Abstract
Background:
Idiopathic pulmonary fibrosis (IPF) is often associated with elevations in pulmonary artery pressures. Although primary pulmonary arterial hypertension (PAH) has been associated with primary graft dysfunction (PGD), the role of secondary PAH in mediating PGD risk in patients with IPF is incompletely understood. The purpose of this study was to evaluate the relationship between mean pulmonary artery pressure (mPAP) and PGD among patients with IPF.
Methods:
We performed a multicenter prospective cohort study of 126 lung transplant procedures performed for IPF between March 2002 and August 2007. The primary outcome was grade 3 PGD at 72 h after lung transplant. The mPAP was measured as the initial reading following insertion of the right-sided heart catheter during lung transplant. Multivariable logistic regression was used to adjust for confounding variables.
Results:
The mPAP for patients with PGD was 38.5 ± 16.3 mm Hg vs 29.6 ± 11.5 mm Hg for patients without PGD (mean difference, 8.9 mm Hg [95% CI, 3.6-14.2]; P = .001). The increase in odds of PGD associated with each 10-mm Hg increase in mPAP was 1.64 (95% CI, 1.18-2.26; P = .003). In multivariable models, this relationship was independent of confounding by other clinical variables, although the use of cardiopulmonary bypass partially attenuated the relationship.
Conclusions:
Higher mPAP in patients with IPF is associated with the development of PGD.
Idiopathic pulmonary fibrosis (IPF) is a fatal and chronically progressive diffuse parenchymal lung disease that is characterized by varying degrees of fibrosis of the lung parenchyma.1 Currently, there are few proven, standardized therapies for IPF2; the only viable treatment that has shown survival benefit for patients with advanced stages of IPF is lung transplantation.3-7 Nonetheless, the survival benefit for lung transplantation in patients with IPF is still complicated by significant early mortality rates, especially within the first year (approximately 28%). The 10-year survival rate after lung transplantation is worse for patients with IPF when compared with other diseases, such as idiopathic pulmonary arterial hypertension (PAH), cystic fibrosis, and α1-antitrypsin-deficiency emphysema.8
Elevated mean pulmonary arterial pressure (mPAP) in patients with IPF has been associated with decreased exercise capacity and worse survival.9 Higher mPAP was also associated with increased mortality following lung transplantation in a large registry study.10 One possible explanation for the link between PAH and early mortality after lung transplantation is primary graft dysfunction (PGD), a severe, acute lung injury occurring early after lung transplantation that is responsible for significant morbidity and mortality.11-20 While previous studies have demonstrated that primary PAH is known to be a risk factor for PGD,14-16,21 the role of secondary PAH in PGD risk is incompletely understood. The purpose of this study was to evaluate the association between mPAP and the development of severe PGD in patients with IPF who underwent lung transplantation. Specifically, we hypothesized that higher mPAP would be associated with an increased risk of PGD in patients with IPF.
Materials and Methods
Study Design
A prospective cohort study was performed using data that was collected from the Lung Transplant Outcome Group (LTOG), which included nine centers in the United States (Columbia University, University of Pennsylvania, Vanderbilt University, Stanford University, University of Alabama at Birmingham, University of Michigan, Johns Hopkins University, Duke University, and University of Pittsburgh). Details of the study sample have been previously published.22,23
Study Population
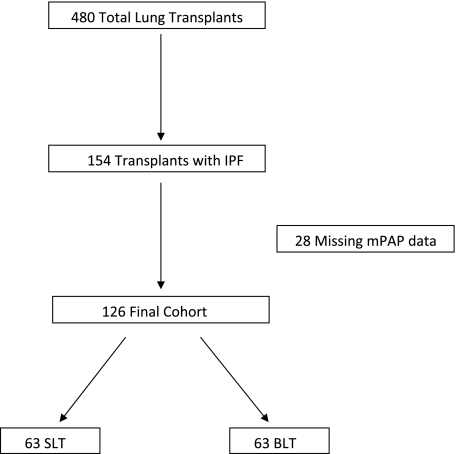
A total of 480 patients who gave consent to the LTOG and who underwent either single-lung transplantation (SLT) or bilateral lung transplantation (BLT) procedures between March 2002 and August 2007 were screened for potential inclusion in the study. One hundred fifty-four patients with IPF as their primary indication for transplant were included.1 Twenty-eight patients were excluded because of missing mPAP data. The overall study population comprised 126 patients (Fig 1).
Figure 1.
Study flow diagram by pulmonary arterial hypertension (PAH) and primary graft dysfunction (PGD) status after lung transplantation. An mPAP of > 40 mm Hg indicated the presence of PAH; an mPAP of ≤ 40 mm Hg indicated that PAH was not present. BLT = bilateral lung transplantation; IPF = idiopathic pulmonary fibrosis; mPAP = mean pulmonary arterial pressure; SLT = single-lung transplantation.
Study subjects had organ preservation and underwent postoperative immunosuppression according to local protocols. Sites during the cohort period were nearly uniform in using low-potassium, extracellular-type preservation fluid. Immunosuppression included induction with IL-2 receptor antagonists at all but one site, followed by maintenance using a calcineurin inhibitor, azathioprine or mycophenolate mofetil, and steroids.
Risk Factor and Outcome Variables
The primary outcome was grade 3 PGD recorded at 72 h, defined by the presence of radiographic infiltrates consistent with pulmonary edema and a Pao2/Fio2 ratio of < 200.11,12,14,20 The primary risk factor was mPAP, reported from initial reading during right-sided heart catheterization performed in the operating room. The mPAP was assessed as a continuous variable. Donor, recipient, and intraoperative variables were evaluated for potential confounding effects on the relationship of the mPAP and PGD. Potentially confounding variables included donor and recipient age, sex, and race; procedure type (single lung vs bilateral); presence of cardiopulmonary bypass; use of nitric oxide; ischemic time; the study center; and the amount of packed RBCs, platelets, and fresh frozen plasma used during the lung transplant procedure. Cardiac output data were not collected prospectively; however, in post hoc analyses, we reviewed charts at a single institution (University of Pennsylvania) to retrospectively collect cardiac output data. These analyses are reported separately. Sensitivity analyses were performed using any grade 3 PGD occurring at grading times during the first 3 days following surgery (T24-T72) as the outcome.
Statistical Analysis
Continuous data were reported as mean values and SDs, while categorical data were presented as counts and percentages. Univariate associations were tested for significance using χ2 or exact tests for discrete variables, as well as the Student t test (for approximately normal data). The relationship of mPAP and predicted probability of PGD was graphed using a fractional polynomial fit.
Multivariable logistic regression was used to adjust for potential confounding variables. Confounding variables introduced into the multivariable model one at a time in order to prevent model overfitting. Confounding was defined as a change in the OR of 15% or greater on adjustment. The Pearson goodness-of-fit test was used for postestimation fit of the logistic model. Center effects were accounted for using a robust variance estimator for cluster-correlated data. All statistical comparisons were performed using STATA version 10.1 (StataCorp; College Station, Texas) and SAS version 9.1 (SAS Institute; Cary, North Carolina).
Results
A total of 480 patients were enrolled, and IPF was diagnosed in 154 of these patients. Twenty-eight patients were excluded because of missing mPAP data (e-Table 1). A total of 126 patients with IPF who underwent either SLT or BLT were included in the overall study population (Fig 1). Table 1 presents baseline donor, preoperative recipient, and intraoperative recipient characteristics among subjects with IPF according to PGD status. Twenty-nine patients (23%) met the criteria for PGD. Black subjects tended to have a slightly greater risk of PGD. Patients with PGD had higher pulmonary artery systolic and diastolic pressures, required larger volumes of packed RBCs, and were more likely to have undergone cardiopulmonary bypass. However, there was no detected difference in the use of intraoperative nitric oxide for patients with or without PGD.
Table 1.
—Baseline Donor and Recipient Preoperative and Intraoperative Characteristics According to PGD Status at 72 h in Patients With IPF
| Subject Characteristics | No PGD (n = 97) | PGD (n = 29) | P Value |
| Donor variables | |||
| Age, mean, y | 36 (33-40) | 37 (31-43) | .86 |
| Gender, female | 55 | 45 | .35 |
| Race/ethnicity | .39 | ||
| White | 72 | 66 | . . . |
| Black | 13 | 14 | . . . |
| Hispanic | 8 | 21 | . . . |
| Asian | 3 | 0 | . . . |
| Other | 3 | 0 | . . . |
| Recipient preoperative and intraoperative variables | |||
| Age, y | 56 (54-59) | 54 (49-59) | .37 |
| Gender, female | 33 | 31 | .84 |
| Race/ethnicity | .08 | ||
| White | 87 | 72 | . . . |
| Black | 8 | 24 | . . . |
| Hispanic | 3 | 0 | . . . |
| Other | 2 | 3 | . . . |
| Procedure type | .29 | ||
| Single-lung transplant | 53 | 41 | . . . |
| Bilateral lung transplant | 47 | 59 | . . . |
| Use of cardiopulmonary bypass | 34 | 69 | .001 |
| Use of inhaled nitric oxide | 26 | 24 | .86 |
| Hemodynamic details, preoperative | |||
| Pulmonary artery systolic pressure, mm Hg | 44 (40-47) | 58 (49-68) | .0004 |
| Pulmonary artery diastolic pressure, mm Hg | 23 (21-25) | 29 (24-34) | .008 |
| Right atrial pressure, mm Hg | 12 (10-13) | 13 (11-15) | .27 |
| Packed RBCs, mL, intraoperatively | 428 (304-552) | 741 (406-1,077) | .03 |
| Platelets, mL, intraoperatively | 164 (48-280) | 406 (79-735) | .08 |
| Fresh frozen plasma, mL, intraoperatively | 202 (103-300) | 354 (200-508) | .13 |
| Ischemic time, min | |||
| First lung | 238 (223-253) | 249 (234-265) | .43 |
| Second lung | 325 (300-351) | 355 (311-399) | .23 |
Data are shown as means with 95% CIs or as percentages. IPF = idiopathic pulmonary fibrosis; PGD = primary graft dysfunction.
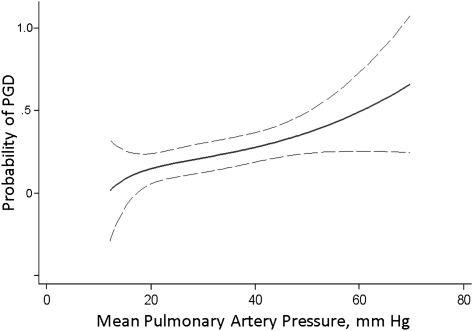
The relationship of mPAP as a continuous variable with PGD among patients with IPF is depicted in Figure 2. The mPAP was higher in patients who developed PGD at 72 h: 38.5 ± 16.3 mm Hg in patients with PGD vs 29.6 ± 11.5 mm Hg in patients without PGD (mean difference, 8.9 mm Hg [95% CI, 3.6-14.2]; P = .001). The increase in odds of grade 3 PGD for each 10-mm Hg increase in mPAP was 1.64 (95% CI, 1.18-2.26; P = .003). In multivariable models, this relationship was independent of all clinical variables, although the use of cardiopulmonary bypass attenuated the relationship (Table 2). In a multivariable model simultaneously adjusting for the use of cardiopulmonary bypass and red cells transfused intraoperatively, the relationship of mPAP with PGD maintained borderline significance (OR, 1.41 [95% CI, 1.00-1.99]; P = .053). The Pearson goodness-of-fit test showed that both the base and multivariable logistic models fit the data reasonably well (P = .363 and P = .282, respectively).
Figure 2.
Relationship of mPAP and probability of PGD, using a fractional polynomial fit with 95% CIs (dashed lines). See Figure 1 legend for expansion of abbreviations.
Table 2.
—Bivariate and Multivariable Analysis of Association of Preoperative mPAP With PGD at 72 h in Patients With IPF
| Variable | OR (95% CI) | P Value |
| Unadjusted base model | 1.64 (1.18-2.26) | .003 |
| Base model adjusted for: | ||
| Recipient age | 1.65 (1.19-2.29) | .003 |
| Donor age | 1.64 (1.13-2.37) | .009 |
| Recipient sex | 1.64 (1.18-2.28) | .003 |
| Donor sex | 1.64 (1.18-2.27) | .003 |
| Recipient race/ethnicity | 1.64 (1.18-2.28) | .003 |
| Donor race/ethnicity | 1.64 (1.18-2.26) | .003 |
| Procedure type | 1.61 (1.16-2.24) | .004 |
| Use of cardiopulmonary bypass | 1.43 (1.01-2.01) | .043 |
| Use of nitric oxide | 1.63 (1.18-2.27) | .003 |
| Packed RBCs, intraoperative | 1.56 (1.11-2.18) | .009 |
| Platelets, intraoperative | 1.66 (1.19-2.32) | .003 |
| Fresh frozen plasma, intraoperative | 1.61 (1.16-2.25) | .004 |
| Total ischemic times | ||
| First lung | 1.61 (1.16-2.24) | .004 |
| Second lung | 1.64 (1.08-2.49) | .019 |
ORs listed are for the risk of PGD for each 10 mm Hg increase in mPAP, adjusted individually for each confounding variable. mPAP = mean pulmonary arterial pressure. See Table 1 for expansion of other abbreviations.
The mortality rates at both 30 days and 90 days for patients with severe PAH (13% and 17%, respectively) appeared greater compared with those of patients without PAH (3% and 5%). However, these results did not achieve statistical significance: The 30-day relative mortality risk was 4.27 (95% CI, 1.01-18.00; P = .055), and the 90-day relative mortality risk was 3.2 (95% CI, 0.99-10.31; P = .057).
The association of mPAP and PGD was similar in the 63 subjects who underwent BLT (OR per 10-mm Hg increase, 1.69 [95% CI, 1.12-2.55]; P = .012) and the 63 patients who underwent SLT (OR per 10-mm Hg increase, 1.46 [95% CI, 0.83-2.57]; P = .19). There was no effect modification on multiplicative interaction testing in the logistic model (P = .53).
In post hoc analyses of cardiac output data, 56 of the 70 patients at the University of Pennsylvania had cardiac outputs recorded. In this subpopulation, 10 subjects had PGD at 72 h. The mean cardiac output among patients with PGD was 5.0 ± 1.8 L/min vs 5.8 ± 2.6 L/min in patients without PGD (mean difference, 0.9 L/min [95% CI, −0.9 to 2.6]; P = .32). The effect of mPAP on PGD risk within this subpopulation was similar to that of the overall group, with an OR of 1.55 for each 10-mm Hg increase in mPAP (95% CI, 0.91-2.65; P = .11), and there was no significant confounding of this relationship by cardiac output (adjusted OR, 1.47 [95% CI, 0.85-2.95]; P = .17).
When sensitivity analyses were performed for the 43 patients (34%) who met the criteria for any grade 3 PGD at any time point between T24 and T72, the mPAP remained associated with PGD (OR per 10-mm Hg increase, 1.33 [95% CI, 1.00-1.77]; P = .048). However, this relationship did not maintain independence when adjusted for the use of blood products or cardiopulmonary bypass.
Discussion
We have demonstrated a strong clinical association between higher mPAP and the risk of PGD among subjects with IPF. The multicenter nature of the study, prospective data collection, and relatively short period of data collection make these findings applicable to the general lung-transplant population.
PAH is common among patients with IPF who are undergoing lung transplantation,24 and previous research has implicated PAH in the risk of PGD.12,14,21,25 Recently, Whitson et al21 demonstrated that increased preoperative mPAP is an independent risk factor for the development of grade 3 PGD within the first 48 h after transplant. Our study adds to these findings by specifically evaluating secondary PAH in patients with IPF in a multicenter prospective cohort study designed to study PGD. The mechanistic underpinnings of the increased risk of PGD among patients with IPF and PAH include greater endothelial injury from hemodynamic forces in addition to inherent abnormalities in coagulation /inflammation, platelet activation, and cell adhesion observed in transplant recipients with both elevated mPAP and PGD.22,23,26 Furthermore, vasoactive mediators such as endothelin-1, platelet-derived growth factor, transforming growth factor-β, and fibroblast growth factor have all been implicated in the pathogenesis of IPF, and these mediators can also contribute to the development of lung injury.27-30
In addition to raising questions about the shared pathophysiologic characteristics of secondary PAH and PGD, our study may have direct clinical implications. Knowledge of elevated mPAP as an independent risk factor for PGD in patients with IPF may help in counseling patients and families on the risks of transplantation. In addition, the identification of higher-risk patients may eventually lead to trials of selective treatment for patients prior to the development of PGD, perhaps with vasoactive or antiplatelet agents. Importantly, given that IPF is often fatal in the absence of lung transplantation, our results should not be interpreted as evidence to exclude certain groups of patients from lung transplantation.
Our study has several limitations. In this study, the individual effects of cardiopulmonary bypass, use of blood products, and PAH cannot be delineated since they often are apparent in the same patients. In particular, the primary reason for the use of cardiopulmonary bypass (eg, planned or urgent) was not recorded in this study. Additionally, there is potential for bias in the measurement of intraoperative mPAP. Although our goal was to record the measurement of mPAP prior to anesthesia induction, the exact timing of the measurement can be inaccurate and thus can reflect variability in the amount of induction, volumes, and sedation. Other hemodynamic details and measurements during right-sided heart catheterization, such as pulmonary capillary wedge pressure, cardiac output, and Fio2 at the time of catheter insertion were not routinely measured in the subjects in this cohort study.31 Measurements of mPAP can vary with right-sided heart function and volume status. Other measures of pulmonary hypertension, such as pulmonary vascular resistance, were not evaluated because pulmonary capillary wedge pressures are not routinely recorded in the operating room. Our post hoc analyses of cardiac output were limited by these missing recorded data and did not have adequate power. Nonetheless, these analyses did not reveal significant confounding of the relationship of mPAP with PGD.
The present study demonstrates the importance of elevated mPAP in patients with IPF as a risk factor for the development of PGD. Future studies are needed to help explain the pathogenic link between PAH and PGD. Furthermore, these results may help in identifying patients with IPF who are at greater risk for PGD prior to lung transplantation.
Supplementary Material
Acknowledgments
Author contributions: Dr Fang: contributed to study conception, data collection, planning of analyses, conduct of analyses, interpretation of analyses, manuscript drafting, and manuscript editing.
Dr Studer: contributed to study conception, data collection, interpretation of analyses, manuscript drafting, and manuscript editing.
Dr Kawut: contributed to study conception, data collection, planning of analyses, interpretation of analyses, manuscript drafting, and manuscript editing.
Dr Ahya: contributed to study conception, data collection, and manuscript editing.
Dr Lee: contributed to study conception, planning of analyses, conduct of analyses, interpretation of analyses, and manuscript editing.
Dr Wille: contributed to data collection, interpretation of analyses, and manuscript editing.
Dr Lama: contributed to data collection, interpretation of analyses, and manuscript editing.
Dr Ware: contributed to data collection, study conception, interpretation of analyses, and manuscript editing.
Dr Orens: contributed to data collection, interpretation of analyses, and manuscript editing.
Dr Weinacker: contributed to data collection, interpretation of analyses, and manuscript editing.
Dr Palmer: contributed to data collection, study conception, interpretation of analyses, and manuscript editing.
Dr Crespo: contributed to data collection, interpretation of analyses, and manuscript editing.
Dr Lederer: contributed to data collection, study conception, interpretation of analyses, and manuscript editing.
Dr Deutschman: contributed to data collection, study conception, interpretation of analyses, and manuscript editing.
Dr Kohl: contributed to data collection, study conception, interpretation of analyses, and manuscript editing.
Dr Bellamy: contributed to planning of analyses, conduct of analyses, interpretation of analyses, and manuscript editing.
Ms Demissie: contributed to data collection, and manuscript editing.
Dr Christie: contributed to study conception, data collection, planning of analyses, conduct of analyses, interpretation of analyses, manuscript drafting, and manuscript editing.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kawut has received funding for consulting, speaking fees, conferences, travel to conferences, research, and serving on steering committees from Gilead, Actelion, United Therapeutics, Pfizer, Gerson Lehrman, and Clinical Advisors. Drs Fang, Studer, Ahya, Lee, Wille, Lama, Ware, Orens, Weinacker, Palmer, Crespo, Lederer, Deutschman, Kohl, Bellamy, and Christie and Ms Demissie have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Additional information: The e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/4/782/suppl/DC1.
Abbreviations
- BLT
bilateral lung transplantation
- IPAH
idiopathic pulmonary arterial hypertension
- IPF
idiopathic pulmonary fibrosis
- LTOG
Lung Transplant Outcome Group
- mPAP
mean pulmonary arterial pressure
- PAH
pulmonary arterial hypertension
- PGD
primary graft dysfunction
- SLT
single-lung transplantation
Footnotes
For editorial comment see page 741
Funding/Support: This study was funded by the National Institutes of Health [Grants NIH HL04243, NIH HL081619, NIH HL087115, NIH HL67771, NIH HL081332] and the Craig and Elaine Dobbin Pulmonary Research Fund.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Collard HR, Loyd JE, King TE, Jr, Lancaster LH. Current diagnosis and management of idiopathic pulmonary fibrosis: a survey of academic physicians. Respir Med. 2007;101(9):2011–2016. doi: 10.1016/j.rmed.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alalawi R, Whelan T, Bajwa RS, Hodges TN. Lung transplantation and interstitial lung disease. Curr Opin Pulm Med. 2005;11(5):461–466. doi: 10.1097/01.mcp.0000175520.41729.4e. [DOI] [PubMed] [Google Scholar]
- 4.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351(9095):24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 5.Charman SC, Sharples LD, McNeil KD, Wallwork J. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant. 2002;21(2):226–232. doi: 10.1016/s1053-2498(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 6.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126(2):469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 7.De Meester J, Smits JM, Persijn GG, Haverich A. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant. 2001;20(5):518–524. doi: 10.1016/s1053-2498(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 8.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132(3):998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 10.Whelan TP, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2005;24(9):1269–1274. doi: 10.1016/j.healun.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Christie JD, Van Raemdonck D, de Perrot M, et al. ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24(10):1451–1453. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114(1):51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127(1):161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 15.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69(6):1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 16.Thabut G, Vinatier I, Stern JB, et al. Primary graft failure following lung transplantation: predictive factors of mortality. Chest. 2002;121(6):1876–1882. doi: 10.1378/chest.121.6.1876. [DOI] [PubMed] [Google Scholar]
- 17.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 18.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB, ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24(10):1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 19.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D, ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131(1):73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Christie JD, Robinson N, Ware LB, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covarrubias M, Ware LB, Kawut SM, et al. Lung Transplant Outcomes Group Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 24.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30(4):715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 25.Bando K, Keenan RJ, Paradis IL, et al. Impact of pulmonary hypertension on outcome after single-lung transplantation. Ann Thorac Surg. 1994;58(5):1336–1342. doi: 10.1016/0003-4975(94)91908-9. [DOI] [PubMed] [Google Scholar]
- 26.Kawut SM, Okun J, Shimbo D, et al. for the Lung Transplant Outcomes Group Soluble p-selectin and the risk of primary graft dysfunction after lung transplantation. Chest. 2009;136(1):237–244. doi: 10.1378/chest.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitzenblum E, Chaouat A, Peacock AJ, Rubin LJ. Pulmonary Circulation: Diseases and Their Treatment. London, England: Arnold; 2004. Pulmonary hypertension due to chronic hypoxic lung disease; pp. 374–386. [Google Scholar]
- 28.Colombat M, Mal H, Groussard O, et al. Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol. 2007;38(1):60–65. doi: 10.1016/j.humpath.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Gagermeier J, Dauber J, Yousem S, Gibson K, Kaminski N. Abnormal vascular phenotypes in patients with idiopathic pulmonary fibrosis and secondary pulmonary hypertension. Chest. 2005;128(6 Suppl):601S. doi: 10.1378/chest.128.6_suppl.601S. [DOI] [PubMed] [Google Scholar]
- 30.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 31.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.