Abstract
Vitamin A (retinol) is required for maintenance of adult mammalian spermatogenesis. In adult rodents, vitamin A withdrawal is followed by a loss of differentiated germ cells within the seminiferous epithelium and disrupted spermatogenesis that can be restored by vitamin A replacement. However, whether vitamin A plays a role in the differentiation and meiotic initiation of germ cells during the first round of mouse spermatogenesis is unknown. In the present study, we found that vitamin A depletion markedly decreased testicular expression of the all-trans retinoic acid-responsive gene, Stra8, and caused meiotic failure in prepubertal male mice lacking lecithin:retinol acyltransferase (Lrat), encoding for the major enzyme in liver responsible for the formation of retinyl esters. Rather than undergoing normal differentiation, germ cells accumulated in the testes of Lrat−/− mice maintained on a vitamin A-deficient diet. These results, together with our previous observations that germ cells fail to enter meiosis and remain undifferentiated in embryonic vitamin A-deficient ovaries, support the hypothesis that vitamin A regulates the initiation of meiosis I of both oogenesis and spermatogenesis in mammals.
Keywords: developmental biology, gamete biology, germ cells, male reproductive tract, meiosis, retinoic acid, retinol, spermatogenesis, spermatogonia
Vitamin A deficiency blocks spermatogonial differentiation during the first round of spermatogenesis in the prepubertal mouse testis.
INTRODUCTION
In the mouse embryo, male germ cells are enclosed by Sertoli cells within the testis cords and arrest as G0/G1 spermatogonia. Within a few days after birth, male germ cells resume mitosis and move toward the basement membrane of the seminiferous cords, where they become undifferentiated spermatogonia that initiate and maintain spermatogenesis. In mice, spermatogenesis begins at the end of the first week of life [1, 2] and continues throughout the lifetime of the adult male. Spermatogenesis is a complex process involving mitotic divisions, meiotic divisions, and dramatic morphological changes ultimately resulting in spermatozoa. Undifferentiated spermatogonia divide to become differentiating spermatogonia (type A1–4 spermatogonia, intermediate spermatogonia, and type B spermatogonia), all of which are observed by Postnatal Day (P) 8. Type B spermatogonia divide to form preleptotene primary spermatocytes, which further develop into haploid spermatids and spermatozoa [3].
The process of spermatogenesis is influenced by both intrinsic and extrinsic factors, including vitamin A (retinol). Vitamin A is required for the maintenance of mammalian spermatogenesis, and most germ cells are lost in vitamin A-deficient (VAD) adult rodents. Vitamin A deficiency results in spermatogenic arrest at the stage of undifferentiated spermatogonia and preleptotene spermatocytes in rats [4, 5] or type A spermatogonia in mice [6], and retinol replacement results in the complete recovery of spermatogenesis [4, 6, 7].
Despite increasing evidence indicating that vitamin A regulates the initiation of meiosis of germ cells in the developing ovary and maintains adult spermatogenesis, direct evidence supporting a role for vitamin A in the initial differentiation and meiotic entry of spermatogonia and, thus, in the initiation of spermatogenesis in the prepubertal testis is still lacking. This can be attributed largely to the lack of a suitable animal model to induce vitamin A deficiency during prepubertal life. Vitamin A is obtained from the maternal diet and is stored primarily as a retinyl ester in the liver and, to a lesser extent, in other tissues. Retinol is esterified primarily through the action of the enzyme lecithin:retinol acyltransferase (LRAT) [8, 9], and Lrat−/− mice are much more susceptible to developing vitamin A deficiency than their wild-type counterparts [10, 11]. In the present study, we use a nutritional approach to produce vitamin A deficiency in the testis of prepubertal Lrat−/− mice before germ cells enter meiosis and describe a role for vitamin A in spermatogonial differentiation and meiotic initiation in these animals.
MATERIALS AND METHODS
Generation of VAD Prepubertal Mice
The Lrat−/− mutant line has been described previously [8]. Female Lrat+/+ and Lrat−/− mice were maintained on a normal chow diet (LabDiet #5015; TestDiet) up to approximately 8 wk of age. Female Lrat+/+ mice were mated with Lrat+/+ males, and female Lrat−/− mice were mated with Lrat−/− males. Mating females were examined daily for vaginal plugs, and the day when the plug was found was considered to be Embryonic Day 0.5. At this time, the females were placed on a purified diet devoid of vitamin A activity [12] and supplemented daily with vitamins D2 (87.5 ng), E (77 μg of RRR-α-tocopherol), and K1 (3.5 μg) [13] without or with added retinyl palmitate (RP; 100 U/day) in soybean oil (VAD diet or vitamin A-sufficient [VAS] diet, respectively). The resulting offspring remained with their mothers and continued to receive the same diet until termination (P6, P10, or P18). A subset of Lrat−/− mothers on the VAD diet were supplemented with RP starting at P5 (rescue experiment). All animal experimentation was reviewed and approved by the University of Wisconsin-Madison College of Agricultural and Life Sciences Animal Care and Use Committee.
RNA Extraction and Quantitative RT-PCR Analysis
Freshly dissected gonads were snap-frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated and reverse transcribed as previously described [14]. Quantitative PCR studies of Stra8 were performed using the Applied Biosystems StepOnePlus Real-Time System with the Power SYBR Green PCR Master Mix (Applied Biosystems). Values were normalized to 18S rRNA expression levels. Values for Stra8 were expressed as a percentage of values determined in samples from Lrat+/+ mice maintained on the VAS diet. The following primer sets were used: for Stra8, 5′-CTGTTGGACCAGATGCTGAA-3′ and 5′-GCAACAGAGTGGAGGAGGAG-3′; for Rn18s, 5′-ACCGCGGTTCTATTTTGTTG-3′ and 5′-AGTCGGCATCGTTTATGGTC-3′. PCR product quality was monitored by using post-PCR melting-curve analyses at the end of the amplification cycles.
Immunofluorescence Studies
Dissected testes were fixed for 1 h in Bouin fixative (VWR Scientific Products) and washed in PBS, dehydrated in 100% ethanol, embedded in paraffin, and sectioned (thickness, 10 μm). Testes from three to four mice from two to four independent litters were analyzed for each treatment group and time point. For immunofluorescence studies, sections were dewaxed and boiled for 10 min in 10 mM citrate buffer (pH 6.0) in a microwave oven. Sections were blocked in PBS containing 5% heat-inactivated serum (matched to the species of the secondary antibody) and 0.1% Triton X-100 for 30 min at room temperature before staining with primary antibodies. Sections were incubated overnight at 4°C with antibodies directed against DEAD box polypeptide 4 (DDX4, also known as MVH; 1:2000; kindly provided by Dr. Toshiaki Noce, Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan), zinc finger and BTB domain containing 16 (ZBTB16, also called PLZF; 1:200, 2A9; Calbiochem), or synaptonemal complex protein 3 (SYCP3; 1:500; a kind gift from Dr. Christa Heyting, Wegeningen University, Wegeningen, The Netherlands) [15]. After three washes in PBS, sections were incubated for 60 min at room temperature with goat anti-rabbit Alexa 488 or goat anti-mouse Alexa 594 (Molecular Probes) diluted 1:500. After three additional washes in PBS, slides were mounted with antifade medium (Biomeda) and analyzed with a Nikon Eclipse TE 2000-U microscope. A negative control that omitted the primary antiserum was included in each experiment.
For quantitative cell analyses, the number of DDX4- and ZBTB16-positive cells per tubule in P6 testes were determined from more than 150 random tubules from three to four mice for each treatment group. The percentage of tubules containing SYCP3-positive cells in P10 testes was determined from more than 150 random tubules from three to four mice for each treatment group, and the total number of positive cells per field was determined from three random fields per mouse testes. To determine the number of ZBTB16-positive cells per tubule in P18 testes, ZBTB16-stained cells in more than 200 random tubules from four mice for each treatment group were counted. Differences between the groups were evaluated using a one-way ANOVA and the Tukey multiple-comparison test, with significance set at P < 0.05.
RESULTS
Adult Lrat−/− Mice Given Dietary Vitamin A Show No Apparent Defects in Male Germ Cell Development
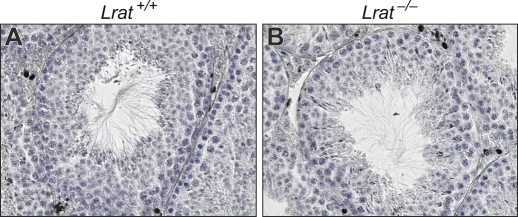
To determine whether the Lrat gene is essential for male germ cell maturation, testicular sections from 3-mo-old Lrat−/− mice maintained on a standard VAS chow diet were stained with hematoxylin. Lrat−/− mice exhibited normal testicular histology, with a full complement of maturing germ cells in their seminiferous tubules (Fig. 1). Seven Lrat−/− males bred to 23 Lrat−/− females all yielded live pups, with an average litter number of 8 ± 1 pups, similar to the number of pups resulting from 27 Lrat+/+ by Lrat+/+ crosses (7 ± 1 pups/litter; mean ± SD).
FIG. 1.
Hematoxylin-stained sections of testes from 3-mo-old Lrat+/+ (A) and Lrat−/− mice (B) show comparable germ cell development. Original magnification ×600.
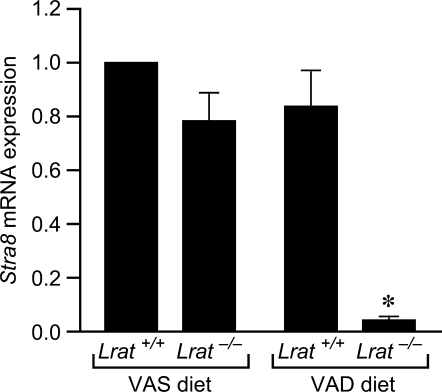
Stra8 Levels Are Decreased at P6 in Testes of Lrat−/− Mice Maintained on the VAD Diet
Stra8 is an all-trans retinoic acid (atRA)-responsive gene [16] that is highly expressed in mouse testis beginning at Day 6 after birth [17], several days before the onset of meiosis I. Stra8 mRNA levels in the testes at P6 did not differ between Lrat+/+ mice receiving either the VAS or VAD diet or from the levels found in Lrat−/− mice fed the VAS diet (Fig. 2). In contrast, Stra8 levels were decreased by approximately 95% in Lrat−/− mice maintained on the VAD diet as compared to the Lrat+/+ VAS controls (Fig. 2). Thus, an increase in Stra8 mRNA was not observed in the testes of VAD Lrat−/− pups at P6, but an increase was seen when these mice were supplemented with dietary vitamin A. These results support the conclusion that vitamin A deficiency was established by P6 in the testes of Lrat−/− mice from mothers fed the VAD diet.
FIG. 2.
Expression of Stra8 is reduced at P6 in the testes of Lrat−/− mice maintained on the VAD diet. Results are expressed relative to values measured in the testes of Lrat+/+ mice maintained on the control VAS diet, arbitrarily set at one. Values are the mean ± SEM of three independent samples. *P < 0.01 vs. control using one-way ANOVA and Tukey post hoc analyses.
Germ Cells in VAD Prepubertal Mouse Testes Fail to Enter Meiosis
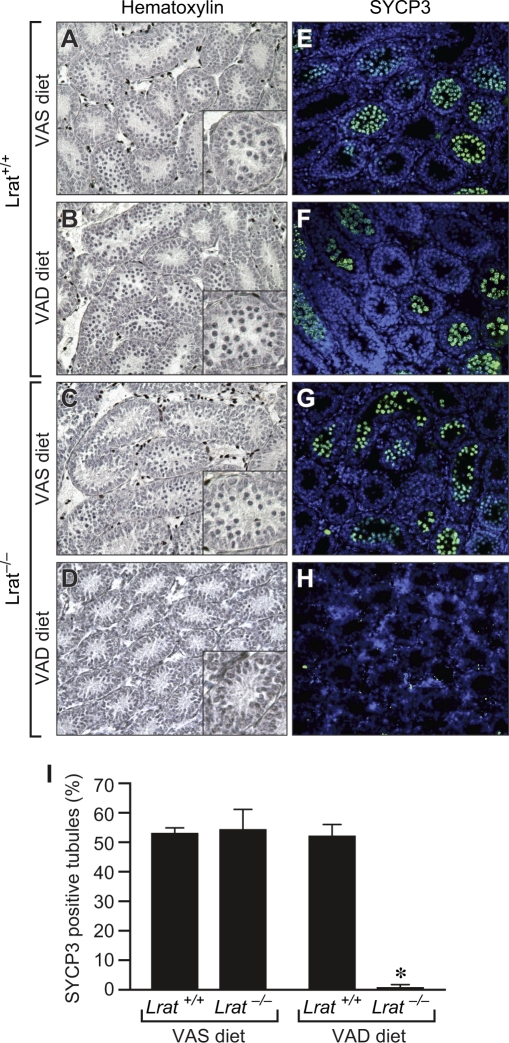
To assess the need for vitamin A for initiation of meiosis of germ cells in the prepubertal testis, tissue sections were prepared at P10 from Lrat+/+ or Lrat−/− mice fed either the VAS or VAD diet and were stained with hematoxylin. Germ cells that had entered meiosis (as evidenced by the presence of condensed chromosomes) were readily observed in the testes of Lrat+/+ mice receiving either the VAS or VAD diet (Fig. 3, A and B) as well as in Lrat−/− mice receiving the VAS diet (Fig. 3C). In contrast, meiotic cells were rarely seen in the testes of Lrat−/− mice maintained on the VAD diet (Fig. 3D). Thus, provision of dietary vitamin A prevented the meiotic failure observed in VAD Lrat−/− pups (Fig. 3, C and D).
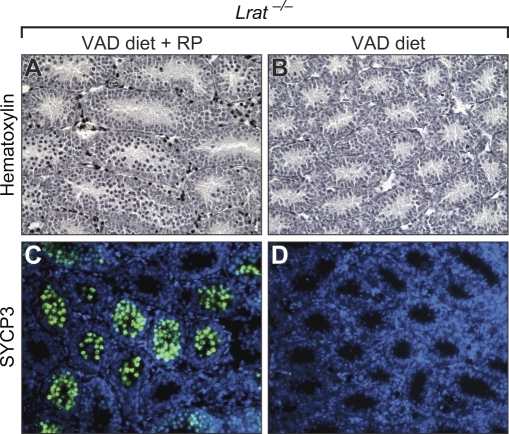
FIG. 3.
Vitamin A deficiency results in meiotic failure of testicular germ cells. Sections at P10 from testes of Lrat+/+ mice fed the VAS (A and E) or VAD (B and F) diet and Lrat−/− mice fed the VAS (C and G) or VAD (D and H) diet were stained with hematoxylin (A–D) or an antibody to SYCP3, a structural component in the synaptonemal complex located along paired meiotic chromosomes (E–H). Quantitation of the percentage of tubules positive for SYCP3 antibody staining is also shown (I). *P < 0.05 vs. all other groups. Original magnification ×200 (A–H) and ×600 (insets in A–D).
We performed immunostaining for SYCP3 to verify these findings. SYCP3 marks the thread-like synaptonemal complex found only in meiotic cells. Similar to the results obtained with hematoxylin staining, SYCP3-labeled cells were found in 53% and 52% of seminiferous tubules in Lrat+/+ mice that had received the VAS or VAD diet, respectively, as well as in 55% of tubules in Lrat−/− mice that had received the VAS diet (Fig. 3, E–G and I). In contrast, SYCP3-positive cells were observed in less than 1% of tubules in the testes of VAD Lrat−/− mice (Fig. 3, H and I). The number of SYCP3-labeled cells per field and the number per labeled tubule were also significantly lower in Lrat−/− mice on the VAD diet (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). Thus, in the prepubertal mouse testis, vitamin A is required for the meiotic entry of germ cells during the first round of spermatogenesis.
Male Germ Cell Development Is Unaltered at P6 in VAD Lrat−/− Pups
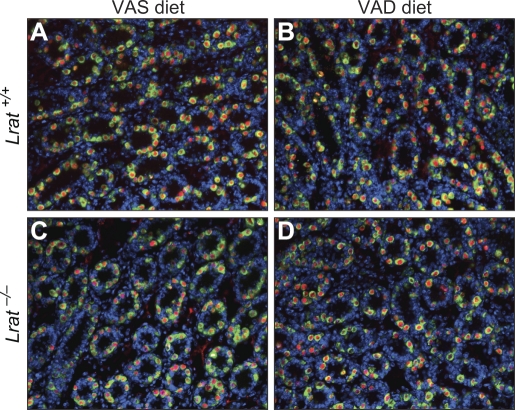
To assess the effect of vitamin A deficiency on male germ cell development, double-staining for DDX4 and ZBTB16 was performed at P6 on testicular sections from Lrat+/+ or Lrat−/− mice fed the VAS or VAD diet. DDX4 marks germ cells, and ZBTB16 labels undifferentiated spermatogonia [18, 19]. The number of germ cells and undifferentiated spermatogonia in testes of Lrat−/− mice maintained on the VAD diet did not differ from the numbers in testes of Lrat+/+ mice maintained on the VAS or VAD diet or in testes of Lrat−/− mice maintained on the VAS diet (Fig. 4 and Supplemental Fig. S2).
FIG. 4.
Germ cell development appears unaltered at P6 in the VAD Lrat−/− testes. Germ cells were identified in the testes of Lrat+/+ mice fed the VAS (A) or VAD (B) diet and in sections from Lrat−/− mice fed the VAS (C) or VAD (D) diet by using antibodies to DDX4 (green; a marker for germ cells) and ZBTB16 (red; a marker of undifferentiated spermatogonia). Nuclei of cells (DNA) were labeled with 4′,6′-diamidino-2-phenylindole (blue). Original magnification ×200.
We next sought to determine whether germ cells in the VAD testes at P6 still retained the ability to enter meiosis. Lrat−/− mice were maintained on the VAD diet as described until P5, at which time a daily supplement of RP was added to the diet. Meiosis was rescued in the testis of these pups, as evidenced by staining with both hematoxylin and SYCP3 (56% tubules positive for SYCP3) (Fig. 5, A and C), whereas no meiosis was evident in testes from Lrat−/− mice not receiving supplemental RP (Fig. 5, B and D). Thus, the developmental potential of testicular germ cells and undifferentiated spermatogonia from VAD Lrat−/− pups at P6 appears to be unaltered, and development can be rescued by the addition of dietary vitamin A.
FIG. 5.
Retinol supplementation prevents meiotic failure because of vitamin A deficiency. Testicular sections from Lrat−/− mouse pups maintained on either the VAD diet from Embryonic Day (E) 0.5 to P5 followed by maternal supplementation from P5 to P10 (A and C) or the VAD diet from E0.5 to P10 (B and D) were stained with hematoxylin (A and B) or an antibody to SYCP3 (C and D). Original magnification ×200.
Germ Cells in VAD Testes Remain Undifferentiated
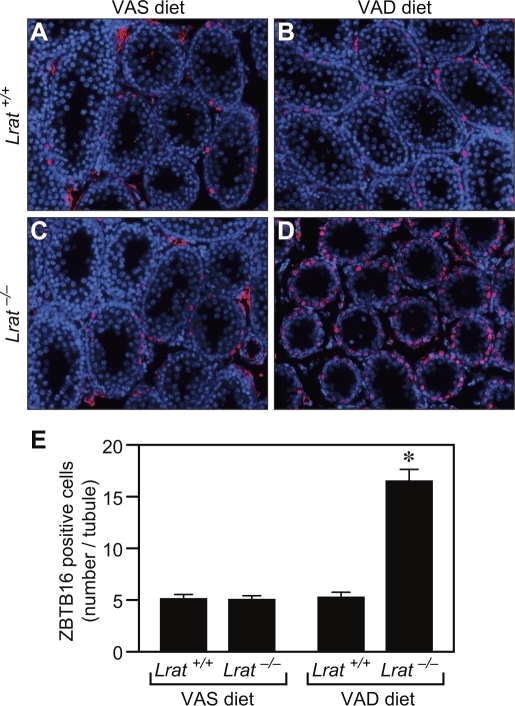
To determine whether the germ cells from VAD Lrat−/− pups that failed to enter meiosis at P6 remained undifferentiated, immunostaining for ZBTB16, a marker of undifferentiated spermatogonia, was performed on testicular sections at P18. In the control group in which Lrat+/+ mice were maintained on a VAS diet, undifferentiated spermatogonia lessened in number with age, corresponding to the time when these cells undergo their expected differentiation (Fig. 6A). Lrat+/+ mice on the VAD diet and Lrat−/− mice on VAS diet also showed a similar reduction in undifferentiated spermatogonia (Fig. 6, B and C). In contrast, the number of germ cells in the VAD Lrat−/− testes that remained as undifferentiated spermatogonia (ZBTB16-positive) lining the basement membrane of the seminiferous tubule was significantly higher than that in the other three groups (Fig. 6, D and E).
FIG. 6.
Germ cells at P18 remain undifferentiated in the mouse VAD Lrat−/− testes. Sections from the testes of Lrat+/+ mice fed the VAS (A) or VAD (B) diet and from Lrat−/− mice fed the VAS (C) or VAD (D) diet were stained with an antibody to ZBTB16 (red); cell nuclei (DNA) were labeled with 4′,6′-diamidino-2-phenylindole (blue). Quantitation of the number of ZBTB16 antibody-labeled cells per tubule is also shown (E). *P < 0.05 vs. all other groups. Original magnification ×200.
DISCUSSION
In the female mouse, meiotic entry of germ cells occurs during embryogenesis, whereas in the male, the entry into meiosis I occurs after birth and throughout adulthood. Previously, we showed that vitamin A deficiency blocks the initiation of meiosis of germ cells in developing ovaries [14]. Until now, it was not possible to determine whether vitamin A is also required for the first meiotic division of the male germ cell, because deficiency could not be induced before spermatogenesis initiates at approximately P7. Using a genetic mouse model that does not efficiently store vitamin A, we now show that meiotic failure of germ cells occurs during the first wave of male spermatogenesis.
Vitamin A deficiency is difficult to produce in wild-type mice, because vitamin A is stored in the liver in the form of retinyl esters [20] and neonates also receive vitamin A in maternal milk [21]. LRAT is the predominant enzyme in the liver responsible for the formation of retinyl esters [8–10], and Lrat−/− mice are more susceptible to developing vitamin A deficiency than wild-type mice [10, 11]. In the present study, vitamin A deficiency was produced in prepubertal life by maintaining pregnant and lactating Lrat−/− female mice on a diet devoid of all forms of vitamin A. Generation of vitamin A deficiency in the testes of pups at P6 was confirmed by measuring the level of the atRA-responsive gene, Stra8. Testicular expression of Stra8 is induced by atRA and serves as an excellent marker for atRA activity [17, 22, 23]. Stra8 expression also is down-regulated by atRA deficiency in a dose-dependent manner in the developing ovary [14]. In normal mice, an increase in Stra8 first occurs in the testes at P6, a few days before the onset of meiosis I. The highest Stra8 levels are reached by P10 [17], a time that coincides with the onset of meiosis I. In Lrat−/− pups maintained on the VAD diet, the expected increase in Stra8 expression that is seen in normal mice did not occur at P6 and was only 5% of the level observed in wild-type mice fed the VAS diet, confirming the establishment of atRA deficiency.
Germ cells fail to enter meiosis in the neonatal VAD Lrat−/− testes at P10, as evidenced by the absence of condensed chromosomes by histological analysis and the lack of SYCP3 expression, a marker found only in meiotic cells. In the adult male, the need for vitamin A to maintain spermatogenesis is well known. The adult VAD testis contains largely undifferentiated spermatogonia, and vitamin A is required for the conversion of these cells to type A1 (differentiated) spermatogonia [24]. Treatment of cultured germline stem cells from P2 male mice with atRA produces an increase in Kit, a marker of spermatogonial differentiation, suggesting that atRA plays a normal role in this process in the neonate [25]. However, evidence that vitamin A is required for meiotic initiation in the neonatal testes in vivo was lacking. The present work proves that vitamin A is required for meiotic initiation during the first round of mouse spermatogenesis.
Meiotic failure in the prepubertal VAD testes cannot be explained on the basis of altered germ cell numbers or impaired ability to initiate the meiotic program. Expression of the germ cell marker, DDX4, and of the undifferentiated spermatogonia marker, ZBTB16, was unaffected by vitamin A deficiency, showing that the number of germ cells in P6 VAD testes was comparable to that in P6 VAS testes. Moreover, supplementation with retinol at P5 rescued the meiotic initiation. This is consistent with our previous observation that germ cell development appeared to be normal in the testis of VAD rat embryos [14]. Taken together, these observations indicate that vitamin A is not required for maintaining the undifferentiated state of male germ cells during embryonic and prepubertal development.
In both the male and female, meiotic failure is accompanied by a marked decrease in Stra8 gene expression. In embryonic ovarian germ cells of Stra8-null mutant mice, initiation of the meiotic prophase is blocked, demonstrating that Stra8 gene function is needed for meiotic entry in mouse embryonic ovaries [26]. Unlike the situation in females, analysis of testes of two Stra8-deficient lines of mice revealed less definitive results. In one Stra8-deficient mouse line, germ cells failed to enter meiosis in the prepubertal testis [27], whereas analysis of a second Stra8-deficient line showed that male germ cells can enter meiosis but fail to complete this process [28]. Therefore, whether meiotic failure in prepubertal VAD testes is a direct result of the absence of Stra8 gene expression in these atRA-deficient germ cells requires further study.
Interestingly, spermatogonia in the prepubertal VAD testes that fail to enter meiosis remain in an undifferentiated state as the pups continue to develop. In the normal P18 testes, germ cells at various stages of spermatogenesis (spermatogonia, primary and secondary spermatocytes, and round spermatids) are present, and the number of undifferentiated spermatogonia gradually declines with the progression of spermatogenesis [1]. In contrast, in the VAD testes at P18, a large number of undifferentiated spermatogonia were found, and they accumulated at the basement membrane of seminiferous tubules. Interestingly, in ovaries of VAD rat embryos, germ cells that fail to undergo meiosis remain undifferentiated [14], similar to the undifferentiated spermatogonia in prepubertal VAD testes, and CYP26B1 maintains low levels of retinoic acid in embryonic testes to block meiotic initiation of germ cells [23, 29, 30]. It appears, therefore, that an environment depleted of retinoic acid maintains germ cells in an undifferentiated state in both sexes. Thus, the VAD Lrat−/− mouse testis provides an enriched source of undifferentiated spermatogonia that may be useful for future studies concerning the regulation of spermatogonial differentiation.
In summary, the present study shows that vitamin A is needed for spermatogonia to enter meiosis I during the first wave of spermatogenesis in prepubertal mice. Spermatogonia remain in an undifferentiated state when vitamin A is absent, resulting in an accumulation of undifferentiated spermatogonia. These results support the hypothesis that vitamin A and its derivative, atRA, regulate the initiation of meiosis I during mammalian spermatogenesis.
Supplementary Material
Footnotes
Supported in part by grants EY008061 (K.P.) and EY019298 (W.B.) from the National Institutes of Health (NIH).
REFERENCES
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977; 74: 68 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auharek SA, de Franca LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat 2010; 216: 577 588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001; 121: 347 354 [DOI] [PubMed] [Google Scholar]
- Huang HFS, Hembree WC. Spermatogenic response to vitamin A in vitamin A-deficient rats. Biol Reprod 1979; 21: 891 904 [DOI] [PubMed] [Google Scholar]
- Mitranond V, Sobhon P, Tosukhowong P, Chindaduangrat W. Cytological changes in the testes of vitamin A-deficient rats. I. Quantitation of germinal cells in the seminiferous tubules. Acta Anat (Basel) 1979; 103: 159 168 [DOI] [PubMed] [Google Scholar]
- Van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod 1990; 43: 363 367 [DOI] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci 1989; 564: 154 172 [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem 2004; 279: 10422 10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise AR, Noy N, Palczewski K, Blaner WS. Delivery of retinoid-based therapies to target tissues. Biochemistry 2007; 46: 4449 4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LM, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem 2005; 280: 40226 40234 [DOI] [PubMed] [Google Scholar]
- O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 2005; 280: 35647 35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, DeLuca HF, Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr 1970; 100: 1049 1052 [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press; 1995: 11 79 [Google Scholar]
- Li H, Clagett-Dame M. Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol Reprod 2009; 81: 996 1001 [DOI] [PubMed] [Google Scholar]
- Lammers JH, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, Heyting C. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol 1994; 14: 1137 1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol 1996; 135: 469 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647 652 [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004; 36: 653 659 [DOI] [PubMed] [Google Scholar]
- McCarthy PT, Cerecedo LR. Vitamin A deficiency in the mouse. J Nutr 1952; 46: 361 376 [DOI] [PubMed] [Google Scholar]
- O'Byrne SM, Kako Y, Dekelbaum RJ, Hansen IH, Palczewski K, Goldberg IJ, Blaner WS. Multiple pathways ensure retinoid delivery to milk: studies in genetically modified mice. Am J Physiol Endocrinol Metab 2009; 298: E862 E870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596 600 [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest 2010; 120: 956 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537 545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430 1434 [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976 14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121: 3233 3242 [DOI] [PubMed] [Google Scholar]
- Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One 2009; 4: e7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007; 148: 4560 4567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.