Abstract
The WNTs are secreted proteins that control essential developmental processes, such as embryonic patterning, cell growth, migration, and differentiation. In mice, three members of the Wnt gene family (Wnt4, Wnt5a, and Wnt7a) have been studied extensively in the female reproductive tract. The present study determined effects of postnatal day and exposure to diethylstilbestrol (DES) on Wnt and Fzd gene expression in the mouse uterus as well as the biological role of Wnt11 in postnatal mouse uterine development and function. Wnt4, Wnt5a, Wnt7a, Wnt7b, Wnt11, Wnt16, Fzd6, and Fzd10 were detected by in situ hybridization in the neonatal mouse uterus. In situ hybridization analyses revealed that Wnt4, Wnt5a, and Wnt16 were localized in the endometrial stroma, whereas Wnt7a, Wnt7b, Wnt11, Fzd6, and Fzd10 were in the uterine epithelia of neonatal mice. Exposure of mice to estrogen or estrogen receptor agonists during critical development periods inhibits endometrial adenogenesis. In the present study, DES-induced disruption of endometrial gland development was associated with reduction or suppression of Wnt4, Wnt5a, Wnt7a, Wnt11, Wnt16, and Fzd10. Ablation of Wnt11, an epithelial-expressed, DES-regulated gene, in the neonatal uterus did not affect endometrial adenogenesis or expression of other Wnt genes. Interestingly, Wnt11-deleted uteri had more endometrial glands on Postnatal Day 10. Although CTNNB1 expression was not affected by ablation of Wnt11, Vangl2 was inhibited in the uteri of Wnt11d/d mice. These results support the idea that a number of different Wnt genes are potential regulators for uterine morphogenesis; however, Wnt11 does not have a direct effect on uterine development.
Keywords: DES, gland development, mouse, uterus, WNT
Multiple Wnt genes regulate postnatal uterine morphogenesis.
INTRODUCTION
Although histogenesis of the uterus is initiated in the fetus, uterine development is not completed until after birth in humans, laboratory rodents, and domestic livestock [1–4]. A major developmental event in the neonatal uterus is adenogenesis, which is defined as the differentiation and development of glands in the endometrium [3, 4]. Histologically, the mouse uterus lacks endometrial glands and consists of a simple luminal epithelium (LE) and relatively undifferentiated mesenchyme at birth [5]. Between birth (Postnatal Day [P] 0) and P3, the three layers of mesenchyme are distinctly segregated into endometrial stroma and the inner circular and prospective outer longitudinal layers of myometrium. By P6, epithelial invaginations appear in the LE that represent the formation of glandular epithelium (GE) buds [6]. By P12, endometrial glands extend from the LE into the surrounding endometrial stroma, and the outer longitudinal layer of the myometrium is fully organized into bundles of smooth muscle cells [5]. The basic adult histoarchitectural configuration of the mouse uterus is established by P15 [7]. Uterine morphogenesis is critical, because disruption of endometrial adenogenesis and mesenchymal specification and differentiation can cause permanent fertility problems in the adult [3, 8, 9]. However, we know little of the genes and regulatory mechanisms governing postnatal development of the uterus.
Neonatal exposure to synthetic estrogen diethylstilbestrol (DES) in mice can lead to multiple patterning defects in the female reproductive tract. In female mice, neonatal DES exposure, acting through high-affinity interaction with estrogen receptor alpha (ESR1), causes uterine malformations, including hypoplasia, stratification of LE, disorganized smooth muscle, and reduced endometrial glands [10–12]. The mechanisms by which hyperstimulation of ESR1 signaling results in abnormal uterine differentiation are not well studied. However, two gene families, Hox and Wnt, which normally regulate developmental processes, were potently repressed by DES during critical periods of reproductive tract patterning and failed to be repressed in Esr1-null mice [10, 13, 14]. Furthermore, mouse mutants of Hoxa10, Hoxa11, Wnt5a, and Wnt7a revealed a requirement for these genes during postnatal uterine development [14–17].
The wingless-type MMTV integration site family member (Wnt) genes are homologous to the Drosophila mealanogaster segment polarity gene wingless (wg). In humans and mice, the Wnt family of genes encodes a group of 19 highly conserved, secreted signaling molecules that are critical regulators of cell fate, growth, and differentiation as well as cell-cell interactions [18]. To be effective in autocrine or paracrine signaling, WNTs associate with their extracellular surface receptors, frizzled (FZD), to mediate intracellular signal transduction pathways [19]. The 10 Fzd genes encode a family of seven transmembrane G protein-coupled receptors that possess an extracellular cysteine-rich domain for WNT binding [20, 21].
In mice, three members of the Wnt gene family (Wnt4, Wnt5a, and Wnt7a) have been identified and studied in the female reproductive tract [22]. Null mutation of these genes or disruption of their expression by endocrine disruptors alters prenatal and postnatal development of the Müllerian duct [4, 23–25]. The role of Wnt4 in postnatal development is not known [22], because Wnt4-null mice fail to form Müllerian ducts and die at birth as a result of numerous defects [26]. Analyses of the Wnt5a and Wnt7a mutants demonstrate the requirement of both genes in fetal uterine development and postnatal endometrial adenogenesis in mice [16, 27]. Wnt5a is expressed throughout the uterine mesenchyme, whereas Wnt7a is expressed only in endometrial LE [16]. Both Wnt5a- and Wnt7a-null mice exhibit malformations in the female reproductive tract and display abnormal morphogenesis of uterine horns, including the absence of uterine glands [16, 17, 22, 27]. Thus, Wnt genes are conserved, critical regulators of female reproductive tract development. In the present study, we examined Wnt and Fzd genes during postnatal uterine morphogenesis, effects of neonatal DES exposure on expression of those genes, and the role of Wnt11 in postnatal uterine morphogenesis.
MATERIALS AND METHODS
Animals and Experimental Design
All animals were handled according to National Institutes of Health guidelines and in compliance with institutionally approved animal protocols. To determine the effects of DES (Sigma) on neonatal uterine morphogenesis and gene expression, female pups were separated from male pups at birth (P0) and injected with 20 μl of corn oil vehicle as a control or with 1 mg kg−1 day−1 (2 μg pup−1 day−1) of DES in corn oil from P1 to P5 [10]. The model of postnatal DES exposure has been well-established to determine cellular and molecular mechanisms regulating uterine development [10, 12, 14, 16, 28]. Pups were necropsied on P1 before the treatment, on P5 approximately 6–8 h after the final treatment, on P10, and on P15 (n = 5 per treatment per day). Two hours before death, pups received an s.c. injection of 100 mg/kg of bromodeoxyuridine (BrdU; Sigma) to assess cell proliferation. At collection, one uterine horn was fixed in fresh 4% paraformaldehyde in PBS at room temperature for 8–12 h and embedded in paraffin; the other horn was snap-frozen in liquid nitrogen and stored at −80°C.
Construction of Targeting Vectors and Generation of Chimeric Mice
The Wnt11 gene is spread over seven exons. Targeted deletions of exons 4 and 5 were used to create the original Wnt11 null allele [29]. The targeting construct, containing Wnt11 fragment with exons 4 and 5 as well as 2 kb of the 5′ arm and 2 kb of the 3′ arm homology regions (Supplemental Fig. S1A, all Supplemental Data are available online at www.biolreprod.org), was transfected into 5–10 × 107 E14TG2a embryonic stem (ES) cells from 129Sv strain using a Bio-Rad gene pulser. ES cell clones were selected positively with G418 (Mediatech, Inc.) for presence of the PGK-neo cassette and negatively with ganciclovir (InvivoGen) for absence of the MC1TK cassette. Southern blot analysis was used to screen genomic DNA with NheI digestion using 3′ external probes with NheI (Supplement Fig. S1B). Correctly targeted clones were microinjected into blastocysts derived to C57BL/6 mice, and F1 agouti male offspring mice were analyzed by PCR genotyping and Southern blot analysis to validate germline transmission of the Wnt11 flox-neo allele.
Generation of a Wnt11 Conditional Null Allele
B6.129-Pgrtm2(cre)Lyd (a.k.a. Pgrcre/+) mice were provided by Drs. Franco DeMayo and John Lydon [30]. B6.Cg-Tg(ACTFLPe)9205Dym (a.k.a. FLPe, Jax #005703) and B6.FVB-Tg(EIIa-cre)C5379Lmgd (a.k.a. EIIa-Cre, Jax #003724) were obtained from the Jackson Laboratory. A PGK-neo cassette flanked by two Frt sites were removed from the Wnt11floxneo/+ allele by crossing the mice to FLPe mice [31], which resulted in generating the Wnt11flox/+ allele. Wnt11flox/flox mice were then obtained by intercrossing heterozygous Wnt11flox/+ mice. The Wnt11floxneo/floxneo and Wnt11flox/flox mice were viable and did not display any abnormalities. To increase the efficiency in production of homozygous null alleles and decrease the incidence of mosaic deletion, Pgrcre/+ Wnt11flox/− mice (Wnt11 conditional null allele, or Wnt11d/d) were generated by crossing Pgrcre/+ Wnt11+/− mice to Pgr+/+ Wnt11flox/flox (control allele, or Wnt11f/f) mice. The PCR strategy to identify of the Wnt11 conditional null allele is shown in Supplemental Figure S1C.
Immunohistochemistry
Immunolocalization of BrdU, ESR1, progesterone receptor (PGR), and CTNNB1 was performed in cross-sections (thickness, 5 μm) of paraffin-embedded uterine sections using specific antibodies and a Vectastain Elite ABC Kit (Vector Laboratories). Antibodies used in the analyses were anti-BrdU (11 170 376 001; Roche), anti-ESR1 (sc-542; Santa Cruz Biotechnologies), anti-PGR (RB-9017-P0; Thermo Fisher), and anti-CTNNB1 (610154; BD Transduction Laboratories). Negative controls were performed in which the primary antibody was substituted with the same concentration of normal immunoglobulin G (Sigma). Antigen retrieval using a boiling citrate buffer was performed as described previously [32].
RT-PCR Analysis
Wnt and Fzd mRNAs were examined in the uteri by RT-PCR as described previously [33–37]. A partial mouse cDNA of 400–700 bp was cloned by RT-PCR using total RNA isolated from mouse uterus, and primer and annealing temperatures used for PCR were as described previously [37]. The amplified PCR products were subcloned into the pCRII cloning vector using a T/A Cloning Kit (Invitrogen) and sequenced in both directions using a DTCS Quick Start Kit and Beckman 2000 Automation Workstation sequencer (Beckman Coulter) to confirm identity.
In Situ Hybridization
In situ hybridization analysis of mouse uteri were conducted using methods described previously [38]. Briefly, deparaffinized, rehydrated, and deproteinated cross-sections (thickness, 5 μm) of the uterine horns from each mouse were hybridized with radiolabeled sense or antisense cRNA probes generated from linearized plasmid DNA templates using in vitro transcription with [35S-α]UTP. After hybridization, washing, and ribonuclease A digestion, slides were dipped in NTB liquid photographic emulsion (Kodak), stored at 4°C for 4–30 days, and developed in Kodak D-19 developer. Slides were then counterstained with Gill modified hematoxylin (Stat Lab), dehydrated through a graded series of alcohol to xylene (Fisher), and protected with a coverslip.
Quantitative Real-Time RT-PCR Analysis
Total RNA was isolated from mouse uterus using the TRIzol reagent (Invitrogen) according to the manufacturer's recommendations. The quantity and quality of total RNA was determined by spectrometry and denaturing agarose gel electrophoresis, respectively. The cDNA was synthesized from total RNA (2 μg) using iScript Select cDNA synthesis Kit (Bio-Rad). Real-time PCR analysis of mRNA expression was performed using a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) with iQ SYBR Green supermix (Bio-Rad) as the detector according to the manufacturer's recommendations. Primers shown in Supplemental Table S1 were designed to amplify cDNAs of approximately 100 bp, and all exhibited similar amplification efficiency (95% ± 3%) as assessed by amplification of cDNA dilution series. PCR cycle parameters were 95°C for 15 sec and 60°C for 1 min for 40 cycles. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (CT) values were determined as the cycle number at which the threshold line crossed the amplification curve. PCR without template or template substituted with total RNA were used as negative controls to verify experimental results. After amplification, the specificity of the PCR was determined by both melt-curve analysis and gel electrophoresis to verify that only a single product of the correct size was present. Data were normalized against Gapdh and are shown as the average fold-increase ± SEM. The fold-changes are equivalent to 2x-y, where x is the CT value of the control and y is the CT value of treatment (DES exposure or ablation of Wnt11).
Statistical Analyses
All quantitative real-time PCR data were subjected to one-way ANOVA, and differences between individual means were tested by a Tukey multiple-range test using Prism 4.0 (GraphPad). Real-time PCR data were corrected for differences in sample loading using the Gapdh data as a covariate. Tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error. A P-value of 0.05 or less was considered to be significant. Data are presented as the least-square mean with SEM.
RESULTS
Effects of DES Exposure in Neonatal Uterus
Neonatal DES exposure causes multiple teratogenic effects, including structural malformations of endometrial adenogenesis [10, 12]. In the present study, exposure of neonatal mice to DES from P1 to P5 disrupted uterine development (Fig. 1A). On P5, expansion of the uterine lumen was present, and the stromal layer was thin and not clearly demarcated in the uterine mesenchymal tissues of DES-treated mice as compared with controls. On P10, DES exposure caused hypertrophic elongated LE with infolding of surfaces, no ductal invagination of endometrial glands into stroma, and disorganized stromal layer. On P15, the uteri of DES-treated mice exhibited epithelial hypertrophy, unclear demarcation and disorganized stroma and myometrium, and reduced or ablated endometrial glands. Analysis of BrdU incorporation revealed that the proliferative cells present in the endometrial and myometrial layers of control uteri were considerably reduced in DES-treated neonatal uteri (Fig. 1B). Active cell proliferation was observed in the endometrium and myometrium of control uteri on P5, was enhanced in the GE on P10, and was still detected in the developing glands and stroma on P15. However, BrdU-positive cells were almost absent on P20 in the control uteri. Thus, DES exposure to neonatal mice affected the critical period of endometrial adenogenesis by inhibiting cell proliferation.
FIG. 1.
Effects of DES exposure in the neonatal uterus. Pups were given control corn oil (CO) or DES injections daily from P1 to P5 (n = 5 per treatment per day). Uterine tissues were collected at the end of treatment and examined for alterations in histology, proliferation, and hormone receptors at the indicated postnatal time points. A) Uterine histology assessed using hematoxylin-and-eosin staining. B) Cell proliferation was detected by incorporated BrdU immunohistochemistry. Immunoreactive ESR1 and PGR were detected using specific antisera against ESR1 (C) and PGR (D). M, myometrium; S, stroma. Bars = 100 μm.
Because most of actions of neonatal DES exposure are mediated through ESR1 [39], we have examined immunoreactive ESR1 in the uteri of DES-treated mice during the neonatal period (Fig. 1C). Immunoreactive nuclear ESR1 was present in all types of cells in the control uterus. DES exposure did not affect ESR1 in the neonatal mouse uterus. Although DES exposure causes hypertrophic LE and disorganized stroma and myometrium, ESR1 was still present in all cell types in the neonatal uterus.
Before birth, PGR is undetectable in the uterus, but a few days after birth, PGR can be localized in LE [40]. Nuclear PGR was detected in the LE on P5, P10, and P15 in the neonatal mouse uterus. Stromal PGR appeared on P15. Neonatal exposure of DES reduced both epithelial and stromal PGR in the neonatal mouse uterus (Fig. 1D).
DES Regulates Wnt and Fzd Genes in Neonatal Mouse
To determine the potential Wnt genes underlying DES-induced uterine malformations, we first screened 19 mouse Wnt genes and 10 mouse Fzd genes by RT-PCR as described previously [37]. RT-PCR analyses found that 10 of the 19 Wnt genes (Wnt2, Wnt2b, Wnt4, Wnt5a, Wnt5b, Wnt7a, Wnt7b, Wnt10a, Wnt11, and Wnt16), and 9 of the 10 Fzd genes (Fzd1, Fzd2, Fzd3, Fzd4, Fzd6, Fzd7, Fzd8, Fzd9, and Fzd10) were expressed in the neonatal mouse uterus (data not shown). Furthermore, only Wnt4, Wnt5a, Wnt7a, Wnt7b, Wnt11, Wnt16, Fzd6, and Fzd10 were detectable by radioactive in situ hybridization analysis of the neonatal mouse uterus; thus, we determined temporal and spatial alterations in those Wnt and Fzd genes by in situ hybridization (Figs. 2 and 3) and real-time RT-PCR analysis (Supplemental Fig. S2).
FIG. 2.
Wnt gene expression was examined by in situ hybridization analysis in uteri from pups given control corn oil (CO) or DES injections daily from P1 to P5 (n = 5 per treatment per day). In each panel portion, representative photomicrographs of in situ hybridization results are presented in dark-field illumination. M, myometrium; S, stroma. Bars = 100 μm.
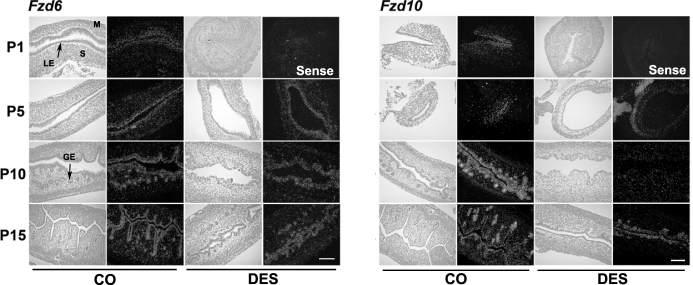
FIG. 3.
Fzd gene expression was examined by in situ hybridization analysis in uteri from pups given control corn oil (CO) or DES injections daily from P1 to P5 (n = 5 per treatment per day). In each panel portion, representative photomicrographs of in situ hybridization results are presented in dark-field illumination. M, myometrium; S, stroma. Bars = 100 μm.
Wnt genes. Wnt4 was detected at low abundance in all cell types in the neonatal mouse uterus but was most abundant in endometrial stroma. Wnt4 was inhibited (P < 0.05) on P5 and P10 in endometrium of mice exposed to DES from P1 but was not affected on P15. Wnt5a was observed predominantly in the endometrium. Exposure to DES after birth transiently increased Wnt5a in the stroma of the uterus on P5 (P < 0.05). However, Wnt5a in endometrium was reduced after DES exposure on P10 and P15 (P < 0.05). Wnt7a was present in the LE on P1 and P5 and in only the LE and superficial ductal GE (sGE) thereafter. DES exposure suppressed Wnt7a in endometrial LE on P5 and P10 but not on P15. Wnt7b was also observed in LE and sGE. Exposure to DES did not affect Wnt7b in the neonatal uterus. Wnt11 mRNA was low in LE at birth and increased in LE and sGE at P10 and P15. Epithelial Wnt11 was suppressed by exposure to DES after birth. Wnt16 was abundantly detected in endometrial stroma and in stroma of the oviduct but not in LE and GE. DES exposure considerably reduced Wnt16 expression in stroma on P5, P10, and P15.
Fzd genes. Fzd6 was detected predominantly in endometrial epithelia in the neonatal mouse uterus. Exposure of mice to DES did not affect Fzd6 in the neonatal uterus. Fzd10 was weakly observed in LE at birth and on P5, was increased in LE and GE on P10, and then declined in LE but remained abundant in GE on P15. Fzd10 was reduced in LE and GE on P5 and P10 in the neonatal mice exposed to DES after birth.
Conditional Ablation of Wnt11 in Neonatal Mouse Uterus
Previous studies have indicated Wnt7a is essential for gland development in the neonatal mouse uterus [27]. Of the 18 other Wnt genes examined our analyses, only Wnt11 exhibited a temporal and spatial expression pattern like that of Wnt7a coupled with downregulation by DES exposure. Wnt11 was detected in uterine epithelium and increased its mRNA levels during the first 2 wk after birth as a critical period for uterine gland development (Figs. 2 and 3 and Supplemental Fig. S2). DES exposure ablated Wnt11 in the neonatal uterus (Figs. 2 and 3 and Supplemental Fig. S2). Furthermore, Wnt11 is required to establish ureteric epithelia for kidney development [29]. Complete germline ablation of Wnt11 leads to perinatal lethality [29]. To further examine the role of Wnt11 in the neonatal uterus, conditional ablation of Wnt11 in the uterus was conducted to circumvent the embryonic lethal phenotype.
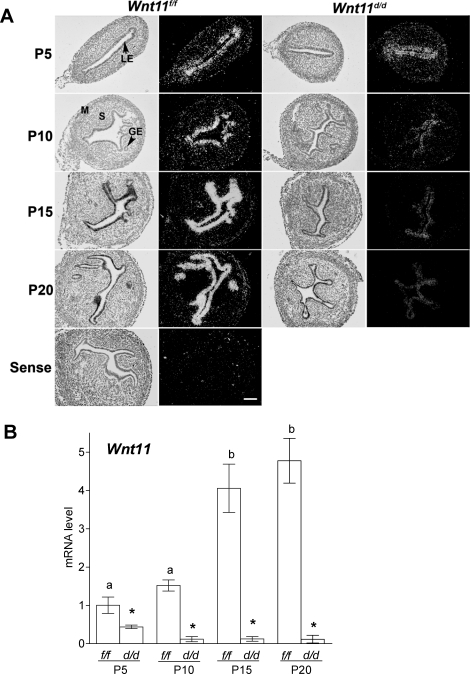
The targeting strategy for Wnt11 is described in Supplemental Figure S1A. We utilized the Pgrcre/+ mouse line in which Cre recombinase is under the control of Pgr promoter [30]. Cre excision activity is restricted to cells that express PGR in progesterone-responsive tissues, such as the uterus, ovary, oviduct, pituitary gland, and mammary gland. Wnt11 was not detected in either the ovary or the pituitary of mice based on RT-PCR analysis (data not shown). However, Wnt11 is expressed by the epithelial cells of the oviduct (data not shown). Analysis of expression of the inserted LacZ transgene in the uterus during development shows that X-gal staining indicated PGR expression begins in the uterine epithelium only after birth (starts on P3) and in the stroma 28 days after birth [41]. In the present study, immunoreactive PGR was detected in LE on P5, whereas stromal PGR was detected on P15 (Fig. 1D). Thus, the Pgrcre/+ mouse line is an excellent model to conditionally ablate Wnt11 in the uterus after birth. To increase the efficiency in production of homozygous Wnt11-null allele, we generated and analyzed Pgrcre/+ Wnt11flox/− mice as Wnt11d/d mice because of weaker Cre excision activity in the first week of the neonatal uterus [41]. Wnt11 gene ablation was validated in the neonatal uterus by measuring Wnt11 expression in the control Wnt11f/f and Wnt11d/d mice (Fig. 4). Although Wnt11 was still detectable in the uteri of Wnt11d/d mice on P5 by real-time PCR, Wnt11 was ablated after P10 (Fig. 4B). We could not examine protein expression of WNT11 because of the unavailability of a high-quality specific antibody. However, in situ hybridization analysis confirmed that Wnt11 was ablated in the neonatal uterus at the mRNA level (Fig. 4A).
FIG. 4.
Wnt11 transcripts were significantly reduced in postnatal mouse uteri from conditionally ablated Wnt11 as determined by in situ hybridization (A) and quantitative RT-PCR (B) analyses (n = 5 per genotype per day). Results are presented as the mean ± SEM. *P < 0.05 for Wnt11f/f versus Wnt11d/d; a and b, P < 0.05, temporally by day in control uteri. M, myometrium; P, postnatal; S, stroma. Bars = 100 μm.
Uterine Gland Development in Wnt11d/d Mice
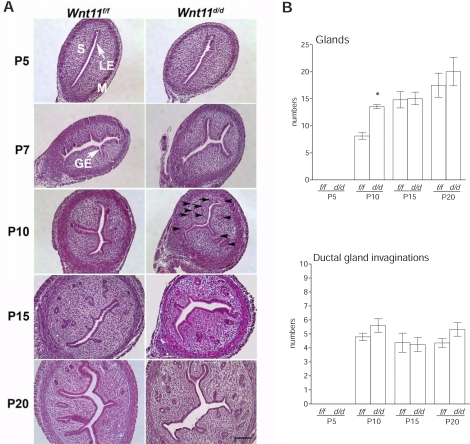
Wnt11d/d pups thrived and did not exhibit any weight differences from wild-type controls (data not shown). Furthermore, female Wnt11d/d mice bred with wild-type male mice were fertile and produced normal numbers of pups. Histological analysis of neonatal uteri was determined in Wnt11f/f and Wnt11d/d mice on P5, P7, P10, P15, and P20 (Fig. 5). Uterine histology revealed that ablation of Wnt11 in the neonatal uterus did not cause inhibition or reduction of endometrial adenogenesis. However, the number of endometrial glands appeared to be greater in the uteri of Wnt11d/d mice at P10 (Fig. 5A, black arrows). Total numbers of uterine glands and ductal gland invaginations were quantified by counting the numbers of glands per section (Fig. 5B). Total number of endometrial glands was significantly increased (P < 0.05) in the uteri on P10 by ablation of Wnt11, but the number of ductal gland invaginations was not affected. We did not see any differences of gland numbers in Wnt11f/f and Wnt11d/d mice on P15 and P20. Cell proliferation was markedly increased in stroma on P5 and in developing glands on P10 in the uteri of Wnt11d/d mice, but not in the uteri on P15 and P20 (Fig. 6).
FIG. 5.
Ablation of Wnt11 in the neonatal mouse uterus alters, but is not essential for, uterine gland development (n = 5 per genotype per day). A) Uterine histology of Wnt11d/d and Wnt11f/f mice. Tissues were stained using hematoxylin and eosin. Black arrows indicate a greater number of endometrial glands at P10. M, myometrium; P, postnatal; S, stroma. Bars = 100 μm. B) Gland numbers and ductal gland invaginations per section of uteri on P5, P10, P15, and P20. Results are presented as the mean ± SEM. *P < 0.05, Wnt11f/f versus Wnt11d/d.
FIG. 6.
Effect of conditional ablation of Wnt11 on cell proliferation in the neonatal mouse uterus (n = 5 per genotype per day). A) Incorporated BrdU was detected by immunohistochemistry. M, myometrium; P, postnatal; S, stroma. Bars = 100 μm. B) Distribution of BrdU in the ablation of Wnt11 uterus. Results are presented as BrdU-positive cells in each cell type ± SEM. *P < 0.05, Wnt11f/f versus Wnt11d/d.
Wnt Genes in Wnt11d/d Mice
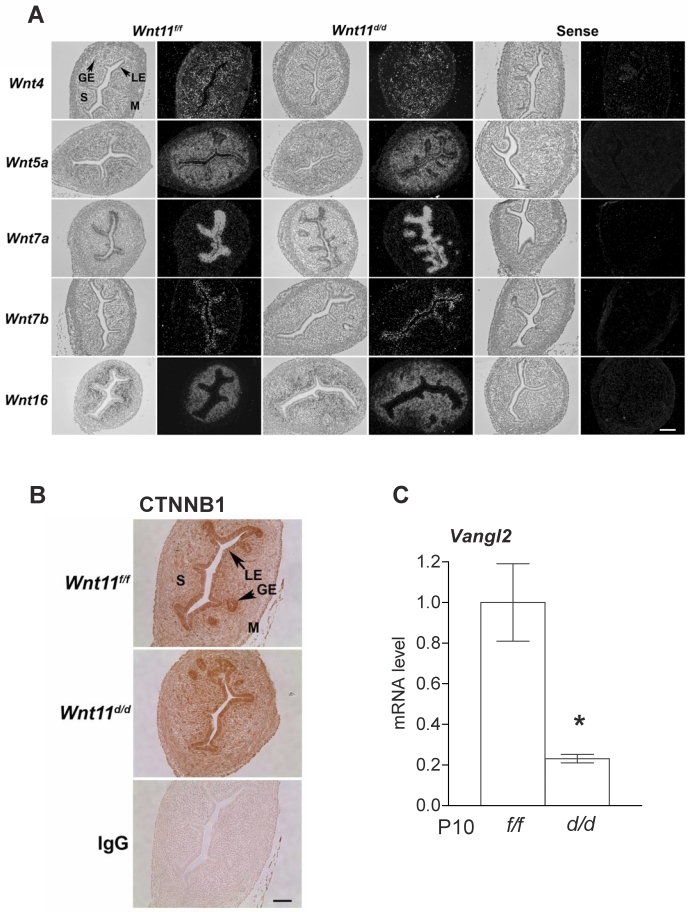
To examine the effect of Wnt11 ablation on other Wnt genes in the neonatal uterus, we used in situ hybridization analysis to determine whether Wnt4, Wnt5a, Wnt7a, Wnt7b, and Wnt16 were altered in the neonatal uterus on P10. However, conditional ablation of Wnt11 did not affect expression of any other Wnt gene in the neonatal uterus (Fig. 7A). Furthermore, expression of CTNNB1, a critical component of the canonical WNT signaling pathway, was not altered by the loss of Wnt11 (Fig. 7B). However, expression of Vangl2, which is downstream noncanonical WNT signaling component, was reduced (P < 0.05) in the uteri of Wnt11d/d mice (Fig. 7C), suggesting that WNT11 acts through noncanonical WNT signaling.
FIG. 7.
In situ hybridization analysis of uterine Wnt genes (A) and immunoreactive CTNNB1 (B) in Wnt11d/d and Wnt11f/f mice on P10 (n = 5 per genotype per day). M, myometrium; P, postnatal; S, stroma. Bars = 100 μm. C) Vangl2 mRNA was analyzed by quantitative RT-PCR in the uteri of Wnt11d/d and Wnt11f/f mice on P10. Results are presented as the mean ± SEM. *P < 0.05, Wnt11f/f versus Wnt11d/d.
DISCUSSION
The present study determined the localization of six members of the Wnt gene family (Wnt4, Wnt5a, Wnt7a, Wnt7b, Wnt11, and Wnt16) and two WNT receptors (Fzd6 and Fzd10) in the neonatal mouse uterus. Wnt4, Wnt5a, and Wnt16 were solely or predominantly present in the endometrial stroma, and Wnt7a, Wnt7b, Wnt11, Fzd6, and Fzd10 were detected in the endometrial epithelia. Although the expression patterns of Wnt4, Wnt5a, and Wnt7a have been reported in the neonatal and/or adult uterus [10, 16, 22, 42, 43], the present study is the first report, to our knowledge, of Wnt7b, Wnt11, Wnt16, Fzd6, and Fzd10 expression in the developing mouse uterus.
In the present study, DES-induced disruption of postnatal uterine development was associated with suppression or reduction of Wnt4, Wnt5a, Wnt7a, Wnt11, Wnt16, and Fzd10 in the neonatal uterus. Fetal and neonatal DES exposure elicits distinct and permanent alterations in the female reproductive tract [44]. DES has a high binding affinity to ESR1 [39]. Indeed, DES disruption of female reproductive tract development and Hoxa10, Hoxa11, and Wnt7a expression is mediated by ESR1 [10]. In the present study, DES exposure did not alter ESR1 expression in neonatal mouse uterus but did alter PGR expression in the LE and stroma. To date, the function of PGR in the neonatal uterus is unknown. Despite the implication that DES may regulate PGR through ESR1, Pgr-null neonatal uteri have apparently normal histoarchitecture [41, 45, 46], so the mechanism by which DES interrupts normal gland development likely involves complex interactions between multiple hormone signaling pathways. In the present study, uterine cell proliferation was highly active during the first 2 wk after birth in both epithelial and stromal cells as assessed by BrdU incorporation. Previous reports [28] and the present results have revealed that exposure of neonatal mice to DES inhibits cell proliferation. Similarly, cell proliferation is reduced in the uterus of Wnt7a-null mice [47], suggesting that reductions in Wnts and their Fzd receptors by DES mediates effects of DES to disrupt uterine development and retard endometrial gland development.
The GE of the mouse uterus begins differentiation between P5 and P7 [6]. During this critical period, endometrial gland differentiation is initiated by the formation of epithelial invaginations. By P10, endometrial glands start tubulogenesis with invaginations from the LE into the surrounding endometrial stroma. On P15, adult histoarchitectural configuration of the mouse uterus is morphologically finished. In fact, results concerning immunoreactive BrdU incorporation showed that active cell proliferation was observed in endometrium and myometrium by P10 but less so on P15 and no longer on P20. Therefore, the initiation of gland development and the stage for active cell proliferation in the uterine development is the most critical time point for endometrial adenogenesis to determine gene regulation and future cell fate.
The functional role of Wnt7a and Wnt5a during the perinatal window has been characterized in the reproductive tract [6, 16, 17, 22]. Analyses of Wnt7a and Wnt5a mutants have demonstrated that both genes are required for endometrial adenogenesis [16, 27]. Although the expression patterns of both genes are different (Wnt5a in stroma and Wnt7a in LE), epithelial-mesenchymal interactions are critical for uterine development with local control and coordination of morphogenetically important cell behaviors, including movement, adhesion, differentiation, and proliferation [2, 48]. Tissue recombination studies in rodents clearly indicate that uterine mesenchyme directs and specifies patterns of epithelial development, and then epithelium is required to support organization of endometrial stroma and myometrial differentiation [40, 49–51]. In the present study, DES exposure completely suppressed Wnt7a in LE during the first 10 days after birth and reduced Wnt5a in stroma even though DES transiently stimulated Wnt5a on P5. Mericskay et al. [16] have proposed that highly regionalized repression of Wnt7a is required to allow LE to change fate, invaginate, and form glands and that Wnt5a is required for this downregulation in the postnatal endometrial adenogenesis. Thus, the present results support that both epithelial and mesenchymal-specific Wnt genes are critical factors for epithelial differentiation and stromal organization for gland formation.
The role of Wnt4 in Müllerian duct initiation is established, but the functional role of Wnt4 during postnatal uterine development is currently unknown because of death of Wnt4-null mice shortly after birth [26]. Wnt4 is present in the mesenchyme at birth and in adults during the estrous cycle [22] and pregnancy [37, 42, 43]. Wnt4 is identified as a downstream target gene of Bmp2 signaling in stromal decidualization [52]. In the present study, Wnt4 remains present with low abundance in the stroma during postnatal uterine development. DES exposure elicited a significant decrease in uterine Wnt4 that exceeded 50% on P5 and P10, suggesting that Wnt4 also has functional role for uterine development after birth as a stromal factor.
The expression of Wnt7b has been shown in human endometrium [53] and adult mouse uterus [37]. Wnt7b-null embryos die at midgestation because of placental abnormalities involving function of the chorion and allantois [54]. Although the functional role of Wnt7b in the adult mouse is currently unknown, estrogen treatment of ovariectomized mice stimulated Wnt7b in the adult uterus [37]. In the present study, Wnt7b was detected in the LE of the neonatal mouse uterus; however, its expression was not affected by DES exposure. Thus, Wnt7b may not be involved DES-induced disruption of uterine development of neonatal mice.
Human WNT16 is detected in placenta [55] and FOXO1-dependent endometrial decidualizing cells [56]. We have also reported that Wnt16 is abundant in the adult mouse uterus during implantation [37]. The present study showed that Wnt16 was highly abundant in the entire stroma, but not in LE and GE, during uterine morphogenesis. Inappropriate exposure of the developing postnatal uterus to DES reduced the expression of Wnt16. Thus, Wnt16 may be a potential regulator for uterine development as an epithelial-mesenchymal paracrine and/or autocrine factor. In support of this, WNT16B enhances cell proliferation and prolonged clonogenicity in primary keratinocytes in human skin [57]. However, to our knowledge, direct studies of Wnt16-null animals have yet to be performed.
To be effective in autocrine and/or paracrine signaling, WNT proteins must associate with their extracellular surface receptors (FZD) to mediate intracellular signal transduction pathways [19]. In the present study, Fzd6 and Fzd10 were observed in the endometrial epithelia. Specifically, Fzd10 was abundant in GE on P10 and P15, and its expression was suppressed by DES exposure. The specific ligand partner for FZD10 in the uterus is not known. However, the disruption of gland formation in DES-exposed uteri could result from downregulation of this receptor rather than altered expression of the key Wnt gene that stimulates its activity in normal gland development.
In the present study, we sought to determine the functional role of Wnt11 in uterine development using conditional ablation of Wnt11 in the uterus. We focused our initial attention on Wnt11, because it was found to be expressed in LE after birth at the critical window associated with onset of GE bud differentiation and invagination into the endometrial stroma. Furthermore, DES exposure of neonatal mice ablated the expression of Wnt11 in the uterus, suggesting that Wnt11 is a critical mediator of DES-induced inhibition of uterine gland development. Wnt11 is required to appropriately establish branching morphogenesis of the ureteric epithelium for kidney development [29, 58–61]. Wnt11-null mice display defects in ureteric branching morphogenesis and consequent kidney hypoplasia [29]. Thus, we hypothesized that epithelial Wnt11 in the neonatal uterus regulates endometrial morphogenesis. However, the present results indicate that ablation of Wnt11 in the neonatal uterus was not detrimental to uterine development. Histoarchitectural analysis showed that normal numbers of glands were observed in the uteri of Wnt11-deleted animals on P15 and P20, although endometrial gland numbers were increased in Wnt11d/d mice on P10 and these glands are active for cell proliferation. Furthermore, Wnt11d/d mice are fertile and have normal numbers of pups (data not shown). Thus, increased number of glands on P10 did not affect any perceived neonatal and adult uterine function.
We also showed that ablation of Wnt11 did not affect the expression of any other Wnt gene or of CTNNB1, a key downstream mediator of canonical WNT pathway in the neonatal uterus. However, Vangl2 was inhibited by ablation of Wnt11. Vangl2 is a noncanonical WNT signaling component and is required for the planar cell polarity [62–65]. Vangl2 mutant mice cause alternations of cell polarity, including composition of the apical surface of the epithelium, more smooth muscle, and reduced stroma as well as Wnt7a expression [66]. These studies propose that the establishment and maintenance of cell polarity through noncanonical WNT signaling are required for development of the female reproductive tract. Wnt5a and probably Wnt16, which are thought to act via the noncanonical WNT pathway, likely regulate uterine development. Although ablation of Wnt11 decreased Vangl2 in the neonatal uterus, Wnt11 may not be able to solely regulate uterine development. Wnt11 may have some functions interacting with other noncanonical Wnt genes in the neonatal uterus.
Collectively, the present results indicate that multiple Wnt genes are expressed in the neonatal mouse uterus and are candidate regulators of postnatal uterine development. DES exposure after birth inhibited epithelial and stromal Wnt genes at a critical time point for endometrial adenogenesis. The spatial expression of Wnt genes are involved in epithelial-mesenchymal interactions as paracrine and autocrine factors to regulate uterine morphogenesis.
Supplementary Material
Footnotes
Supported by NIH/NICHD HD0588222, P30 ES09106, ACS-IL 139038, and SIU-SOM CRC (to K.H.).
REFERENCES
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287 302 [PubMed] [Google Scholar]
- Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol 1976; 47: 137 194 [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68: 85 122 [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat 1989; 186: 21 42 [DOI] [PubMed] [Google Scholar]
- Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17beta-estradiol. Endocrinology 1985; 117: 2229 2237 [DOI] [PubMed] [Google Scholar]
- Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod 2004; 70: 1870 1876 [DOI] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, Broaddus RR, Lewis MT, Lydon JP, Jeong JW, DeMayo FJ. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod 2010; 82: 991 999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289 300 [PubMed] [Google Scholar]
- Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol 2001; 238: 224 238 [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res 1980; 40: 3988 3999 [PubMed] [Google Scholar]
- Yoshida A, Newbold RR, Dixon D. Effects of neonatal diethylstilbestrol (DES) exposure on morphology and growth patterns of endometrial epithelial cells in CD-1 mice. Toxicol Pathol 1999; 27: 325 333 [DOI] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES). Dev Biol 1998; 197: 141 154 [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in deregulation of Wnt7a during uterine morphogenesis. Nat Genet 1998; 20: 228 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996; 122: 2687 2696 [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 2004; 131: 2061 2072 [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 1998; 395: 707 710 [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837 1851 [PubMed] [Google Scholar]
- Dale TC. Signal transduction by the Wnt family of ligands. Biochem J 1998; 329 (pt 2): 209 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu T, Slusarski DC, Yang-Snyder J, Malbon CC, Moon RT, Wang H. Activation of a frizzled-2/beta-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc Natl Acad Sci U S A 1999; 96: 14383 14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem 1996; 271: 4468 4476 [DOI] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 1998; 76: 91 99 [DOI] [PubMed] [Google Scholar]
- Kitajewski J, Sassoon D. The emergence of molecular gynecology: homeobox and Wnt genes in the female reproductive tract. Bioessays 2000; 22: 902 910 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4: 969 980 [DOI] [PubMed] [Google Scholar]
- Sassoon D. Wnt genes and endocrine disruption of the female reproductive tract: a genetic approach. Mol Cell Endocrinol 1999; 158: 1 5 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signaling. Nature 1999; 397: 405 409 [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201 3211 [DOI] [PubMed] [Google Scholar]
- Huang WW, Yin Y, Bi Q, Chiang TC, Garner N, Vuoristo J, McLachlan JA, Ma L. Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol 2005; 19: 669 682 [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003; 130: 3175 3185 [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58 66 [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 2000; 25: 139 140 [DOI] [PubMed] [Google Scholar]
- Taylor KM, Gray CA, Joyce MM, Stewart MD, Bazer FW, Spencer TE. Neonatal ovine uterine development involves alterations in expression of receptors for estrogen, progesterone, and prolactin. Biol Reprod 2000; 63: 1192 1204 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Gray CA, Spencer TE. The activin-follistatin system in the neonatal ovine uterus. Biol Reprod 2003; 69: 843 850 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Spencer TE. Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology 2004; 145: 3247 3257 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Carpenter KD, Welsh TH, Jr, Burghardt RC, Spicer LJ, Spencer TE. The IGF system in the neonatal ovine uterus. Reproduction 2005; 129: 337 347 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Spencer TE. Estrogen disruption of neonatal ovine uterine development: effects on gene expression assessed by suppression subtraction hybridization. Biol Reprod 2005; 73: 752 760 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, II, Rucker EB, III, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod 2009; 80: 989 1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Stagg AG, Joyce MM, Jenster G, Wood CG, Bazer FW, Wiley AA, Bartol FF. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 1999; 140: 4070 4080 [DOI] [PubMed] [Google Scholar]
- Korach KS, Chae K, Levy LA, Duax WL, Sarver PJ. Diethylstilbestrol metabolites and analogs. Stereochemical probes for the estrogen receptor binding site. J Biol Chem 1989; 264: 5642 5647 [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (mullerian) epithelial differentiation. Dev Biol 2001; 240: 194 211 [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178 186 [DOI] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol 2004; 18: 1238 1250 [DOI] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 2004; 18: 3035 3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Bigsby R, Brody JR. Ontogeny of sex steroid receptors in mammals. Parker MG. Nuclear Hormone Receptors: Molecular Mechanisms, Cellular Functions, Clinical Abnormalities. London: Academic Press; 1991: 235 268 [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266 2278 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000; 289: 1751 1754 [DOI] [PubMed] [Google Scholar]
- Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod 2004; 71: 444 454 [DOI] [PubMed] [Google Scholar]
- Sharpe PM, Ferguson MW. Mesenchymal influences on epithelial differentiation in developing systems. J Cell Sci Suppl 1988; 10: 195 230 [DOI] [PubMed] [Google Scholar]
- Cunha GR. Alterations in the developmental properties of stroma during the development of the urogenital ridge into ductus deferens and uterus in embryonic and neonatal mice. J Exp Zool 1976; 197: 375 388 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 1983; 39: 559 598 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Brody JR. Role of uterine epithelium in the development of myometrial smooth muscle cells. Biol Reprod 1989; 40: 861 871 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 2007; 282: 31725 31732 [DOI] [PubMed] [Google Scholar]
- Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL. Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer 1997; 75: 1131 1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol 2001; 237: 324 332 [DOI] [PubMed] [Google Scholar]
- Fear MW, Kelsell DP, Spurr NK, Barnes MR. Wnt-16a, a novel Wnt-16 isoform, which shows differential expression in adult human tissues. Biochem Biophys Res Commun 2000; 278: 814 820 [DOI] [PubMed] [Google Scholar]
- Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell-cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 2007; 21: 2334 2349 [DOI] [PubMed] [Google Scholar]
- Teh MT, Blaydon D, Ghali LR, Briggs V, Edmunds S, Pantazi E, Barnes MR, Leigh IM, Kelsell DP, Philpott MP. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci 2007; 120: 330 339 [DOI] [PubMed] [Google Scholar]
- Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 2006; 299: 466 477 [DOI] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signaling during kidney development. Development 2004; 131: 3345 3356 [DOI] [PubMed] [Google Scholar]
- Michael L, Sweeney DE, Davies JA. The lectin Dolichos biflorus agglutinin is a sensitive indicator of branching morphogenetic activity in the developing mouse metanephric collecting duct system. J Anat 2007; 210: 89 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 2004; 14: 550 557 [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant loop-tail. Nat Genet 2001; 28: 251 255 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003; 423: 173 177 [DOI] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res 2005; 96: 292 299 [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development 1998; 125: 1149 1159 [DOI] [PubMed] [Google Scholar]
- Vandenberg AL, Sassoon DA. Noncanonical Wnt signaling regulates cell polarity in female reproductive tract development via Van Gogh-like 2. Development 2009; 136: 1559 1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.