Abstract
The bacterial Sm-like protein Hfq facilitates RNA-RNA interactions involved in post-transcriptional regulation of the stress response. Specifically, Hfq helps pair noncoding RNAs (ncRNAs) with complementary regions of target mRNAs. To probe the mechanism of this pairing, we generated a series of Hfq mutants and measured their affinity for RNAs like those with which Hfq must associate in vivo. We tested the mutants’ DsrA-dependent activation of rpoS, and their ability to stabilize DsrA ncRNA against degradation in vivo. Our results suggest that Hfq has two independent RNA-binding surfaces. In addition to a well-known site around the core of the Hfq hexamer, we observe interactions with the distal face of Hfq, a new locus with which mRNAs and poly(A) sequences associate. Our model explains how Hfq can simultaneously bind a ncRNA and its mRNA target to facilitate the strand displacement reaction required for Hfq-dependent translational regulation.
Hfq protein from Escherichia coli was first described in connection with Qβ-phage replication1,2. Hfq has recently emerged as a central player in post-transcriptional gene regulation as mediated by bacterial ncRNAs3–6. Escherichia coli Hfq mutants show disrupted signaling in stress response pathways7,8, arising from the need for Hfq to mediate base-pairing between regulatory ncRNAs and their mRNA targets. Examples of these partnerships include DsrA-rpoS7,9,10, OxyS-fhlA11,12, OxyS-rpoS13, RprA-rpoS14, RyhB-sodB15–17 and Spot42-galETKM18.
Complexes between ncRNAs and their mRNA targets function in several ways. Most commonly, complexed structures lead to translational activation or repression by remodeling mRNA regulatory regions containing the ribosome-binding site (RBS) and/or start codon. Alternatively, the interaction can enhance decay of the target mRNA16 or simply block translation11. Clearly, Hfq facilitates base-pairing between ncRNAs and their targets, but how it does so is poorly understood. How the chaperone function relates to other Hfq activities such as the control of poly(A) tail elongation19,20 and regulation of mRNA stability21,22 is also unknown.
Hfq shares sequence similarity to the eukaryotic Lsm proteins23–27. In the Conserved Domain Database28 Hfq is listed under the Sm and Sm-like protein family as well as among the eubacterial Hfqs. An alignment of the conserved Lsm and Hfq motifs shows that the Sm1 and Sm2 regions overlap with the Hfq motif (Fig. 1). Crystallographic characterization of Hfq revealed a classical Sm fold, as predicted from sequence alignment and homology modeling27,29. Whereas eukaryotic Sm proteins form heteroheptameric rings30–32, Hfq forms homo hexamers similar to the archaeal Sm proteins33,34. RNA-binding contacts are observed for both Hfq and Sm proteins in cocrystal structures with short (A+U)-rich oligonucleotides, and show that these small RNA substrates interact with their protein partners in a similar manner (Fig. 1).
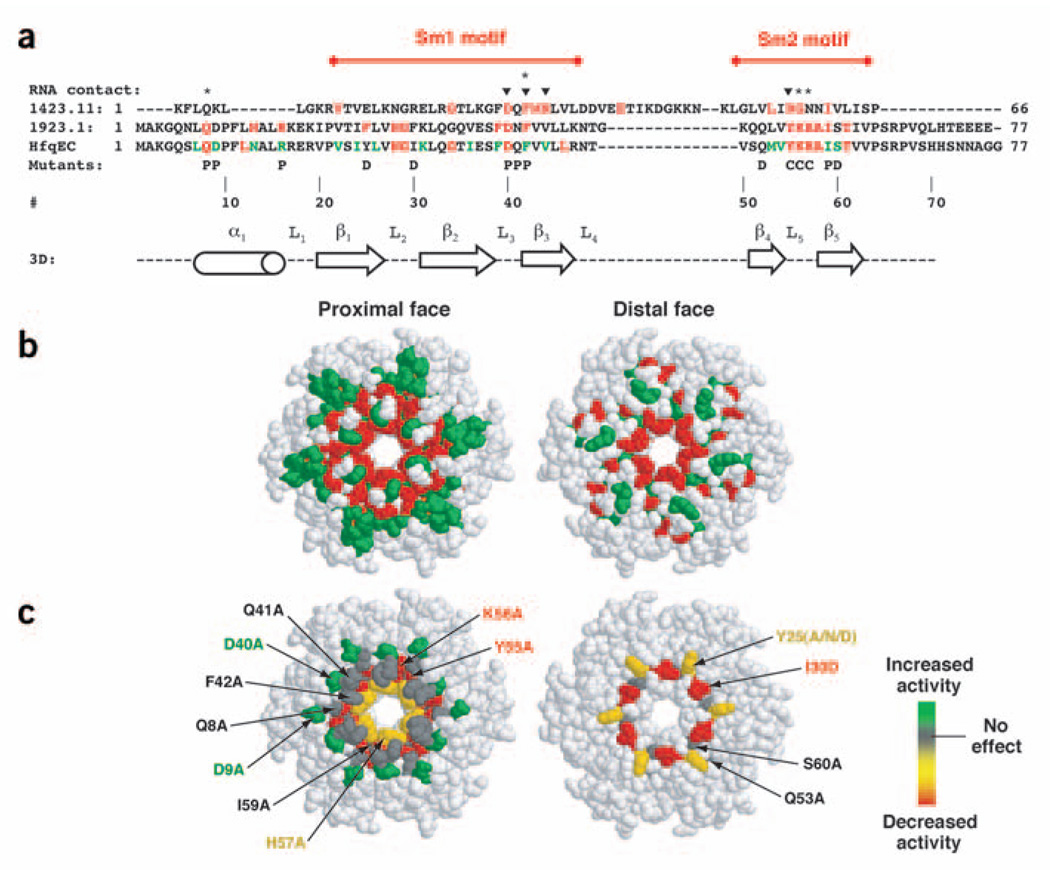
Figure 1.
Sequence alignments and structure of Hfq. (a) Sequences 1412.11 and 1923.1 are entries from the conserved domain database representing the superfamily of Lsm proteins and Hfq, respectively. The two conserved domains have been aligned to properly juxtapose the motifs present in both protein families. Sequence elements in red represent the most highly conserved portions of the domain. HfqEC is the E. coli Hfq protein, colored based on an alignment of 25 bacterial Hfq homologs showing complete identity and sequence similarity in red and green, respectively. Above the alignment, triangles mark sites where RNA is known to interact with Lsm proteins and diamonds indicate known RNA-binding interactions with Hfq. The bottom line of the alignment shows the sites mutated as part of the study; P, location on the proximal face; D, location on the distal face; C, central cavity mutation. Numbering is provided for reference and is based on the E. coli Hfq sequence. Secondary structure information derives from the crystal structure of HfqEC (PDB entry 1HK9)27. (b) Space-filling representation of the HfqEC crystal structure (PDB entry 1HK9) color-coded with the sequence conservation data from the HfqEC alignment in a. The distal face representation shows the same image rotated 180° relative to the proximal face around a vertical axis in the plane of the page. (c) Space-filling representation of the HfqEC crystal structure upon which the composite RNA binding data from Table 1 have been superimposed.
Two parallel but nonexclusive models have been proposed to explain how Hfq promotes intermolecular base-pairing. In the first model, Hfq acts explicitly as an RNA chaperone, partially unfolding one or both RNAs35,36. In the second model, Hfq binds both RNAs, increasing their local concentration to induce the interaction. Several studies have probed RNA structural changes upon Hfq binding. DsrA ncRNA showed no substantial secondary structure changes37. The rpoS mRNA 5′ untranslated region (5′ UTR) was not assayed for structural changes in this study, but more recent work indicates that the rpoS leader sequence also remains unchanged upon binding Hfq (R. Lease and S. Woodson, Johns Hopkins University, personal communication).
Several studies suggested that more than one RNA can simultaneously assemble onto Hfq. Work by two groups has recently shown that stable ternary complexes containing DsrA, rpoS mRNA and Hfq can form (P.J.M., E. Espinosa, Indiana University, and A.L.F., unpublished data; R. Lease and S. Woodson, Johns Hopkins University, personal communication). These complexes represent product-like states wherein the two RNAs are already base-paired with one another on Hfq. Similar ternary complexes have been observed in coimmunoprecipitation experiments25. It seems possible, therefore, that the single RNA-binding site observed crystallographically is not sufficient for Hfq’s ability to promote intermolecular base-pairing.
We addressed these questions through a mutational analysis of Hfq, probing in vitro binding to several model RNAs that represent species with which Hfq must interact. Hfq mutants were assayed in vivo using a reporter assay and RNA lifetime experiments. Together, the results support a model wherein at least two independent RNA-binding sites exist on the Hfq hexamer, and juxtaposition of bound RNAs facilitates base-pairing.
RESULTS
Hfq mutagenesis
To identify amino acids essential for RNA binding, we constructed a series of E. coli Hfq missense mutants (Fig. 1). Hfq Y55A, Hfq K56A and Hfq H57A contain mutations that cluster around the central cavity of the hexamer, a region that interacts with short (A+U)-rich sequences in the Hfq–RNA cocrystal structure29. Other mutants, such as Hfq D40A, Hfq Q41A and Hfq F42A lie along the proximal surface of the torus. Minimal binding site studies have shown that Hfq preferentially binds (A+U)-rich sequences adjacent to double-stranded regions37,38. Thus, these sites represent a potential contact surface for the duplex were it to lay down onto Hfq adjacent to the site at which the (A+U)-rich sequence binds. Hfq Q8A and Hfq D9A represent a series of highly conserved residues that lie on the proximal face of the torus that might contact RNAs a bit farther from the central cavity. Finally, the Hfq Y25A, Hfq Y25N, Hfq Y25D, Hfq I30D, Hfq Q53A and Hfq S60A mutations represent sites on the distal face of the Hfq hexamer. All mutants were expressed as His6-tag fusion proteins. In control experiments, the His6-tag had no substantial effect on the binding properties of Hfq in vitro (as assayed by gel shift and calorimetry) and His6-tagged Hfq fully complements an hfq− strain in vivo.
RNA-binding properties of mutant Hfqs
Previous data from our labs suggested that two or more RNA-binding surfaces might be pre sent on Hfq37. When poly(A) was used in an attempt to compete away DsrA in a gel shift assay, a supershift was observed rather than competitive binding37. Therefore, to assess the effect of these mutations on RNA binding, several different RNA substrates were tested, including DsrA, RNA-U (a 7-nucleotide (nt) RNA with the sequence AU5G), rpoS mRNA 5′ UTR and A27 (a 27-nt synthetic poly(A) oligomer). DsrA and RNA-U represent the ncRNAs, all of which contain (A+U)-rich sequences as part of their minimal binding element. This comparison is reinforced by the fact that short poly(U) RNAs have been shown to supplant DsrA in competitive binding experiments37. rpoS mRNA 5′ UTR and A27 represent the target mRNAs regulated by the ncRNA, corresponding to the 5′ and 3′ termini of the mRNA, respectively.
RNA binding was probed using gel mobility shift assays and isothermal titration calorimetry. In the case of DsrA, the RNA shifts, and then supershifts, as it binds first one then a second equivalent of Hfq hexamer (Fig. 2a). The rpoS mRNA 5′ UTR construct that we used also probably binds at least two equivalents of the Hfq hexamer. Because the initial species never accumulates significantly we have treated the rpoS mRNA-binding data as a single transition providing an apparent Kd (Fig. 2b).
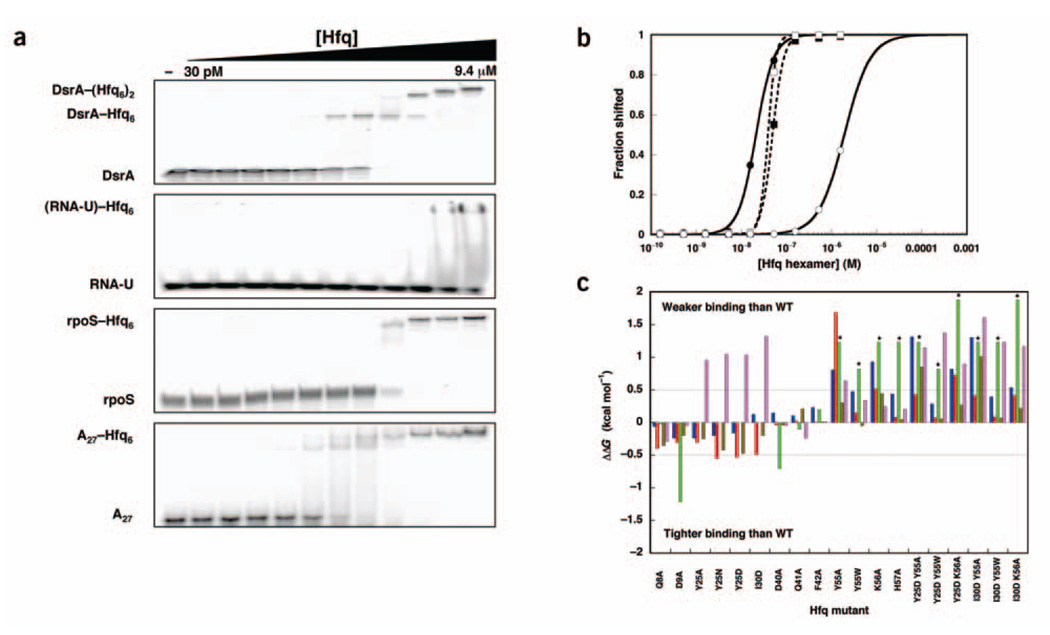
Figure 2.
In vitro analysis of RNA binding to mutant Hfq proteins. (a) Gel shift experiments showing the binding of wild-type Hfq to DsrA, RNA-U, rpoS mRNA 5′ UTR and A27. (b) Quantification of the gel shift experiments in a. (b) Quantification of the gel shift experiments in a. Closed circles, DsrA K1; open circles, RNA-U; closed squares, rpoS mRNA 5′ UTR; open squares, A27. Lines represent nonlinear least-squares fitting to a cooperative binding model. (c) Histogram showing the free energy of binding relative to wild-type (WT) Hfq for each Hfq mutant. ΔΔGs between −0.5 and 0.5 kcal mol−1 indicate insignificant effects on RNA binding. Asterisks indicate data that are lower limits of the actual effect as the interactions were too weak to be measured. Blue, DsrA–Hfq6; red, DsrA–(Hfq6)2; green, RNA-U; brown, rpoS mRNA 5′ UTR; lavender, A27.
We consolidated the data from the 4 RNA substrates for each mutant Hfq, represented as the ΔΔG relative to binding wild-type Hfq (Fig. 2c). DsrA binds to wild-type Hfq with an affinity of 21 ± 1 nM (reported as hexamer, 126 nM monomer). Under the same conditions, the second binding event occurs with a Kd of 94 nM (reported as hexamer, 564 nM monomer). This value is within two-fold of those previously determined37. DsrA affinity is affected by residual RNA often found to copurify with Hfq. The RNase A treatment in the current purification significantly reduced residual RNA and is probably the origin of the tighter affinities measured here.
Most of the mutations had relatively small effects on DsrA-binding affinity (Table 1), the largest being on the order of 20-fold decreased affinity, corresponding to a ΔΔG of ~1.8 kcal mol−1. The modest effects of the point mutants could have resulted from polyvalent interaction between the larger RNAs and Hfq masking defects in one of the binding domains37. If this were true, the smaller RNAs (RNA-U and A27) would provide a more sensitive gauge of whether the residue participates in the RNA-protein interaction.
Table 1.
Comparison of in vitro affinity data with in vivo activities for Hfq mutants
| Kd (nM hexamer) | |||||||
|---|---|---|---|---|---|---|---|
| Mutant | DsrAa | RNA-U |
RpoS mRNA 5′ UTR |
A27 | Hfq accum. |
rpoS::lacZ assay |
DsrA t1/2 (min) |
| Wild type | 21 ± 1 | 2,500 ± 200 | 49 ± 1 | 39 ± 1 | Yes | 5.7 ± 0.7 | 55 ± 3b |
| 94 ± 5 | |||||||
| Q8A | 19 ± 1 | 2,400 ± 100 | 27 ± 1 | 24 ± 1 | Yes | 3.2 ± 0.2 | – |
| 48 ± 2 | |||||||
| D9A | 14 ± 1 | 320 ± 40 | 35 ± 2 | 36 ± 1 | Yes | 7 ± 1 | – |
| 56 ± 1 | |||||||
| Y25A | 19 ± 1 | – | 32 ± 1 | 196 ± 3 | Yes | – | – |
| 35 ± 1 | |||||||
| Y25N | 15 ± 1 | – | 24 ± 1 | 229 ± 2 | Yes | 4.5 ± 0.6 | – |
| 37 ± 2 | |||||||
| Y25D | 16 ± 1 | – | 22 ± 1 | 224 ± 2 | Yes | 4.0 ± 0.9 | – |
| 38 ± 1 | |||||||
| I30D | 26 ± 1 | – | 35 ± 1 | 363 ± 3 | Yes | 5.2 ± 0.9 | – |
| 41 ± 2 | |||||||
| D40A | 27 ± 1 | 760 ± 90 | 46 ± 1 | 36 ± 1 | No | 0.8 ± 0.1 | – |
| 88 ± 2 | |||||||
| Q41A | 25 ± 1 | 2,100 ± 100 | 70 ± 2 | 26 ± 1 | Yes | 6.4 ± 0.9 | – |
| 98 ± 4 | |||||||
| F42A | 31 ± 1 | 3,500 ± 300 | 50 ± 1 | 40 ± 1 | Impaired | 2.5 ± 0.2 | – |
| 95 ± 4 | |||||||
| Q53A | – | – | – | – | Yes | 3.0 ± 0.6 | – |
| – | |||||||
| Y55A | 82 ± 4 | >20,000 | 82 ± 3 | 115 ± 2 | Yes | 0.8 ± 0.1 | 29 ± 5 |
| 1,600 ± 100 | |||||||
| Y55W | 47 ± 1 | >10,000 | 45 ± 1 | 69 ± 1 | Yes | 4.5 ± 0.8 | – |
| 121 ± 8 | |||||||
| K56A | 101 ± 2 | >20,000 | 104 ± 3 | 59 ± 1 | Yes | 1.0 ± 0.2 | 0.9 ± 0.1c |
| 224 ± 9 | |||||||
| H57A | 44 ± 1 | >20,000 | 53 ± 1 | 55 ± 1 | Yes | 5.4 ± 0.5 | – |
| 108 ± 3 | |||||||
| I59A | – | – | – | – | Yes | 4.2 ± 0.5 | – |
| – | |||||||
| S60A | – | – | – | – | Yes | 5.0 ± 0.3 | – |
| – | |||||||
| Y25D Y55A | 194 ± 9d | >20,000 | 206 ± 6 | 270 ± 12 | – | – | – |
| Y25D Y55W | 34 ± 1 | >10,000 | 54 ± 1 | 400 ± 4 | – | – | – |
| 106 ± 7 | |||||||
| Y25D K56A | 84 ± 3 | >60,000 | 77 ± 2 | 177 ± 3 | – | – | – |
| 320 ± 24 | |||||||
| I30D Y55A | 190 ± 34d | >20,000 | 270 ± 22 | 590 ± 11 | – | – | – |
| I30D Y55W | 41 ± 12 | >20,000 | 55 ± 1 | 312 ± 9 | – | – | – |
| 110 ± 13 | |||||||
| I30D K56A | 52 ± 1 | >60,000 | 71 ± 2 | 280 ± 10 | – | – | – |
| 190 ± 19 | |||||||
DsrA exhibits two sequential transitions—a shift and a supershift. The first value corresponds to K1 and the second value to the supershift, K2. Close inspection of the rpoS mRNA 5′-UTR shifts reveals two apparent shifted species similar to those seen with DsrA. However, the two transitions are sufficiently coupled that they cannot be fit separately.
Hfq− strains have a DsrA t1/2 of 5 ± 2 min.
These data show a biphasic time dependence. A total of 90% of the DsrA RNA decays with a t1/2 of 0.9 min. The final 10% of the RNA shows a much longer t1/2, −35 min.
No clear distinction between K1 and K2 behavior was possible owing to abnormal smearing of the bands. Data were fit to a single transition.
Under the same gel conditions, RNA-U exhibits poor affinity for the wild-type Hfq hexamer (2.5 ± 0.2 µM hexamer, 15 µM monomer). This result is consistent with minimal binding studies that have shown Hfq preferentially binds (A+U)-rich sequences adjacent to base-paired elements37. Despite the weak interactions, binding is perturbed by specific mutations. Hfq D9A showed eight-fold tighter binding to RNA-U. Mutant proteins Hfq Y55A, Hfq Y55W, Hfq K56A and Hfq H57A were sufficiently defective that no binding was observed, even at the highest protein concentrations.
Because RNA-U bound so weakly, we compared its affinity to those of a pair of RNAs that should not specifically bind Hfq—the substrate and ribozyme strands of hammerhead ribozyme 16 (ref. 39). These RNAs showed no gel shift behavior up to 30 µM Hfq monomer. The interaction of RNA-U with the mutant Hfqs clearly approaches that of pure nonspecific binding under these conditions. At the same time, the deleterious effects of mutations within the Sm2 core motif suggest that RNA-U binding occurs at the crystallographically observed site.
With respect to rpoS mRNA 5′ UTR binding, the collection of mutations we prepared showed few effects. The largest defect observed across the series, with Hfq K56A, was only two-fold. This result indicates that perhaps not all RNAs bind via the central core and the Sm2 motif. Distal face mutations also did not significantly affect rpoS mRNA 5′-UTR binding. Two double mutants (Hfq Y25D Y55A and Hfq I30D Y55A) that combine defects in the Sm2 motif with those on the distal face show reduced affinity for rpoS mRNA 5′ UTR, however. These data suggest that rpoS mRNA 5′ UTR may interact with both faces of Hfq.
Very different results were observed in the case of A27 binding. Gel shift experiments measured an affinity of 39 ± 1 nM for wild-type Hfq hexamer (234 nM in monomer). Of the proximal face mutants, the most impaired was Hfq Y55A, but this protein had only a three-fold effect on binding. These results suggest that the Sm2 region at the center of the torus does not interact in any significant way with A27 and is consistent with the additive binding behavior previously observed between DsrA domain II and poly(A)37. The distal face mutants showed different behavior, however. Mutations at Tyr25 and Ile30 affected A27 binding, leading to a ten-fold loss in affinity for Hfq Y25D and Hfq I30D. Double mutants showed no additional changes in affinity for A27 when Sm2 motif changes were combined with distal face mutations. We therefore infer that poly(A) interacts with the distal face of Hfq.
Competition experiments reveal two independent binding sites
Previous experiments with wild-type Hfq showed what seemed to be mutual binding of A27 and DsrA to Hfq37. In light of the results described above, we carried out a series of competition experiments using gel shift assays to look at the effect of A27 addition to binary complexes containing either DsrA or rpoS mRNA 5′ UTR prebound to Hfq (Fig. 3 and Table 2). We observed markedly different behavior for wild-type Hfq, Hfq Y25D and Hfq K56A. Concomitant binding of A27 and either DsrA or rpoS mRNA 5′ UTR was observed at concentrations of wild-type Hfq sufficient to promote the formation of the DsrA–(Hfq6)2 species, consistent with our previous findings37. When the distal face mutant Hfq Y25D was used, additive binding was completely abolished, indicating that A27 binding was independent of DsrA and rpoS mRNA 5′ UTR (data not shown). When the Sm2 mutant Hfq K56A was used, a third outcome resulted. The addition of A27 displaced DsrA; it similarly displaced rpoS mRNA 5′ UTR, albeit less efficiently. The band resulting from DsrA displacement by A27 migrated more slowly in the native gel than unbound DsrA, with mobility similar to that of DsrA dimer (data not shown).
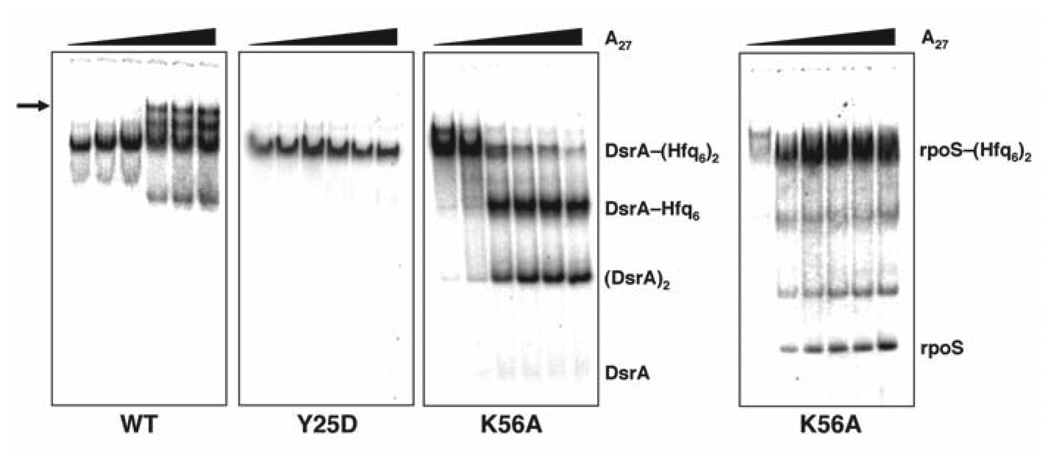
Figure 3.
Native gel analysis showing the effect of A27 addition to the DsrA–(Hfq6)2 complex. DsrA–(Hfq6)2 was preassembled at appropriate concentrations of Hfq (based on the measured affinity constants in Table 2) using 32P-labeled DsrA before the addition of unlabeled A27 (0, 0.03, 0.1, 0.3, 1 or 3 µM). Gels compare the behavior of wild-type (WT) Hfq to Hfq Y25D and Hfq K56A as labeled. A supershifted species is observed (arrow) only in the case of WT Hfq. For Hfq Y25D, the addition of A27 had no effect on DsrA binding. For Hfq K56A, A27 displaced DsrA in the form of the homodimer, DsrA2, based on its gel migration against authentic standards.
Table 2.
Summary of A27 competition experiments
| Complex | Mutant | [Hfq] (nM hexamer) |
Outcome upon A27 competition |
|---|---|---|---|
| DsrA–Hfq6 | |||
| Wild type | 42 | No effect | |
| Y25D | 25 | No effect | |
| K56A | 125 | Abnormal competition | |
| DsrA–(Hfq6)2 | |||
| Wild type | 125 | Supershift | |
| Y25D | 83 | No effect | |
| K56A | 250 | Abnormal competition | |
| rpoS–(Hfq6)2 | |||
| Wild type | 83 | Supershift | |
| Y25D | 50 | No effect | |
| K56A | 125 | No effect |
The competition experiments lead to two conclusions. First, Hfq K56A binds DsrA improperly, allowing for competition by A27. Second, this abnormal binding mode possibly leads to altered folding of the ncRNA and assembly of DsrA dimers—a trait that could lead to adverse consequences in vivo.
Isothermal titration calorimetry
An alternative way to measure Hfq-RNA interactions is with isothermal titration calorimetry (ITC)40–42. This method offers several advantages over the gel shift experiments. Reaction stoichiometry is more readily determined from the data. Furthermore, detailed thermodynamic information is obtained more accurately than from electrophoretic methods, because the enthalpic contribution of the free energy is measured directly.
A typical ITC experiment is shown in Supplementary Figure 1 online. Consistent with the gel shift experiments, DsrA, rpoS mRNA 5′ UTR and poly(A) showed tight binding to Hfq hexamer with Kd values in the low nanomolar range. Because of the weak interaction between Hfq and RNA-U (~2.5 µM from gel shift analysis), we could not achieve the necessary sample concentrations to study its binding accurately using this method. Hfq Y55A showed a 50-fold reduction in binding affinity whereas the Hfq K56A showed a 30-fold reduction in binding affinity for DsrA, whereas Hfq H57A had nearly wild-type affinity. Both results are consistent with the gel shift experiments (Supplementary Table 1 online).
The apparent stoichiometries from the ITC experiments are consistent with Hfq acting as a preformed hexamer and binding a single equivalent of DsrA or rpoS mRNA 5′ UTR. Thus, the ITC data imply that the binding of a second equivalent of Hfq to an RNA (K2) may result from working under trace RNA conditions, as typically done in gel shift assays. An unexpected result was observed in poly(A) experiments, however. Fitting of the ITC data suggests that A18 (a shorter, 18-nt poly(A) RNA was used in these experiments to diminish the likelihood that multiple Hfqs might bind to the longer RNA43) binds two identical sites per Hfq hexamer.
Binding enthalpies (ΔH°) of −40 to −80 kcal mol−1 were measured for the same three substrates with corresponding binding entropies (ΔS°) of −100 to −250 cal mol−1 K−1 (Supplementary Table 1 online). These values show that the enthalpy of the interaction is quite favorable, but largely offset by highly unfavorable entropy. As these parameters combine the energetic contribution of the binding phenomenon with any potential RNA rearrangements, a detailed interpretation requires further study.
Accumulation and complementation analysis of Hfq mutants
Within a living cell, the interaction of Hfq with mRNAs and ncRNAs is much more complex than that probed with our in vitro model system. To determine whether the Hfq mutants function in vivo we used an rpoS::lacZ reporter system in an hfq− strain of E. coli (Fig. 4)7. Expression of this reporter requires both transcription and translation of rpoS7. The assay tests for two phenomena. First, plasmid-borne mutant Hfqs must accumulate, and second, they must facilitate proper post-transcriptional regulation of rpoS. Because DsrA is the predominant ncRNA involved in the translational regulation of rpoS at low temperatures, all assays were conducted at 30 °C (ref. 44). To prevent overexpression of Hfq, the mutants were expressed under control of the inducible araBAD promoter45. In agreement with previous work7, in the absence of arabinose or with vector alone, very little Hfq accumulated (Fig. 4b) and RpoS::LacZ expression was low (Fig. 4c). Addition of 150 µ arabinose induced wild-type expression levels of Hfq and normal translation of RpoS::LacZ (Fig. 4c).
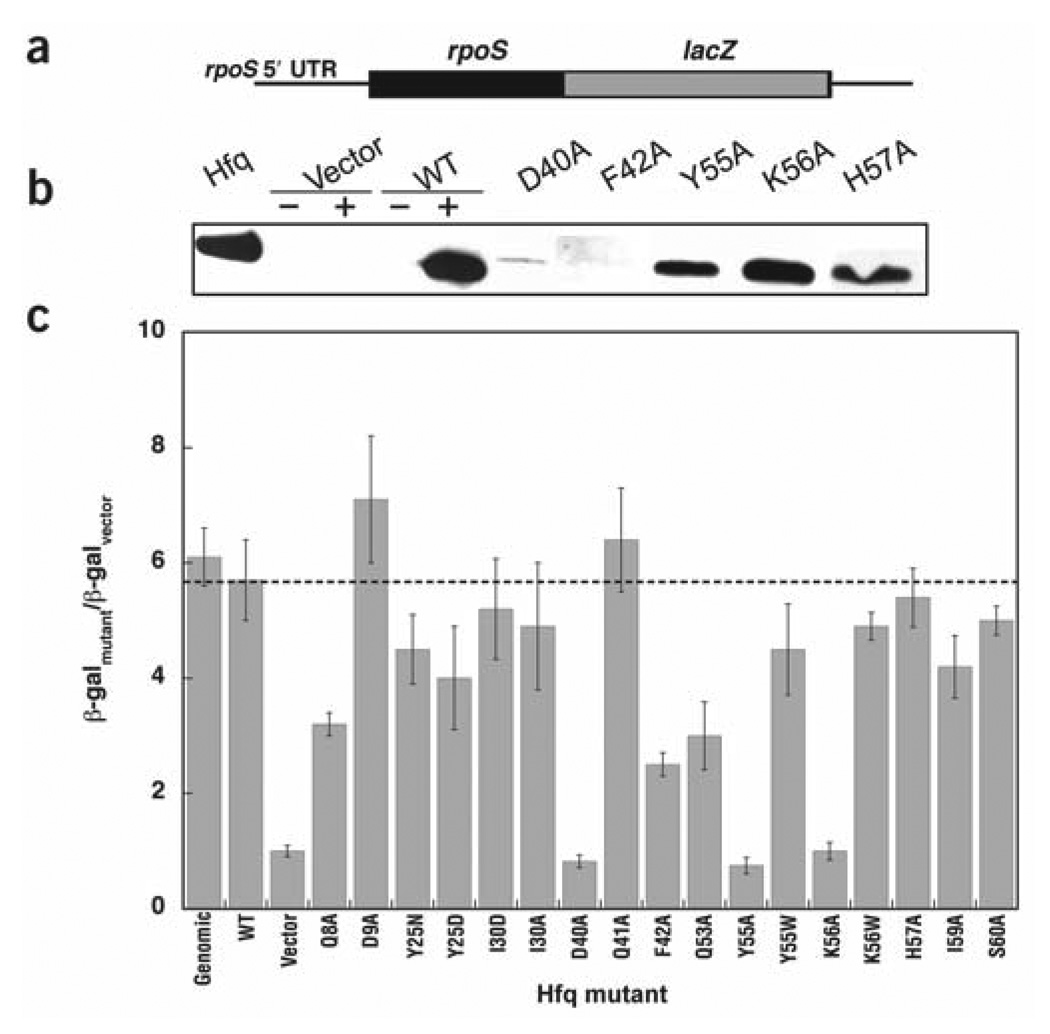
Figure 4.
Assay for in vivo accumulation and rpoS activation. (a) Schematic diagram of the reporter construct used for the Hfq complementation assay. (b) Western blot showing the accumulation of Hfq in vivo. In the control lanes, + and − refer to the presence or absence of 150 µM arabinose, added to induce Hfq from an araBAD promoter. Vector represents the pBAD plasmid45 without the hfq gene. The Hfq lane shows the endogeneous Hfq levels in an hfq+ strain of E. coli. (c) Histogram showing the results of in vivo translational activation of the RpoS::LacZ fusion protein for wild-type (WT) and mutant Hfq proteins. Values <4 are considered significant defects based on the error in this assay. β-gal, β-galactosidase activity.
As misfolded proteins tend to be degraded and cleared from the cell, western blotting was used to ensure that mutant Hfqs accumulated normally at the same arabinose concentration. Most of the mutants provided normal expression under these conditions, as can be seen for representative mutants like Hfq Y55A and Hfq K56A. Hfq D40A and Hfq F42A (Fig. 4b, lanes 6 and 7) showed abnormal accumulation, however. Circular dichroism (CD) and dynamic light scattering verified that these proteins fold and hexamerize in vitro, but they may be sufficiently destabilized to pose a problem in vivo. Because there was a significant decrease in the Hfq concentration in these cells, any loss of activity in the reporter complementation assay (Fig. 4c) could result from either defective RNA binding or decreased protein accumulation.
As expected from the in vitro experiments, Hfqs containing mutations in the Sm2 motif, including Hfq Y55A and Hfq K56A, did not complement the hfq− phenotype. As these proteins were present at wild-type levels based on western blot analysis, these results suggest that loss of in vivo activity for these mutants was not due to decreased accumulation. Notably, Hfq H57A and Hfq Y55W, mutants that do not bind RNA-U but retain wild-type DsrA and rpoS mRNA 5′ UTR affinity in vitro, retained their in vivo activity. This finding suggests that the small changes in DsrA binding observed in vitro for Hfq Y55A and Hfq K56A are more diagnostic of the in vivo behavior than RNA-U binding. Other mutations within the phylogenetically conserved Sm2 region showed no defects in our assay.
Assessment of DsrA stability in vivo
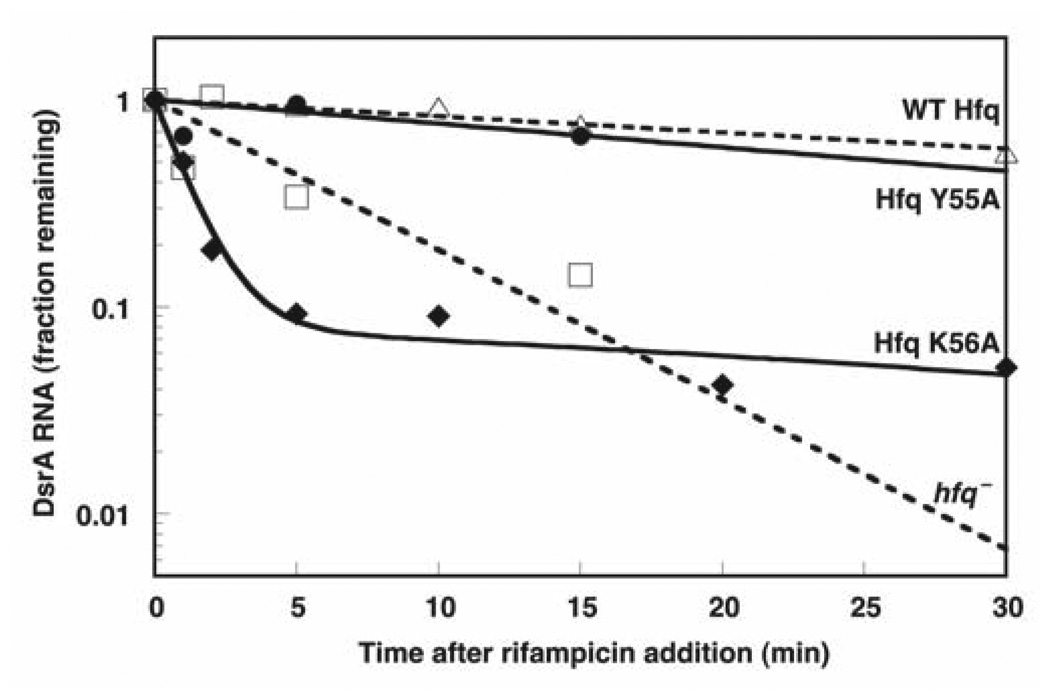
RNA lifetime analysis has been used as another measure of Hfq activity in vivo7,16,38. In the presence of Hfq, DsrA has been shown to degrade significantly more slowly than when Hfq is absent7. We measured DsrA lifetimes for both Hfq mutants that had significantly reduced activity in complementation assays that could not be explained by simple accumulation defects. Hfq Y55A and Hfq K56A show markedly different behavior after inhibition of transcription with rifampicin (Fig. 5). Whereas Hfq Y55A led to long-lived RNA similar to wild-type Hfq, in the presence of Hfq K56A, DsrA degraded rapidly. The behavior occurred with biphasic kinetics. DsrA degraded in the fast phase, accounting for ~90% of the total, was less stable than in the absence of Hfq entirely. The remaining 10% of the RNA was reasonably long-lived.
Figure 5.
Lifetime analysis of DsrA in vivo. Data are based on QRT-PCR for wild-type (WT) Hfq and selected mutants using primers specific for full-length DsrA. Solid lines (Hfq mutants) and dashed lines (Hfq and hfq−) are least-squares fits to the data from which the half-lives were calculated. Hfq K56A has been fit to a double-exponential decay whereas the other data are fit to a single-exponential model.
DISCUSSION
Site-directed mutagenesis was used to probe the interaction between Hfq and four RNAs that represent some of the ncRNAs and mRNAs with which Hfq interacts in vivo. The effects of these mutations were assayed both in vitro and in vivo. Sites of mutation were chosen based on structural and phylogenetic information. Structurally, we can group the mutants into three categories: those affecting the central cavity (Y55A, K56A and H57A), the proximal face (Q8A, D9A, D40A, Q41A, F42A and I59A) or the distal face (Y25X, I30X, Q53A and S60A). Notably, mutations at these loci had distinct effects on behavior, both in vitro and in vivo.
Two central cavity mutations severely impair function
Previous studies had implicated the central cavity as essential for Hfq and Sm protein function. Only two cavity mutants, Hfq Y55A and Hfq K56A, showed consistent in vitro defects in binding to DsrA. Both mutations target highly conserved residues within the Sm2 motif. Mutations at other conserved Sm2 residues (His57, Ile59 and Ser60) did not significantly impair in vitro or in vivo behavior.
The minimal substrate RNA-U showed much greater sensitivity to Sm2 region mutations, whereas rpoS mRNA 5′ UTR and A27 were unaffected. The known RNA-binding cavity along the inner rim of the Hfq hexamer thus seems only to interact with the (A+U)-rich elements of the ncRNAs, and does not represent the primary binding surface for poly(A) sequences or mRNAs. Additional RNA-binding surfaces must be present on Hfq, supporting the idea that Hfq functions by colocalizing ncRNAs and their mRNA targets. Hfq probably facilitates the strand exchange reaction largely by putting the RNAs in close proximity, and possibly by presenting appropriate interaction surfaces toward one another.
The DsrA half-life experiments provide an additional window into the complexities of the Hfq system. Previous work has shown that in the absence of Hfq DsrA stability is markedly decreased in vivo7. This effect is thought to be due to the overlap of the Hfq-binding and RNase E cleavage sites on DsrA. Although both Hfq Y55A and Hfq K56A are inactive for DsrA-mediated regulation of rpoS, their abilities to stabilize DsrA in vivo are diametrically opposed. Hfq Y55A behaves like wild-type Hfq whereas Hfq K56A provides DsrA almost no protection from RNase E. The notable ability of Hfq Y55A to stabilize DsrA suggests that, although DsrA binds to Hfq Y55A in vivo, it cannot correctly interact with rpoS mRNA. This difference between Hfq Y55A and Hfq K56A could relate to the stability of a ternary complex involving Hfq, DsrA and rpoS mRNA (P.J.M., E. Espinosa, Indiana University, and A.L.F., unpublished data).
Proximal face mutations show few specific effects
Most of the proximal face mutations showed little effect on in vitro and in vivo activity. Where disagreement was observed (Hfq D40A and Hfq F42A), the data are rectified by protein accumulation defects in vivo. Although it is unclear why these two mutants fail to accumulate, the result clearly explains their inability to complement the hfq− strain.
Distal face mutations alter binding only to poly(A)
Distal face mutants were generated to test the hypothesis that RNA- or protein-binding interactions might occur on that face5,37. Hfq Y25D and Hfq I30D bound A27 with five- to ten-fold reduced affinity, but did not show altered binding to any of the other three RNA substrates.
The distal face mutants complemented the rpoS activation function of wild-type Hfq in vivo, and effectively bound DsrA and rpoS mRNA 5′ UTR in vitro. Hfq is known to be involved in modulating polyadenylation, however. Because all mutations that altered poly(A) binding localized to the distal face, one might infer that polyadenylation control uses this surface of the protein. Further studies are required to find whether these mutations differentially affect polyadenylation.
Archaeal Sm proteins are believed to aggregate into long rodlike structures46. Even in the absence of extended rods, a dodecameric structure could have explained well how Hfq hexamers bring together RNAs in a pairwise fashion. Such a model requires the distal face to be involved in an Hfq-Hfq contact. The addition of six aspartates in lieu of hydrophobic residues at this potential interaction surface should have been quite destabilizing were such face-to-face dodecamerization important. Our results do not absolutely preclude the formation of such species, but they do argue against it.
rpoS mRNA 5′-UTR binding remains mysterious
An unresolved issue from this work is where rpoS mRNA binds Hfq. The work of Lease and Woodson indicates that several U-rich sequences within the rpoS mRNA 5′ UTR become protected from nuclease digestion upon Hfq binding (R. Lease and S. Woodson, Johns Hopkins University, personal communication). Such results imply an interaction with the central cavity, but our data suggest that, if the mRNA binds the central cavity, additional contacts must mask effects of the Y55A and K56A mutations. In addition to the 5′ UTR used in these studies, rpoS mRNA in vivo would contain the coding region and the 3′ UTR. Does the mRNA naturally contact both faces of Hfq? If so, additional binding determinants might be present along the exterior edge of the torus. Our current study has not probed those regions. Furthermore, the C-terminal extension of Hfq could contribute to RNA binding in ways we have not yet recognized.
Implications for understanding Sm and Lsm proteins
How do these data reflect on our understanding of the eukaryotic Sm and Lsm proteins, with which Hfq shares a common ancestor? In the Pyrococcus abyssi Sm1 core complex, RNA was shown to bind facially, near the end of strand β2 (ref. 47), and binding was significantly impaired in a Y34V mutant. This binding surface corresponds to the L3 region in the Sm1 motif of Hfq, adjacent to Asp40, Gln41 and Phe42. Although the mutations at these sites do not exhibit the marked RNA-binding defect seen in the P. abyssi Sm1 system, the accumulation defect of Hfq D40A implies that this region along the proximal face of the torus is still critical for function of the resulting RNP complex. The eukaryotic SmG protein uses this L3 region to contact RNAs in spliceosomal small nuclear ribonucleoprotein (snRNP) cores48,49. Our data are consistent with a second site of contact between the RNAs and Hfq, but the exact location of the L3 contacts may have evolved after the homohexameric Hfq complex diverged to form the heteromeric aggregates observed in modern spliceosomes. Current models of U1 snRNPs also imply significant contact between the duplex regions of the RNA and the outer rim of the torus. Further exploration will probe these surfaces.
In summary, these experiments reveal that Hfq contains at least two distinct RNA-binding surfaces. Mutations that alter ncRNA binding affinity cluster around the Sm2 domain, but do not significantly perturb affinity for rpoS mRNA 5′ UTR. Additionally, distal face mutations are the only ones tested that affect A27 binding. Poly(A) RNA binding therefore uses contacts on the back face of the torus. Such an organization could spatially and functionally separate Hfq’s effects on polyadenylation from those mediating base-pairing between ncRNAs and their mRNA targets.
METHODS
Plasmid construction for rpoS mRNA 5′ UTR
The 5′ UTR from E. coli rpoS (nucleotides −134 to +3) was obtained by PCR using the primers rpoSA1 and rpoSA2 (Supplementary Table 2 online) using Pfu Turbo (Stratagene). The PCR product was ligated into pUC19 using BamH1 and EcoRI. The resulting DNA was transformed into XL-10 Gold cells (Stratagene). Plasmid pJEF-10301 was isolated by miniprep (Qiagen) and sequenced. Large-scale isolation of plasmid DNA was done using the Qiagen Gigaprep protocol, and the vector was prepared for runoff transcription by exhaustive digestion with SspI.
RNA preparation for in vitro binding
RNA-U, A18 and A27 were purchased from Dharmacon Research and deprotected following the manufacturer’s protocol. RNA quality was assessed by denaturing PAGE and gel-purified as necessary. Other RNAs (DsrA and rpoS mRNA 5′ UTR) were transcribed in vitro and gel-purified as described37.
Site-directed mutagenesis
Using the QuikChange procedure (Stratagene), all mutants were prepared in two separate backgrounds. The pET-21b background has a C-terminal His6-tag and was used for in vitro analysis. The pBAD24 background is under an arabinose promoter system and was used for in vivo analysis. All mutations were verified by sequencing.
Hfq expression and purification
Wild-type Hfq is heat-stable and the standard purification protocol exploits this property25,50. As several of the mutant proteins were found to be heat-sensitive, all mutants (as well as wild-type Hfq) were purified by Co2+-affinity chromatography, using a C-terminal His6-tag. Expression was induced by addition of 1 mM IPTG to cultures grown to A600 = 0.4–0.6. Induction proceeded for 4 h at 37 °C before harvesting. Cell pellets (0.5-l equivalents) were resuspended in 25 ml lysis buffer (50 mM HEPES, pH 7.5, 500 mM NH4Cl, 20 mM imidazole, 5% (w/v) glycerol) with EDTA-free Complete protease inhibitor cocktail (Stratagene) and lysed by ultrasonication. Lysate was treated with DNase I (100 U) and RNAse A (100 µg) and incubated on ice for 1 h. Centrifugally clarified lysate was passed over a Hi-trap metal chelation column (Amersham-Pharmacia) preloaded with CoSO4. The column was washed with five volumes of lysis buffer, then five volumes of wash buffer 1 (50 mM HEPES, pH 7.5,1 M NH4Cl, 5% (w/v) glycerol). Hfq was eluted with five volumes of elution buffer 1 (50 mM HEPES, pH 7.5, 500 mM NH4Cl, 250 mM imidazole, 5% (w/v) glycerol) followed by five volumes of elution buffer 2 (50 mM HEPES, pH 7.5, 8 M urea, 1 M NH4Cl, 50 mM EDTA, 5% (w/v) glycerol). Protein was concentrated to 0.5–1.0 mg ml−1 and dialyzed against storage buffer (50 mM HEPES, pH 7.5, 250 mM NH4Cl, 1 mM EDTA, 10% (w/v) glycerol). Concentrations of protein and any residual RNA were assessed by the Warburg-Christian method51. Purity was assessed by SDS-PAGE. Mutants were checked by CD spectroscopy using a Jasco-J715 spectrometer and by dynamic light scattering using a Malvern Nano-S Zetasizer to ensure proper folding and oligomerization relative to wild-type Hfq.
Electrophoretic mobility shift assays
5′ end–labeled RNAs were annealed at 90 °C for 120 s, cooled to 37 °C, and incubated with Hfq for 30 min in 50 mM HEPES, pH 7.5, 250 mM NH4Cl. Immediately before loading, samples were diluted with an equal volume of native loading buffer (10% (w/v) sucrose, xylene cyanol, bromophenol blue) or denaturing loading buffer (7 M urea, 1× TBE, xylene cyanol, bromophenol blue). Samples (15 µl) were resolved at 5 W on either 5% (w/v) polyacrylamide native gels or 5% (w/v) polyacrylamide/7 M urea gels. Dried gels were visualized by phosphorimaging (Molecular Dynamics) using a Typhoon 9210 imaging system (Amersham-Pharmacia). Quantification was done using ImageQuant 5.2 (Molecular Dynamics) and Kaleidagraph 3.0 (Synergy). Data were fit using nonlinear least-squares analysis to a cooperative binding model. Cooperativity values (n) tended to fall between 2 and 3. Higher n-values yielded comparable goodness-of-fit parameters. In the case of the A27 competition assays, complexes of DsrA or rpoS mRNA 5′ UTR with Hfq were preassembled.
Isothermal titration calorimetry
Calorimetry was done on a MicroCal VP-ITC. Samples were dialyzed into reaction buffer (50 mM HEPES, pH 7.5, 250 mM NH4Cl, 10% (w/v) glycerol) and degassed before loading each experiment. RNAs (21–25 µM) were titrated into 1.4 ml of 0.6–3.5 µM Hfq hexamer over 35 8-µl injections with constant stirring at 310 r.p.m., 4-min injection spacings, and thermostatting at 25 °C. Data were corrected by subtraction of a baseline defined by the terminal 10–15 injection points after saturation of the binding event. Data were analyzed using Origin 7.0 (MicroCal) as described52.
Western blot analysis
Total cellular extracts of E. coli were separated on tris-tricine SDS 16% (w/v) polyacrylamide gels53 and electroblotted54 to PVDF membrane. Equal loading across lanes was verified by staining with Ponceau S (Sigma). The membrane was probed with rabbit anti-Hfq polyclonal anti-sera7. Antibody–antigen complex was visualized with goat anti-rabbit immunoglobin horseradish-peroxidase-conjugated antibody (Pierce) and ECL reagent kit (Pierce).
Purification of RNA for half-life studies
DsrA half-life was determined as described37,55. Cultures of wild-type and hfq− strains were grown at 30 °C to A600 = 0.4–0.6. Rifampicin (100 µg µl−1 final concentration) was added and cells were collected at the indicated time points. These samples were added immediately to prechilled tubes containing 0.1 volume phenol stop solution (5% (v/v) buffered phenol, pH 7.4, in ethanol)56. Cells were pelleted, resuspended in 200 µl TE (10 mM Tris, pH 8.0, 1 mM EDTA), and passed over QIAshredder mini columns (Qiagen). RNA was extracted with Trizol reagent (Invitrogen), precipitated with isopropoanol, and washed with 75% (v/v) ethanol. RNA was incubated with 30 U of RQ1 DNase (Promega) at 37 °C for 20 min followed by DNase inactivation at 60 °C for 10 min. Trizol extraction and isopropanol precipitation were repeated before resuspension of RNA in RNase-free water. Concentrations were determined by measuring absorbance at 260 nm.
Quantitative RT-PCR (QRT-PCR)
Relative RNA concentrations were determined by quantitative RT-PCR using a Roche LightCycler TaqMan PCR system (Applied Biosystems). Primer and probe design was based on the E. coli dsrA and 5S RNAs using Primer Express 1.5 (ABI). The dsrA primers and probes were designed to detect only full length DsrA. Primers and probes were chemically synthesized (IDT). Probes were 5′ end–labeled with 6-carboxyfluorescein and 3′ end–labeled with ‘black hole quencher’ BHQ1 (Biosearch Technologies). Each 10 µl RT-PCR mixture contained 50 ng RNA, LightCycler-RT-PCR reaction mix hybridization probe (Roche), LightCycler RT-PCR enzyme mix (omitted in RT-negative reactions), 300 nM each of the forward and reverse primers, and 250 nM Taqman probe. Both RT-positive and RT-negative reactions were tested in duplicate. Reactions were performed using a Roche LightCycler with optimized cycle conditions. DsrA RNA abundance relative to 5S ribosomal RNA was determined using the −ΔΔCt method as described by the manufacturer (ABI). 5S RNA was used as the internal control.
In vivo RpoS::LacZ fusion activity
RpoS::LacZ fusion activity was determined as described7. Briefly, cells were grown at 30 °C in LB medium with the appropriate antibiotic. Total β-galactosidase units were determined as described57 and plotted against the culture A600. The slope of the linear regression (differential rate of expression) was used as the specific activity. Mutant activities were determined at least three times and s.d. calculated.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by grants from the US National Institutes of Health (GM065430 to A.L.F., GM056448 to D.S., and T32-GM07757 to Indiana University/P.J.M.). A.L.F. is a Cottrell Scholar of Research Corporation. The Typhoon 9210 imaging system was purchased with a grant from the US National Science Foundation (DBI-0244815). The authors thank T. Stone (Indiana University) for technical support in the Physical Biochemistry Instrumentation Facility, and J. Fitzgerald, A. Kerzmann, N. Anderson and S. Kamel (Indiana University) for assistance in the cloning and sequencing of the constructs.
Footnotes
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Hori K, Yanazaki Y. Nucleotide sequence specific interaction of host factor I with bacteriophage Q β RNA. FEBS Lett. 1974;43:20–22. doi: 10.1016/0014-5793(74)81095-1. [DOI] [PubMed] [Google Scholar]
- 2.Van Emmelo J, De Boever J, Gillis E, Fiers W. A host factor required for Q-β-RNA-replication in "Escherichia coli”. Arch. Int. Physiol. Biochim. 1973;81:393–394. [PubMed] [Google Scholar]
- 3.Storz G. An Expanding Universe of Noncoding RNAs. Science. 2002;296:1260–1263. doi: 10.1126/science.1072249. [DOI] [PubMed] [Google Scholar]
- 4.Repoila F, Majdalani N, Gottesman S. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol. Microbiol. 2003;48:855–861. doi: 10.1046/j.1365-2958.2003.03454.x. [DOI] [PubMed] [Google Scholar]
- 5.Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- 7.Sledjeski DD, Whitman C, Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnleitner E, et al. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Microb. Pathog. 2003;35:217–228. doi: 10.1016/s0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 9.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lease RA, Cusick ME, Belfort M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl. Acad. Sci. USA. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altuvia S, Zhang A, Argaman L, Tiwari A, Storz G. The Escherichia coli OxyS regulatory RNA represses fhlA translation by blocking ribosome binding. EMBO J. 1998;17:6069–6075. doi: 10.1093/emboj/17.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argaman L, Altuvia S. fhlA repression by OxyS RNA: kissing complex formation at two sites results in a stable antisense–target RNA complex. J. Mol. Biol. 2000;300:1101–1112. doi: 10.1006/jmbi.2000.3942. [DOI] [PubMed] [Google Scholar]
- 13.Zhang A, et al. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 1998;17:6061–6068. doi: 10.1093/emboj/17.20.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majdalani N, Chen S, Murrow J, St John K, Gottesman S. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 2001;39:1382–1394. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- 15.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masse E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 2003;17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vecerek B, Moll I, Afonyushkin T, Kaberdin V, Blasi U. Interaction of the RNA chaperone Hfq with mRNAs: direct and indirect roles of Hfq in iron metabolism of Escherichia coli. Mol. Microbiol. 2003;50:897–909. doi: 10.1046/j.1365-2958.2003.03727.x. [DOI] [PubMed] [Google Scholar]
- 18.Moller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002;16:1696–1706. doi: 10.1101/gad.231702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajnsdorf E, Regnier P. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc. Natl. Acad. Sci. USA. 2000;97:1501–1505. doi: 10.1073/pnas.040549897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Derout J, et al. Hfq affects the length and the frequency of short oligo(A) tails at the 3′ end of Escherichia coli rpsO mRNAs. Nucleic Acids Res. 2003;31:4017–4023. doi: 10.1093/nar/gkg456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vytvytska O, Moll I, Kaberdin VR, von Gabain A, Blasi U. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev. 2000;14:1109–1118. [PMC free article] [PubMed] [Google Scholar]
- 22.Folichon M, et al. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 2003;31:7302–7310. doi: 10.1093/nar/gkg915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arluison V, et al. Structural modelling of the Sm-like protein Hfq from Escherichia coli. J. Mol. Biol. 2002;320:705–712. doi: 10.1016/s0022-2836(02)00548-x. [DOI] [PubMed] [Google Scholar]
- 24.Moller T, et al. Hfq: a bacterial Sm-like protein that mediates RNA–RNA interaction. Mol. Cell. 2002;9:23–30. doi: 10.1016/s1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang A, Wassarman KM, Ortega J, Steven AC, Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Zhulin I, Wartell RM. Predicted structure and phyletic distribution of the RNA-binding protein Hfq. Nucleic Acids Res. 2002;30:3662–3671. doi: 10.1093/nar/gkf508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauter C, Basquin J, Suck D. Sm-like proteins in eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchler-Bauer A, et al. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 2003;31:383–387. doi: 10.1093/nar/gkg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq– RNA complex: a bacterial Sm-like protein. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambach C, et al. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell. 1999;96:375–387. doi: 10.1016/s0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 31.Achsel T, et al. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999;18:5789–5802. doi: 10.1093/emboj/18.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achsel T, Stark H, Luhrmann R. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc. Natl. Acad. Sci. USA. 2001;98:3685–3689. doi: 10.1073/pnas.071033998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toro I, et al. RNA binding in an Sm core domain: X-ray structure and functional analysis of an archaeal Sm protein complex. EMBO J. 2001;20:2293–2303. doi: 10.1093/emboj/20.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toro I, Basquin J, Teo-Dreher H, Suck D. Archaeal Sm proteins form heptameric and hexameric complexes: crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus. J. Mol. Biol. 2002;320:129–142. doi: 10.1016/S0022-2836(02)00406-0. [DOI] [PubMed] [Google Scholar]
- 35.Moll I, Leitsch D, Steinhauser T, Blasi U. RNA chaperone activity of the Sm-like Hfq protein. EMBO Rep. 2003;4:284–289. doi: 10.1038/sj.embor.embor772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissmann TA, Touati D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brescia CC, Mikulecky PJ, Feig AL, Sledjeski DD. Identification of the Hfq binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA. 2003;9:33–43. doi: 10.1261/rna.2570803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Blasi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003;9:1308–1314. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stage-Zimmermann TK, Uhlenbeck OC. Hammerhead ribozyme kinetics. RNA. 1998;4:875–889. doi: 10.1017/s1355838298980876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plum GE, Breslauer KJ. Calorimetry of proteins and nucleic acids. Curr. Opin. Struct. Biol. 1995;5:682–690. doi: 10.1016/0959-440x(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 41.Ladbury JE, Chowdhry BZ. Sensing the heat: the application of isothermal titration calorimetry to thermodynamic studies of biomolecular interactions. Chem. Biol. 1996;3:791–801. doi: 10.1016/s1074-5521(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 42.Leavitt S, Freire E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 2001;11:560–566. doi: 10.1016/s0959-440x(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 43.de Haseth PL, Uhlenbeck OC. Interaction of Escherichia coli host factor protein with oligoriboadenylates. Biochemistry. 1980;19:6138–6146. doi: 10.1021/bi00567a029. [DOI] [PubMed] [Google Scholar]
- 44.Sledjeski DD, Gupta A, Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 45.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mura C, Kozhukhovsky A, Gingery M, Phillips M, Eisenberg D. The oligomerization and ligand-binding properties of Sm-like archaeal proteins (SmAPs) Protein Sci. 2003;12:832–847. doi: 10.1110/ps.0224703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thore S, Mayer C, Sauter C, Weeks S, Suck D. Crystal structures of the Pyrococcus abyssi Sm core and its complex with RNA: common features of RNA-binding in Archaea and Eukarya. J. Biol. Chem. 2003;278:1239–1247. doi: 10.1074/jbc.M207685200. [DOI] [PubMed] [Google Scholar]
- 48.Urlaub H, Raker VA, Kostka S, Luhrmann R. Sm protein-Sm site RNA interactions within the inner ring of the spliceosomal snRNP core structure. EMBO J. 2001;20:187–196. doi: 10.1093/emboj/20.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark H, Dube P, Luhrmann R, Kastner B. Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature. 2001;409:539–542. doi: 10.1038/35054102. [DOI] [PubMed] [Google Scholar]
- 50.Arluison V, et al. The C-terminal domain of Escherichia coli Hfq increases the stability of the hexamer. Eur. J. Biochem. 2004;271:1258–1265. doi: 10.1111/j.1432-1033.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 51.Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- 52.Mikulecky PJ, Takach JC, Feig AL. Entropy-driven folding of an RNA helical junction: an isothermal titration calorimetric analysis of the hammerhead ribozyme. Biochemistry. 2004;43:5870–5881. doi: 10.1021/bi0360657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brescia CC, Kaw MK, Sledjeski DD. The DNA binding protein H-NS binds to and alters the stability of RNA in vitro and in vivo. J. Mol. Biol. 2004;339:505–514. doi: 10.1016/j.jmb.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc. Natl. Acad. Sci. USA. 2002;99:9697–9702. doi: 10.1073/pnas.112318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller JH. Experiments in Bacterial Genetics. New York: Cold Spring Harbor Laboratory, Cold Spring Harbor; 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.