Abstract
Helicobacter pylori outer membrane proteins, such as the blood group antigen–binding adhesin (BabA), are associated with severe pathological outcomes. However, the in vivo role of BabA during long-term infection is not clear. In this study, Mongolian gerbils were infected with H. pylori and necropsied continuously during 18 months. Bacterial clones were recovered and analyzed for BabA expression, Leb-binding activity, and adhesion to gastric mucosa. BabA expression was completely absent by 6 months post-infection. Loss of BabA expression was attributable to nucleotide changes within the babA gene that resulted in a truncated BabA. In response to the infection, changes in the epithelial glycosylation pattern were observed that were similar to responses observed in humans and monkeys. Furthermore, infections with BabA-expressing and BabA-nonexpressing H. pylori showed no differences in colonization, but infection with the BabA-expressing strain exhibited histological changes and increased inflammatory cell infiltration. This suggests that BabA expression contributes to severe mucosal injury.
Helicobacter pylori infect humans and primates and cause considerable morbidity and mortality worldwide. The ideal animal model will respond to the organism in a similar manner to humans and thus facilitate detailed analysis of bacterial-host interactions. Mongolian gerbils are small and inexpensive, and they develop histological findings that mimic the lesions induced by H. pylori infection in humans, including peptic ulcer disease and gastric cancer [1–5]. This makes Mongolian gerbils a relevant model for studying inflammatory processes caused by H. pylori infection.
Adherence of H. pylori to gastric mucosa is essential for the development of mucosal inflammatory responses [6]. The best-described adhesins are the blood group antigen-binding adhesin (BabA) [7] and the sialic acid–binding adhesin (SabA) [8]. These proteins facilitate adherence to the fucosylated ABH/Lewis b (Leb) blood group antigens [9, 10] and to the inflammation-associated sialyl-Lewis x (sLex), respectively [8]. BabA expression has been associated with severe mucosal cellular inflammation and an increased risk of peptic ulcer disease and gastric cancer [11–13]. However, patients infected by strains with low BabA expression and without Leb-binding ability are reported to exhibit a higher level of mucosal injury and more-severe clinical outcomes, compared with patients infected with strains with high BabA-expression levels and Leb-binding activity [11]. Experimental infection of Rhesus monkeys or mice is associated with loss of BabA expression [14, 15]. A recent study on 2 H. pylori–infected gerbils also described loss of BabA expression [15]; however, the in vivo effects of BabA during long-term infection within gastric mucosa remain unclear. In this study, we investigated the effect of BabA over an 18-month period in Mongolian gerbils with long-term H. pylori infection. We found BabA expression to increase initially, to decrease over time, and to be completely absent after 6 months. Gerbils exhibited changes in glycosylation in the gastric mucosa that were similar to changes in humans and Rhesus monkeys, which further emphasizes the relevance of gerbils as a model for H. pylori infection, adherence, and host responses.
MATERIALS AND METHODS
Bacterial Strains, Animals, Inoculation, and Euthanasia
The H. pylori strains used in this study were the clinical isolate TN2GF4 [5], strain 17875/Leb (BabA+/SabA-), and 17875/sLex (BabA-/SabA+) (17875ΔbabA1::kanΔbabA2::cam) [8]. The latter 2 strains are derivatives of CCUG17875 (CCUG).
Gerbil housing conditions are described in the Supplementary Data. Six-week-old male Mongolian gerbils (MGS/Sea; Harlan Sprague Dawley) were orogastrically inoculated 3 times (days 0, 1, 2) with 1.0 mL of H. pylori (109 colon-forming units/mL) or sterile brain-heart infusion (BHI) broth using gastric intubation needles after 16 h of fasting [16]. No specific pretreatments were administered prior to orogastric H. pylori inoculation. Five to seven H. pylori–inoculated gerbils and 3–5 age- and sex-matched uninfected gerbils (control group) were used for each time point. At necropsy, an ∼1-mm2 piece of gastric mucosa from the antrum was collected for culturing H. pylori [16] and polymerase chain reaction (PCR).
Histopathological findings
Gastric tissues were fixed in 10% neutral buffered formalin and sliced along the longitudinal axis into 4–7 slips of equal width, embedded in paraffin, and cut into 4-μm sections. Sections were stained with hematoxylin and eosin for morphological observation and with Genta stain for detection of H. pylori [17]. The degree of mononuclear cell (MNC) and polymorphonuclear cell (PMN) infiltrations and H. pylori density was graded from 0 (absent/normal) to 5 (maximal intensity) [17]. These scores were evaluated in all slips from each stomach, and the results were averaged.
Immunohistochemistry
Immunostaining of the gastric mucosa for Leb and H1 was performed with mouse monoclonal antibodies to Leb (1:100) (Seikagaku) and H1 (1:100) (Gene Tex) using the immuno-enzyme polymer method (Nichirei Biosciences). Alternatively, H1 and sLex were detected with mouse monoclonal antibodies BG-4 (1:40) (Signet) and α-sialyl-Lewis x (1:25) (Calbiochem), respectively, and Alexa Fluor 568-goat-anti-mouse immunoglobulin (Ig) G (1:300) (Molecular Probes). Immunostaining for H. pylori was done with rabbit anti-H. pylori polyclonal antibody (DAKO) and peroxidase-labeled antirabbit IgG polyclonal antibody (DAKO).
Detection of Bacterial Proteins
Protein extracts were obtained by suspending bacteria in B-PER reagent (Pierce Biotechnology). Immunoblotting was performed using standard methods. Primary antibodies anti-BabA (AK277), anti-BabB (AK276), anti-OipA a (AK282), anti-SabA (AK278), anti-AlpA (AK214), and anti-AlpB (AK262) were diluted 1:5000 and anti-UreB, anti-CagA, and-VacA antibodies (Austral Biologicals) were diluted 1:2000. Secondary anti-rabbit IgG was diluted 1:2000 (Cell Signaling Tech). Signals were detected with LumiGLO reagents (Cell Signaling Tech) and radiographic film exposure. The specificity of these antisera in immunoblots has been confirmed previously [18–20]. Quantitation of relative protein expression levels are described in the Supplementary Data.
Radioimmunoassay (RIA) and Scatchard Analysis
Binding activities to 125-I-labeled H1/Leb receptor glycoconjugate were measured in duplicates with glycoconjugates LNDI-HSA-Leb and LNF1-HSA-H type 1 (Isosep) [21]. Receptor binding affinity to H1 was analyzed by Scatchard analysis [21].
Sequencing of Outer Membrane Protein (OMPs) Genes and Promoter Regions
The babA gene and promoter regions, the babB, sabA, and oipA genes, were amplified from genomic DNA by PCR [22–24], purified, and directly sequenced at Macrogen (Seoul, Korea). PCR was also used for genomic localization of the bab genes. Sequences of the gene-specific primers are available on request.
Adherence H. pylori to Gastric Mucosa
Gastric tissue were deparaffinised and incubated in 0.5 M Na-Citrate buffer (pH 6) at 99°C for 40 min, and when needed, pretreatment with 0.1 U Neuraminidase (Sigma-Aldrich) at 37°C for 90 min was applied prior to blocking in (1× phosphate-buffered saline [PBS] + 0.05% Tween 20 + 1% Bovine serum albumine) at 25°C for 1 h. Fluorescein isothiocyanate (FITC)-labeled bacteria [9] were added to the tissues for 2 h or overnight at 25°C, washed (1× PBS + 0.05% Tween 20), and analyzed for binding using Zeiss AxioImager Z1 microscope, apotome, and Axiovision software, version 4.5.
Statistical Analyses
Statistical analyses included the Mann-Whitney rank-sum test, the non-paired Student's t test, Fisher's exact test, and Spearman's rank test, depending on the data set being analyzed. Results are presented as median values when the data were not distributed normally, and mean values ± standard errors are presented when a normal distribution was observed. A P value of <.05 indicated statistical significance.
RESULTS
Strain TN2GF4 Induced Gastric Inflammation, Gastric Ulcer, and Hyperplasia in Mongolian Gerbils
Gerbils were infected with H. pylori TN2GF4 or BHI broth and necropsied at 1, 3, 6, and 18 months after inoculation. Bacteriological and histological examination did not reveal H. pylori infection or histological gastritis in BHI-inoculated control gerbils. H. pylori infection was confirmed by culture, histological examination, and/or PCR in all H. pylori–inoculated gerbils. One month after infection, MNC and PMN infiltrations were observed in the gastric mucosa, primarily in the submucosa of infected animals. Histological changes gradually increased, with maximal cellular infiltration after 6 months (Figure 1A). Gastric ulcers developed in 1 (17%) of 6 gerbils by 3 months, 3 (50%) of 6 by 6 months, and 3 (43%) of 7 by 18 months after H. pylori inoculation. Hyperplastic lesions were observed in 1 (17%) of 6 gerbils by 6 months and 1 (14%) of 7 by 18 months. Mucosal thickness increased to a maximum at 6 months and did not change until 18 months. H. pylori density did not change during the observation period (data not shown). None of the infected gerbils developed gastric cancer after 18 months, which was similar to previous findings for this strain of H. pylori [25].
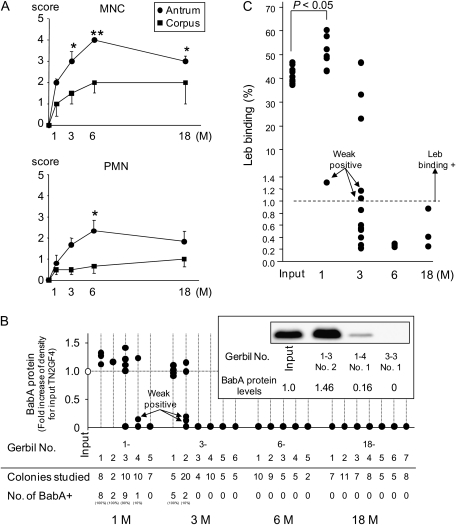
Figure 1.
A, Cellular inflammation in the gastric mucosa of Helicobacter pylori infected gerbils. Mononuclear cell (MNC) and polymorphonuclear cell (PMN) infiltration scores were graded from 0 (absent/normal) to 5 (maximal intensity) in corpus and antrum Mongolian gerbil tissue, presented as means ± standard errors. *P <.05; **P <.01 compared with 1 month. Both MNC and PMN infiltration peaked significantly 6 months after infection in antrum Mongolian gastric mucosa. B, Loss of blood group antigen–binding adhesin (BabA) expression during infection. An mean of 7 H. pylori colonies (range, 2–20 colonies) were picked from each gerbil as output strains, and the BabA expression levels of each output strain recovered from gerbils were measured by immunoblot analysis. The levels are presented as fold-induction of BabA expression, compared with input strain TN2GF4. BabA expression increased after 1 month after infection and diminished thereafter. Immunoblot results, representing BabA-expression level in the input strain and in the different output strains, are also shown. C, Leb-receptor binding activity in clones recovered after infection. Binding to the 125I-Leb conjugate was measured for 27 selected output clones (10 randomly selected BabA expressing clones, 14 randomly selected BabA nonexpressing clones, and all 3 BabA weak-expressing clones) and for the input strain TN2GF4 and 10 randomly selected single clones of the input strain TN2GF4 with radioimmunoassay. Binding activity is given as (%) of bound 125I-Leb.
BabA Expression was Lost after Long-term Infection
Expression of virulence-associated proteins was detected with immunoblot analysis of the H. pylori input strain, TN2GF4 (Supplementary Data). The BabA, OipA, AlpA, AlpB, CagA, and VacA proteins were expressed, but the BabB and SabA proteins were not. On average, 7 H. pylori colonies were isolated from each gerbil as output clones and analyzed for BabA expression. On the basis of results from immunoblots, the output clones were categorized as strains expressing BabA (output BabA+) and strains not expressing BabA (output BabA-), and the 3 output clones exhibited faint BabA expression (output BabA-weak) (Figure 1B). After 1 month, output BabA+ clones was found in 4 (80%) of 5 gerbils, and the level of BabA expression increased ∼20%, compared with the input strain, TN2GF4 (P <.01). By 3 months, the number of BabA-expressing clones had reduced markedly, and output BabA+ and output BabA-weak clones were found in 2 (33%) of 6 gerbils. By 6 and 18 months, none of the output clones expressed BabA (Figure 1B).
The DNA sequences of the full-length babA gene and its promoter region (-170 to +57 base-pairs) from TN2GF4 and the output clones from each gerbil, corresponding to 1–6 months of infection, were obtained. All of the analyzed output BabA+ clones (n = 11) and all output BabA-weak clones (n = 3) contained intact babA genes. Output BabA- clones displayed a number of frame-shift mutations in the babA gene (Table 1). Thus, the down-regulation of BabA expression was not attributable to phase shift variation via slipped strand mispairing mechanisms (SSM) nor via recombination with the babB gene previously observed in monkeys and mice [14, 15]. Instead, loss of BabA expression was caused by nucleotide changes that introduced a stop codon in the babA gene.
Table 1.
Changes in the Nucleotide Sequence of the babA Gene in Output Clones
| Time, months | Gerbil no. | Clone no. | BabA expression | babA nucleotide sequence | Length ofBabA peptide sequence |
| 1 | 1-1 | 1, 3 | + | No change | Intact |
| 1 | 1-2 | 1, 2 | + | No change | Intact |
| 1 | 1-3 | 2, 4 | + | No change | Intact |
| 1 | 1-4 | 5 | + | No change | Intact |
| 3 | 3-1 | 1, 2 | + | A1381G | Intact |
| 3 | 3-2 | 1, 8 | + | No change | Intact |
| 1 | 1-4 | 1 | Weak | No change | Intact |
| 3 | 3-2 | 2, 3 | Weak | No change | Intact |
| 1 | 1-3 | 1, 3 | - | Deletion (916: 80 bp) | 320 aa |
| 1 | 1-4 | 3 | - | C993T | 330 aa |
| 1 | 1-5 | 1, 2 | - | G1965A | 654 aa |
| 3 | 3-3 | 1, 3 | - | Deletion (498: 31 bp) | 176 aa |
| 3 | 3-4 | 2, 7 | - | Deletion (2009: 70 bp) | 518 aa |
| 3 | 3-5 | 1, 2 | - | Deletion (1545:19 bp; 1995: 84 bp) | 518 aa |
| 3 | 3-6 | 1, 2 | - | Insertion (615A: 1 bp) | 204 aa |
| 6 | 6-1 | 1, 2 | - | Deletion (254 :1 bp) | 92 aa |
| 6 | 6-2 | 1, 2 | - | Insertion (950T: 1 bp) | 347 aa |
| 6 | 6-3 | 1, 2 | - | Insertion (615G: 1 bp) | 204 aa |
| 6 | 6-4 | 1, 2 | - | Deletion (635: 1 bp), A1381G | 223 aa |
| 6 | 6-5 | 1, 2 | - | Insertion (1698C: 1 bp), G58A | 587 aa |
NOTE. The nucleotide sequences of the full-length babA genes from the input TN2GF4 and from output clones of each gerbil 1, 3, and 6 months after infection were determined. Gerbil number, name of recovered clones, and BabA expression is presented in the table. BabA, blood group antigen–binding adhesin; bp, base-pair.
The genomic sequences of the 5' region of the oipA and sabA genes in the input and output strains were identical (data not shown). The babB gene was out of frame in the input TN2GF4 strain and in all output clones (the number of CT repeat was 9 or 10), except output clones from 1 gerbil that was infected for 18 months. DNA sequencing confirmed that the babB gene was brought in frame via SSM and a change in the number of CT repeats. The babA gene in strain TN2GF4 is situated in a locus downstream of hypD, and the babB gene is situated at a locus downstream of s18; the locations of these genes were unchanged in the output clones. No remarkable changes in other protein expression levels were detected in the output clones relative to TN2GF4 (Supplementary Data).
Leb-binding Activity and BabA Expression in the Output Clones
The BabA adhesin recognizes fucosylated blood group antigens, such as ABH/Leb and H1 antigen [9]. We randomly selected 27 output clones, including 3 output BabA-weak clones and the input strain TN2GF4 and 10 randomly selected single clones of the input strain TN2GF4, and monitored binding to 125I-Leb. Among the Leb-binding clones, the Leb binding significantly increased in the 1-month output clones and decreased in output clones by 3 months (Figure 1C). All BabA+ clones and the input strain exhibited Leb-binding activity; 3 BabA-weak clones exhibited very weak Leb binding (1.05% to 1.31%). BabA- clones had no Leb-binding activity.
H. pylori Adherence and the Expression of H1-Antigen in the Gerbil Gastric Mucosa
None of the gerbils exhibited immunoreactivity to Leb. In uninfected control gerbils, immunoreactivity for H1 was restricted to the luminal surface and/or intracytoplasmic mucin of surface mucous cells covering the gastric lumen or superficial foveilae (Figure 2). In H. pylori–inoculated gerbils, immunoreactivity toward H1 diminished in inflamed gastric mucosa from 3 months and was completely absent after 6 months (Figure 2). Because no Leb was detected in the gerbil mucosa, we also included analysis of BabA-mediated adherence of H. pylori TN2GF4 to the H1 receptor.
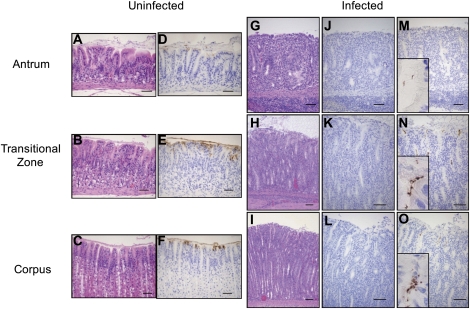
Figure 2.
Histological findings for uninfected control gerbils and gerbils at 3 months after infection. A, D, G, J, and M correspond to antrum gerbil mucosa; B, E, H, K, and N correspond to the transitional zone (mucoparietal glands); and C, F, I, L, and O correspond to the corpus mucosa. The morphology is presented by hematoxylin and eosin staining of uninfected (A–C) and infected (G–I) gerbil gastric tissue. The corresponding regions were immunostained with monoclonal H1 antibodies and detected with a brown chromogen. D–F represent unifected gerbils and J–L represent Helicobacter pylori infected mucosa. Expression of H1 antigen was found in D–F (ie, uninfected mucosa). No staining or faint staining of H1 was seen in J–L (ie, H. pylori–infected mucosa). H. pylori bacteria cells were detected with α–H. pylori polyclonal antibody and stained brown (M–O). Enlargement of areas with H. pylori staining are inserted in figures M–O. Scale bars represent 50 μm.
Binding of TN2GF4 to 125I-Leb and H1 receptor conjugate was measured. The BabA-expressing strain, 17875/Leb, which exhibits receptor-binding activity to both Leb and H1, was used as a positive control. Strains TN2GF4 and 17875/Leb had binding activity that was almost identical to that of the H1 antigen, but the Leb-binding activity was higher in strain 17875/Leb (Figure 3A). H1-binding affinity was measured according to Scatchard and showed that TN2GF4 (Ka = 8.06E + 10) had 7-fold lower H1-binding affinity than did 17875/Leb (Ka = 5.36E + 11) (Figure 3B).
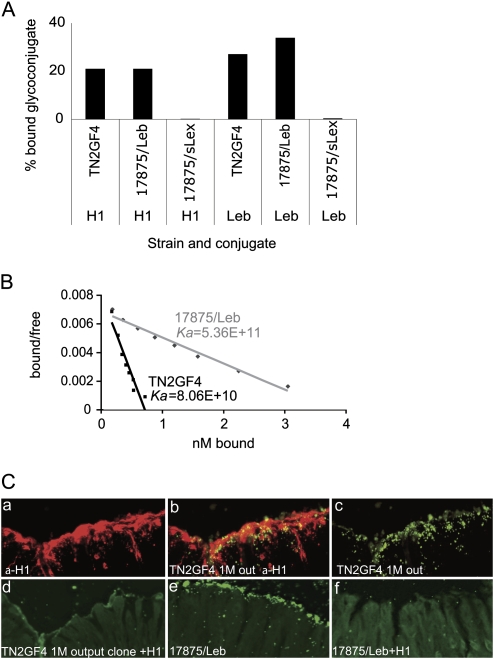
Figure 3.
A and B show Leb- and H1-receptor binding properties of strain TN2GF4. A, One representative set of 3 independent radioimmunoassay measurements of Leb- and H1-binding activity are presented as percentage bound glycoconjugate for strains TN2GF4 (input), 17875/Leb, and 17875/sLex (negative control). B, Receptor-binding affinity conjugate was measured according to Scatchard. The slope of the curve represents the H1 receptor-binding affinity. C, Adherence of output TN2GF4 to Mongolian gerbil gastric mucosa. Tissue sections of Mongolian gerbils gastric mucosa 1 month after infection were incubated with: (a) α-H1 monoclonal antibody and fluorochrome Alexa 488 for detection of H1 expression; (b) co-localization of FITC-labelled bacteria cells of a 1-month output TN2GF4 (BabA+) clone isolated from the corresponding gerbil and H1 expression; (c) FITC-labelled bacteria cells of a 1-month output TN2GF4 (BabA+) clone isolated from the corresponding gerbil; (d) FITC-labelled bacterial cells of the 1-month output TN2GF4 clone that were pre-incubated with H1 prior to incubation with the tissue sections or FITC-labelled bacterial cells of strain 17875/Leb; (e) FITC-labelled bacterial cells of strains 17875/Leb; (f) FITC-labelled bacterial cells of strains 17875/Leb that were pre-incubated with H1 prior to incubation with the tissue sections.
To study adherence of TN2GF4 to the gerbil gastric mucosa, bacterial cells were applied to gerbil gastric tissue sections. We observed binding of TN2GF4 to the regions where the α-H1 monoclonal antibody detected the H1 antigen (Figure 3C). Preincubation with H1-conjugate prior to application of TN2GF4 to the tissue sections abolished binding (Figure 3C–D). Also, strain 17875/Leb bound to the gerbil gastric mucosa and, similar to TN2GF4 binding, could be inhibited with H1 receptor conjugate (Figure 3C–F). In contrast, TN2GF4 exhibited vivid binding to human gastric mucosa, and the BabA-non-expressing out-put clone was devoid of binding (data not shown). In summary, TN2GF4 exhibit binding to the Mongolian gerbil gastric mucosa via the H1 antigen.
Expression of the Inflammation-Associated sLex Antigen in Mongolian Gerbil Tissue
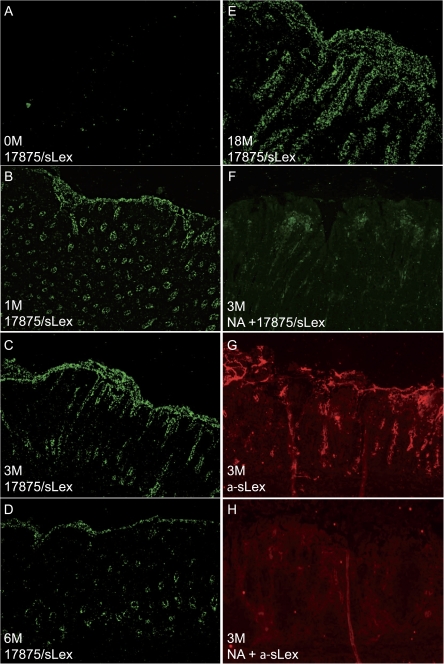
The sLex antigen is only expressed in minute levels in healthy gastric mucosa, but expression increases upon inflammation in both humans and primates [8, 26]. We analyzed the ability of H. pylori SabA adhesin to recognize and bind sLex in gerbil tissue. Because strain TN2GF4 is devoid of SabA expression, the SabA-expressing/BabA-non-expressing strain 17875/sLex was used as a marker for sLex. Fluorescently labelled 17875/sLex was applied to gerbil gastric tissue sections, and binding was measured in gerbils infected for 1, 3, 6, and 18 months (Figure 4B–E). Only background staining was detected in healthy gerbil gastric tissue (Figure 4A). Tissue sections corresponding to inflamed gastric mucosa were treated with neuraminidase, which cleaves sialic acid moieties and thus abolishes SabA-mediated H. pylori binding (Figure 4F). sLex expression was also detected with monoclonal antibodies directed against sLex in infected gerbils, whereas only background staining was detected in healthy gerbil gastric tissue or after neuraminidase treatment (Figure 4G and 4H). Thus, Mongolian gerbils exhibit changes in sialylation that are similar to those exhibited by humans and primates, and H. pylori binds specifically to sialic acid present in inflamed gastric gerbil mucosa. The host response and changes in glycosylation during infection demonstrate that gerbils constitute a relevant model for H. pylori infection and host inflammatory responses.
Figure 4.
Expression of sialyl-Lewis x (sLex) antigen in inflamed gerbil gastric mucosa and Helicobacter pylori adherence. sLex expression in gerbil mucosa was probed with either sLex monoclonal antibody or with the SabA-expressing H. pylori strain 17875/sLex. A, Uninfected gerbil gastric tissue overlaid with FITC-labelled 17875/sLex. B–E, Gerbil mucosa obtained at 1, 3, 6, and 18 months after inoculation and probed with strain 17875/sLex. F, Neuraminidase-treated gerbil tissue sections obtained at 1 month after infection and incubated with 17875/sLex. G, gerbil gastric mucosa obtained 1 month after infection and detected with monoclonal sLex antibodies and Alexa 568. H, Neuraminidase-treated gerbil tissue sections obtained 1 month after infection and incubated with sLex monoclonal antibodies and Alexa 568.
Re-inoculation of Output Strains Recovered from Gerbils
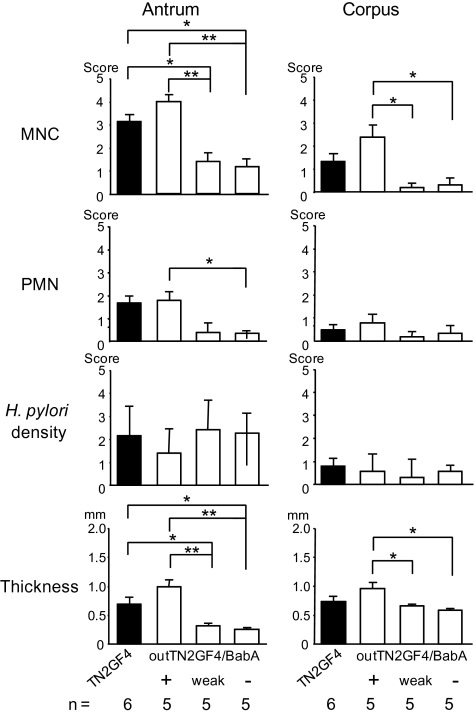
As shown above, there appears to be an inverse relationship between the degree of cellular inflammation and the expression of BabA in H. pylori–infected gerbils. All colonies isolated from gerbils with gastric ulcers were devoid of BabA expression. To further investigate the relationship between BabA and disease outcome, we challenged gerbils with 3 output clones of different BabA phenotypes: a BabA-expressing clone (outTN2GF4/BabA+), a BabA low-expressing clone (outTN2GF4/BabA-weak), and a BabA nonexpressing clone (outTN2GF4/BabA-). Gerbils were sacrificed at 1 and 3 months after infection. All strains infected gerbils, as confirmed by culture, histological findings, and/or PCR. No histological differences were observed among strains by 1 month (data not shown). By 3 months, MNC infiltration and mucosal thickness were significantly reduced in the outTN2GF4/BabA-weak and outTN2GF4/BabA- challenge groups, compared with the outTN2GF4/BabA+ group (Figure 5). Cellular infiltration and mucosal thickness were slightly but not significantly increased in the outTN2GF4/BabA+ challenge group, compared with the TN2GF4 group (Figure 5). In contrast, H. pylori density was similar, irrespective of the group at both 1 and 3 months. These data show that BabA plays a role, either directly or indirectly, in inducing cellular inflammation.
Figure 5.
Cellular inflammation and mucosal thickness in gastric mucosa of gerbils re-inoculated with recovered Helicobacter pylori 3 months after infection. Polymorphonuclear cell (PMN), mononuclear cell (MNC), and H. pylori density scores were graded from 0 (absent/normal) to 5 (maximal intensity) in corpus and antrum Mongolian gerbil tissue 3 months after inoculation. Mucosal thickness was measured in millimeters. Data are presented as mean values ± standard errors. *P <.05; **P <.01.
None of the colonies recovered from the outTN2GF4/BabA- challenge group expressed detectable levels of BabA at either 1 or 3 months (Table 2), and none exhibited genotypic changes in the babA gene, compared with the inoculated outTN2GF4/BabA- strain (data not shown). All colonies recovered from the outTN2GF4/BabA-weak challenge group at 1 month and 25 (93%) of 27 colonies recovered at 3 months exhibited faint BabA expression, similar to that of inoculated outTN2GF4/BabA-weak strains. Two colonies from 1 gerbil at 3 months did not exhibit detectable levels of BabA, and sequence analysis showed that both colonies had an insertion within the babA gene (insertion of C at position 233), confirming that the loss of BabA expression was caused by frame-shift nucleotide changes in the babA gene. Thus, there is a difference in down-regulation of BabA between high-expressing output clones and weak-expressing output clones. All 1-month weak-output clones and almost all 3-month weak-output clones expressed BabA, in contrast to the high BabA–expressing clones, for which the down-regulation of BabA was pronounced after only 1 month. For the outTN2GF4/BabA+ challenge group, results were similar to those for the first round of infection; BabA expression decreased with time after infection, whereas inflammation increased (Table 2). There were no significant changes in OipA, AlpAB, BabB, or SabA expression levels among recovered colonies, irrespective of the group (data not shown).
Table 2.
Blood Group Antigen–Binding Adhesin (BabA) Expression in Output Strains Recovered from the Second Route of Infection
| Group | Time, months | Gerbil no. | No. of colonies studied | BabA+, no. (%) of colonies | BabA-weak, no. (%) of colonies |
| BabA+ | 1 | 1-1P | 24 | 24 (100) | 0 |
| 1 | 1-2P | 12 | 12 (100) | 0 | |
| 1 | 1-3P | 5 | 4 (80) | 0 | |
| 1 | 1-4P | 7 | 3 (43) | 0 | |
| 1 | 1-5P | 7 | 2 (29) | 0 | |
| 3 | 3-1P | 20 | 3 (15) | 0 | |
| 3 | 3-2P | 9 | 0 | 0 | |
| 3 | 3-3P | 5 | 0 | 0 | |
| 3 | 3-4P | 1 | 0 | 0 | |
| 3 | 3-5P | 13 | 0 | 0 | |
| BabA-weak | 1 | 1-1W | 10 | 0 | 10 (100) |
| 1 | 1-2W | 10 | 0 | 10 (100) | |
| 1 | 1-3W | 9 | 0 | 9 (100) | |
| 1 | 1-4W | 7 | 0 | 7 (100) | |
| 1 | 1-5W | 1 | 0 | 1 (100) | |
| 1 | 1-6W | 5 | 0 | 5 (100) | |
| 1 | 1-7W | 3 | 0 | 3 (100) | |
| 3 | 3-1W | 10 | 0 | 8 (80) | |
| 3 | 3-2W | 10 | 0 | 10 (100) | |
| 3 | 3-3W | 2 | 0 | 2 (100) | |
| 3 | 3-4W | 5 | 0 | 5 (100) | |
| 3 | 3-5W | No colonies recovered | |||
| BabA- | 1 | 1-1N | 6 | 0 | 0 |
| 1 | 1-2N | 5 | 0 | 0 | |
| 1 | 1-3N | 2 | 0 | 0 | |
| 1 | 1-4N | 3 | 0 | 0 | |
| 1 | 1-5N | 3 | 0 | 0 | |
| 3 | 3-1N | 10 | 0 | 0 | |
| 3 | 3-2N | 5 | 0 | 0 | |
| 3 | 3-3N | 5 | 0 | 0 | |
| 3 | 3-4N | 3 | 0 | 0 | |
| 3 | 3-5N | 5 | |||
NOTE. Helicobacter pylori clones used for infection, time of infection, name of gerbil, number of colonies studied from each gerbil, and percentage of the studied colonies with high and weak BabA expression are given in the table.
DISCUSSION
Our data demonstrated that BabA-expressing H. pylori caused severe injury to gastric mucosa, whereas BabA low-expressing or nonexpressing strains caused only mild inflammation. This outcome suggests that high BabA expression is associated with severe inflammatory response. Both BabA expression and cognate H1-receptor binding activity disappeared during the course of infection in correspondence with severe inflammation. Whether BabA expression results in a survival disadvantage in the gerbil stomach because of close contact to the gastric epithelium and exposure to the host response or whether loss of BabA is a result of gene products not required for growth or survival, because its natural receptor diminishes in response to inflammation, is not yet known. H1 was expressed in uninfected mucosa and decreased in inflamed mucosa. Down-regulation of H1 expression in the gerbil stomach was analogous to human H1 expression, which progressively decreases during progression to gastric metaplasia and gastric carcinoma [27].
BabA exhibit higher binding affinity to Leb antigen than to the H1 antigen [7, 9]. No Leb was found in the gerbil gastric mucosa, but we demonstrated that H. pylori TN2GF4 exhibited H1 binding to gerbil epithelium up to 3 months after infection. However, H. pylori TN2GF4 colonized gerbils for 6 and 18 months. Gastric mucosal tissue sections derived from these gerbils did not express H1, suggesting that additional adhesins are involved in colonization. We show that, in a manner similar to that for humans, expression of sLex increases in the gerbil gastric mucosa in response to inflammation. Strain TN2GF4 did not express the SabA adhesin, but we showed that an additional SabA-expressing H. pylori strain mediated binding to the cognate sLex receptor. These similarities in host response between human and gerbils emphasize that gerbils are a relevant model for H. pylori infection and host responses. Additional studies will clarify whether, in addition to H1 and sLex, additional carbohydrate moieties are involved in the adherence of H. pylori to gerbil gastric mucosa. Similarly, mapping of carbohydrate moieties present on gerbil gastric epithelial cells may identify additional glycoproteins or glycolipids that function as receptors.
Infection of BabA nonexpressing strains in gerbils was associated with reduced inflammation and suggests that loss of BabA expression and H1 binding ability observed in inflamed gerbil mucosa were coincidental. Importantly, the loss of BabA expression in inflamed gerbil mucosa was similar to that observed in experimentally infected primates [14]. In humans, expression of BabA has been associated with severe mucosal cellular inflammation and an increased risk of peptic ulcer disease and gastric cancer [11–13]; whereas patients infected with BabA-nonexpressing strains present with a higher level of mucosal injury than do patients infected with BabA-expressing strains [11]. However, it is not known how long these patients had been infected, how far the disease had progressed, or the level of BabA expression during infection. In this context, we found it highly interesting that down-regulation of BabA in BabA-high expressing strain occurred after only 1 month of infection, in contrast to findings for the BabA-weak expressing strains, a majority of which still expressed BabA after 3 months. Clones with weak BabA expression may avoid close contact with the epithelium and thereby avoid exposure to the host bactericidal response and maintenance of BabA expression.
In Rhesus monkeys and mice, loss of BabA occurred via SSM mechanisms or by babA and babB homologous recombination [14, 15]. The babA gene in strain TN2GF4 does not contain repetitive nucleotide sequences, which are required for SSM. Here, BabA down-regulation was attributable either to point mutations, deletions, or insertions that lead to truncation. Although rare, a similar observation has been reported for human H. pylori isolates [28]. Homologous recombination of bab genes has been observed in clinical human isolates but at a low frequency [22, 29, 30], and it has also recently been reported as a likely mechanism for generating Leb-nonbinding babA output clones of gerbils [15]. We did not find such bab genes in the gerbil output clones, but because recombination occurs at low frequencies, it cannot be excluded that such allelic variants are present in the output populations.
In conclusion, BabA expression is not likely to be essential for colonization, but the elicited gerbil host response confirms a role for BabA adhesin as a virulence factor and its impact in the induction of a severe inflammatory response. The loss of BabA expression in inflamed gerbil mucosa was similar to that seen in humans and primates, and the fact that gerbils exhibit responses, such as changes in the glycosylation pattern and H. pylori receptor, that are similar to those in humans and Rhesus monkeys demonstrates the relevance of the gerbil model for studying the effects of adherence on H. pylori infection and host responses.
Supplemental Material
Supplementary data are available at http://www.oxfordjournals.org/our_journals/jid/online.
Funding
The Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center, and National Institutes of Health grant number DK 62813 (to Y.Y.), Vetenskapsrådet (VR/M) and Cancerfonden (to A.A).
Acknowledgments
We thank Dr Masafumi Nakao (Osaka, Japan), for providing H. pylori strain TN2GF4, and Drs Rainer Haas and Stefan Odenbreit (Munich, Germany), for providing αBabA, αBabB, and αSabA.
References
- 1.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31(Suppl 9):24–8. [PubMed] [Google Scholar]
- 3.Ikeno T, Ota H, Sugiyama A, et al. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–60. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura K, Maeda S, Nakao M, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–10. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–8. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 6.Guruge JL, Falk PG, Lorenz RG, et al. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci U S A. 1998;95:3925–30. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilver D, Arnqvist A, Ögren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 8.Mahdavi J, Sondén B, Hurtig M, et al. Helicobacter pylori SabA adhesin in persistent infection chronic inflammation. Science. 2002;297:573–8. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspholm-Hurtig M, Dailide G, Lahmann M, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–22. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 10.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–5. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto S, Olaniyi OO, Arnqvist A, et al. Helicobacter pylori BabA expression, gastric mucosal injury, clinical outcome. Clin Gastroenterol Hepatol. 2007;5:49–58. doi: 10.1016/j.cgh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka Y, Kikuchi S, El-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–24. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 13.Yamaoka Y, Ojo O, Fujimoto S, et al. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775–81. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solnick JV, Hansen LM, Salama NR, Boonjakuakul JK, Syvanen M. Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:2106–11. doi: 10.1073/pnas.0308573100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Styer CM, Hansen LM, Cooke CL, et al. Expression of the BabA adhesin during experimental infection with Helicobacter pylori. Infect Immun. 2010;78:1593–600. doi: 10.1128/IAI.01297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaoka Y, Kita M, Kodama T, et al. Helicobacter pylori infection in mice: role of outer membrane proteins in colonization and inflammation. Gastroenterology. 2002;123:1992–2004. doi: 10.1053/gast.2002.37074. [DOI] [PubMed] [Google Scholar]
- 17.El-Zimaity HM, Graham DY, Al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 18.Kudo T, Nurgalieva ZZ, Conner ME, et al. Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J Clin Microbiol. 2004;42:2279–81. doi: 10.1128/JCM.42.5.2279-2281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odenbreit S, Kavermann H, Puls J, Haas R. CagA tyrosine phosphorylation and interleukin-8 induction by Helicobacter pylori are independent from AlpAB, HopZ and bab group outer membrane proteins. Int J Med Microbiol. 2002;292:257–66. doi: 10.1078/1438-4221-00205. [DOI] [PubMed] [Google Scholar]
- 20.Walz A, Odenbreit S, Mahdavi J, Borén T, Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700–8. doi: 10.1093/glycob/cwi049. [DOI] [PubMed] [Google Scholar]
- 21.Aspholm M, Kalia A, Ruhl S, et al. Helicobacter pylori adhesion to carbohydrates. Methods Enzymol. 2006;417:293–339. doi: 10.1016/S0076-6879(06)17020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbeck JC, Hansen LM, Fong JM, Solnick JV. Genotypic profile of the outer membrane proteins BabA BabB in clinical isolates of Helicobacter pylori. Infect Immun. 2006;74:4375–8. doi: 10.1128/IAI.00485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pride DT, Meinersmann RJ, Blaser MJ. Allelic Variation within Helicobacter pylori babA and babB. Infect Immun. 2001;69:1160–71. doi: 10.1128/IAI.69.2.1160-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheu BS, Sheu SM, Yang HB, Huang AH, Wu JJ. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut. 2003;52:927–32. doi: 10.1136/gut.52.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudo T, Lu H, Wu JY, et al. Pattern of transcription factor activation in Helicobacter pylori-infected Mongolian gerbils. Gastroenterology. 2007;132:1024–38. doi: 10.1053/j.gastro.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linden S, Borén T, Dubois A, Carlstedt I. Rhesus monkey gastric mucins: oligomeric structure, glycoforms and Helicobacter pylori binding. Biochem J. 2004;379(Pt 3):765–75. doi: 10.1042/BJ20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HS, Choe G, Kim WH, Kim HH, Song J, Park KU. Expression of Lewis antigens and their precursors in gastric mucosa: relationship with Helicobacter pylori infection and gastric carcinogenesis. J Pathol. 2006;209:88–94. doi: 10.1002/path.1949. [DOI] [PubMed] [Google Scholar]
- 28.Hennig EE, Allen JM, Cover TL. Multiple chromosomal loci for the babA gene in Helicobacter pylori. Infect Immun. 2006;74:3046–51. doi: 10.1128/IAI.74.5.3046-3051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bäckström A, Lundberg C, Kersulyte D, Berg DE, Borén T, Arnqvist A. Metastability of Helicobacter pylori bab adhesin genes and dynamics in Lewis b antigen binding. Proc Natl Acad Sci U S A. 2004;101:16923–8. doi: 10.1073/pnas.0404817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pride DT, Blaser MJ. Concerted evolution between duplicated genetic elements in Helicobacter pylori. J Mol Biol. 2002;316:629–42. doi: 10.1006/jmbi.2001.5311. [DOI] [PubMed] [Google Scholar]