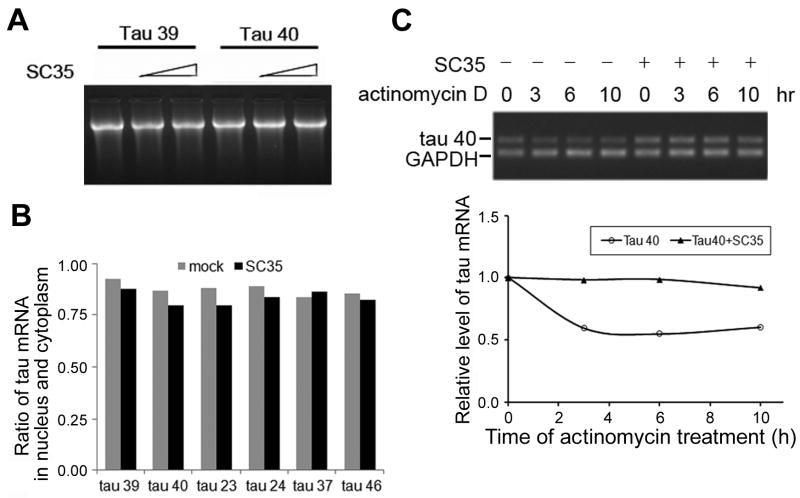
To determine the mechanism by which SC35 increased 4R-tau expression, we determined whether SC35 promotes tau transcription. To this aim, we immuno-purified SC35 protein by using Protein G beads cross-linked with anti-HA antibody and then added the purified SC35 to an in vitro transcription system. We found that addition of SC35 did not alter the amount of mRNA of tau39 or tau40 synthesized in vitro (Fig. 3A). These results suggest that SC35 is unlikely to regulate tau transcription.
Figure 3.
SC35 has no detectable effect on transcription but regulates the processing of tau mRNA. A, mRNAs of tau39 and tau40 were biosynthesized in vitro in the absence or in the presence of purified SC35. The synthesized mRNA was then analyzed by gel electrophoresis. B, HEK-293FT cells were co-transfected with pCEP4/SC35 and different isoforms of tau. The total tau mRNA extracted from the nuclear and the cytoplasmic fractions was measured by RT-PCR, and the ratio of nuclear:cytoplasmic tau mRNA was calculated and presented as an average from two separate experiments. C, HEK-293T cells were treated with actinomycin D (2 μg/μL) for 0, 3, 6, and 10 h after transfection with tau40 alone or together with SC35 for 48 h. RNAs were then extracted and tau40 mRNA was measured by RT-PCR and analyzed by agarose gel electrophoresis (upper panel). The levels of tau40 mRNA are plotted after normalization to control GAPDH RNA on the basis of densitometry analysis (lower panel).
For protein expression, mRNA needs to be transported to the cytoplasm from nucleus before protein translation. To investigate whether SC35 affects tau mRNA transport, we co-transfected HEK-293FT cells with pCEP4/SC35 and different tau isoforms, we fractionated the transfected cells into nuclear and cytoplasmic fractions, extracted RNA from both fractions, and then measured the mRNA level of tau by RT-PCR. We found that SC35 did not change the nuclear to cytoplasmic ratio of tau mRNA (Fig. 3B), suggesting that SC35 does not affect tau mRNA transport.
To identify if SC35 affects the stability of 4R-tau mRNA, we overexpressed SC35 with tau40 into HEK-293T cells and measured the turn-over of tau40 mRNA after new RNA synthesis was suppressed by actinomycin D, a transcription inhibitor. We observed a rapid tau decrease in tau40 mRNA in the presence of actinomycin D, but the tau40 mRNA reduction was prevented when SC35 was co-expressed (Fig. 3C). These results suggest that co-expression of SC35 inhibits the degradation of tau40 mRNA (Fig. 3C). Thus, it appears that SC35 increases 4R-tau expression via stabilization of the mRNA.
3.4 SC35 binds to tau mRNA through exon 10
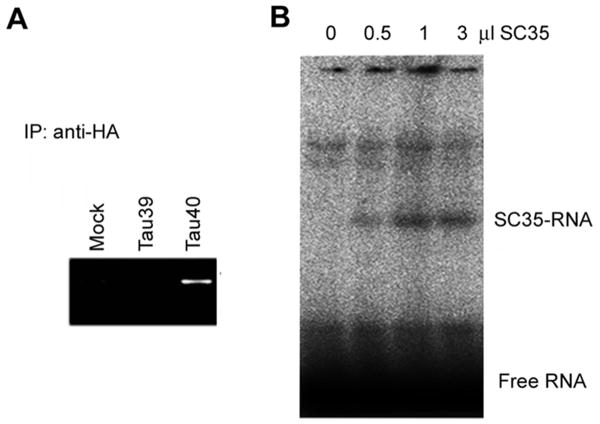
To understand why SC35 promoted the expression of 4R-tau but not of 3R-tau, we investigated the binding between SC35 and tau mRNA. To this aim, we immunoprecipitated SC35 48 h after HEK-293T cells were co-transfected with pCEP4/HA-SC35 and pEGFP/tau39 or pEGFP/tau40. Tau mRNA co-immunoprecipitated with SC35 was then amplified by RT-PCR and examined by agarose gel electrophoresis. We found only tau40, but not tau 39, in the SC35 immunoprecipitates (Fig. 4A), suggesting that SC35 binds to tau40 mRNA, but not tau39 mRNA.
Figure 4.
SC35 binds to exon 10 of tau40 mRNA. A, HEK-293T cells were harvested 48 h after co-transfection with pCEP4/HA-SC35 and either pEGFP/tau39 or pEGFP/tau40, and SC35 from the cell lysates was immunoprecipitated by using anti-HA antibody. Tau mRNA of the immunoprecipitates was amplified and detected by agarose gel electrophoresis. B, Various amounts of purified SC35 were incubated with γ 32P-labeled tau exon 10 oligomers for 40 min, followed by gel electrophoresis and autoradiography.
To localize the binding site of tau mRNA to SC35, we labeled a 30-nt RNA oligomer that was SC35-like enhancer of tau exon 10 with 32P and incubated it with various amounts of purified SC35. Electrophoresis of the incubated mixtures in native gels revealed a radioactive band that was consistent to the size of the SC35-RNA complex (Fig. 4B). The band radioactivity correlated to the amounts of SC35 added into the incubation mixtures. These results suggest that SC35 binds to tau mRNA through the RRM domain of exon 10.
Discussion
The SR proteins play important roles in both constitutive splicing and alternative splicing [7,30,31]. As an important member of SR proteins, SC35 regulates the alternative splicing of tau. Overexpression of SC35 promotes tau exon 10 inclusion [7]. We recently demonstrated that SC35 promoted tau exon 10 inclusion by acting on the SC35-like enhancer of tau exon 10 (unpublished data). In the present study, by using intronless tau constructs, we found that SC35 enhanced 4R-tau but not 3R-tau expression at both the mRNA and protein levels. We identified, for first time, that SC35 stabilized mRNA and promoted 4R-tau expression by acting on the exon 10 of tau mRNA. Our results suggest a broader role for SC35 in the expression of tau in addition to its function in the alternative splicing of tau exon 10. Because only 4R-tau expression was found to be up-regulated by SC35, our findings also provide a novel molecular mechanism involved in the regulation of 3R-tau and 4R-tau expression.
Increasing evidence suggests that the SR proteins not only regulate splicing of pre-mRNA, but also are involved in other events from gene to protein cascade, including transcription, transportation, and translation. SC35 is reported to play an active role in transcriptional elongation in a gene-specific manner [25]. In the present study, we found that SC35 increased the level of 4R-tau mRNA, but this increase was not resulted from increased transcription as demonstrated by our in vitro transcription data. Further studies indicated that SC35 increased the level of tau mRNA by binding to exon 10 of tau mRNA and consequently stabilizing the mRNA. Therefore, the ability of SC35 to stabilize tau mRNA was dependent on the presence of exon 10. The expression of only those isoforms that contained exon 10 (i.e., 4R-taus), but not the isoforms without exon 10, was elevated by SC35 as a result of the mRNA stabilization. Our observation is consistent with the fact that there is a SC35-like enhancer at the 5′ of tau exon 10 [32]. However, overexpression of SC35 did not affect the level of tau37 at both mRNA and protein, suggesting tau37 mRNA might have a weak SC35 acting site between exon 2 and exon 4, which need to be identified.
It is known that some SR proteins, i.e. ASF and 9G8, shuttle between nucleus and cytoplasm and help mRNA transport from nucleus to cytoplasm [33,34]. In the present study, we found that overexpression of SC35 did not affect the distribution of tau mRNA between the nucleus and the cytoplasm, suggesting a minimal role of SC35 on the transportation of tau mRNA.
The activity of SC35 to regulate alternative splicing is tightly regulated by its phosphorylation. GSK-3β and Dyrk1A phosphorylate SC35 and modulate its function in the alternative splicing of tau exon 10 ([31] and our unpublished data). Both kinases are up-regulated in AD brain [35,36]. Whether SC35's activity to stabilize the 4R-tau mRNA is also regulated by its phosphorylation catalyzed by GSK-3β and Dyrk1A remains to be elucidated. Understanding of the molecular mechanism by which up-regulation of these kinases modulates the alternative splicing and the level of tau in the brain will provide novel insight into the molecular pathogenesis of AD and probably other related tauopathies.
Acknowledgments
This work was supported in part by funds from Nantong University; New York State Office for People With Developmental Disabilities; a National Natural Science Foundation of China Grant 81030059; a Grant from Basic Research Program of Jiangsu Education Department (10KJA310040); a U.S. Alzheimer's Association Grant (IIRG-10-173154) and US National Institutes of Health Grant (AG027429 partial).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22:70–7. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. [PubMed] [Google Scholar]
- 5.Iqbal K, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer's disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59:750–3. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem. 2008;283:28660–9. doi: 10.1074/jbc.M802645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. The Journal Of Cell Biology. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santacruz K, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 11.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–26. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 12.Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–33. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 13.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8:393–9. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9:4225–30. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2:1389–97. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez M, Estivill X, de la Luna S. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J Cell Sci. 2003;116:3099–107. doi: 10.1242/jcs.00618. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005;1739:104–15. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739:240–50. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Sergeant N, Delacourte A, Buee L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta. 2005;1739:179–97. doi: 10.1016/j.bbadis.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong XY, Wang P, Han J, Rosenfeld MG, Fu XD. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell. 2009;35:1–10. doi: 10.1016/j.molcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu XD, Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990;343:437–41. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- 23.Fu XD, Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992;256:535–8. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- 24.Xiao R, Sun Y, Ding JH, Lin S, Rose DW, Rosenfeld MG, Fu XD, Li X. Splicing regulator SC35 is essential for genomic stability and cell proliferation during mammalian organogenesis. Mol Cell Biol. 2007;27:5393–402. doi: 10.1128/MCB.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S, Coutinho-Mansfield G, Wang D, Pandit S, Fu XD. The splicing factor SC35 has an active role in transcriptional elongation. Nat Struct Mol Biol. 2008;15:819–26. doi: 10.1038/nsmb.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–90. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert SL, Pehrson JR, Sharp PA. XIST RNA associates with specific regions of the inactive X chromatin. J Biol Chem. 2000;275:36491–4. doi: 10.1074/jbc.C000409200. [DOI] [PubMed] [Google Scholar]
- 29.Sun BK, Deaton AM, Lee JT. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell. 2006;21:617–28. doi: 10.1016/j.molcel.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 30.D'Souza I, Schellenberg GD. Determinants of 4-repeat tau expression. Coordination between enhancing and inhibitory splicing sequences for exon 10 inclusion. J Biol Chem. 2000;275:17700–9. doi: 10.1074/jbc.M909470199. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez F, Perez M, Lucas JJ, Mata AM, Bhat R, Avila J. Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35. Implications for Alzheimer's disease. J Biol Chem. 2004;279:3801–6. doi: 10.1074/jbc.M311512200. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3:8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanford JR, Gray NK, Beckmann K, Caceres JF. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 2004;18:755–68. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J Alzheimers Dis. 2010;19:1221–9. doi: 10.3233/JAD-2010-1317. [DOI] [PubMed] [Google Scholar]
- 36.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–9. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]