Abstract
Background. Male circumcision reduces human immunodeficiency virus (HIV) and herpes simplex virus type 2 (HSV-2) acquisition, and HSV-2 infection is associated with an increased risk of HIV acquisition. To assess the cellular basis for these associations, we estimated immunologic cellular densities in foreskin tissue.
Methods. Immunostained CD1a+ dendritic cell and CD4+ and CD8+ T cell densities were quantified in foreskin samples obtained from medical circumcision in Rakai, Uganda (35 HIV-infected, HSV-2-infected men; 5 HIV-infected, HSV-2-uninfected men; 22 HIV-uninfected, HSV-2-infected men; and 29 HIV-uninfected, HSV-2-uninfected men.
Results. CD1A+ dendritic cell densities did not vary by HIV or HSV-2 status. Compared with densities in HIV-uninfected, HSV-2-uninfected men (mean, 26.8 cells/mm2), CD4+ T cell densities were similar in the HIV-infected, HSV-2-infected group (mean, 28.7 cells/mm2), were significantly decreased in the HIV-infected, HSV-2-uninfected group (mean, 11.2; P < .05), and were increased in the HIV-uninfected, HSV-2-infected group (mean, 68.7; P < .05). Dermal CD8+ T cell densities were higher in the HIV and HSV-2-coinfected group (mean, 102.9) than in the HIV-uninfected, HSV-2-uninfected group (mean, 10.0; P < .001), the HIV-infected, HSV-2-uninfected group (mean, 27.3; P < .001), and the HIV-uninfected, HSV-2-infected group (mean, 25.3; P < .005).
Discussion. The increased CD4+ cellular density in the HIV-uninfected, HSV-2-infected men may help to explain why HSV-2–infected men are at increased risk of HIV acquisition. The absence of this increase in men coinfected with both HIV and HSV-2 is likely in part the result of the progressive loss of CD4+ cells in HIV infection. Conversely, HIV and HSV-2 coinfection appears to synergistically increase CD8+ T cell densities.
Three clinical trials in Africa have demonstrated that adult male circumcision can decrease acquisition of human immunodeficiency virus (HIV), and two of these trials have also shown that circumcision decreases acquisition of herpes simplex virus type 2 (HSV-2) [1–5]. However, the mechanisms for this protective effect are not fully understood. One proposed mechanism is that the foreskin contains high numbers of HIV target cells, increasing susceptibility to infection, and it is possible that the foreskin cellular density may vary depending on the presence of sexually transmitted infections (STIs), such as HSV-2 [6–9].
Multiple epidemiological studies report a higher prevalence and incidence of HIV infection among HSV-2–infected populations [10–12]. These studies led to the hypothesis that infection with HSV-2 could increase HIV infectivity and/or susceptibility by increasing the density of HIV-infected or -susceptible target cells at sites of herpetic ulcerations and that treatment with anti–HSV-2 medication might decrease HIV transmission and acquisition [10–12]. This hypothesis was tested in three large trials of HSV-2 suppressive therapy, and it was found that HSV-2 suppression had no significant effect on HIV acquisition or transmission [13–15]. The negative trial outcomes could be explained by persistent high levels of HIV-susceptible lymphocytes at sites of prior herpetic lesions but not at unaffected areas [8, 16].
There are limited data regarding the cellular immunologic composition of the foreskin of HIV-1– and HSV-2–infected men [7, 17]. With use of foreskin samples from two circumcision trials in Rakai, Uganda, both HIV and HSV-2 infection were shown to be associated with foreskin inflammation. In this study, HIV and HSV-2 coinfection was associated with the most dramatic increase in inflammation, and this inflammation was primarily focal [9]. An improved understanding of the lymphocytic and dendritic cellular composition of this inflammation in the foreskin may provide insight into the association between HIV-1 and HSV-2 and the protective mechanisms of circumcision. Therefore, the densities of CD1A+ dendritic cells and CD4+ and CD8+ T lymphocytes were assessed quantitatively in the foreskins of men with and without HIV and HSV-2 infection in Rakai, Uganda [3, 18]
MATERIALS AND METHODS
Study Population
The Rakai circumcision trials are described in detail elsewhere [3, 18]. In brief, uncircumcised men aged 15–49 years were enrolled in two trials of male circumcision for HIV infection and STI prevention. One trial evaluated the effect of circumcision on the risk of HIV acquisition among initially HIV-uninfected men [3]. The second trial evaluated the effect of circumcision of HIV-infected men on transmission of HIV to their uninfected female partners [18]. Uncircumcised HIV-infected men were eligible if they had a CD4 cell count ≥350 cells/mm3, no active STIs, and no history of AIDS-defining illness. Men were randomized to either the intervention arm, which received immediate circumcision, or the control arm, in which circumcision was delayed for 24 months. Information on sociodemographic characteristics, sexual risk behaviors, hygiene practices, symptoms and signs of genitourinary infection, and physical examimations were collected at enrollment and follow-up visits at 6, 12, and 24 months. Men scheduled for surgery who had visible signs of lesions were treated, and circumcision was delayed until the lesions were healed. Foreskin tissue samples obtained at circumcision were selected at random for this study [9]. The trials were approved by four institutional review boards: the Science and Ethics Committee of the Uganda Virus Research Institute (Entebbe, Uganda), the HIV subcommittee of the National Council for Research and Technology (Kampala, Uganda), the Committee for Human Research at Johns Hopkins University Bloomberg School of Public Health (Baltimore, MD), and the Western Institutional Review Board (Olympia, WA). The trials were overseen by two independent Data Safety Monitoring Boards and were registered with ClinicalTrials.Gov (NCT00425984 and NCT00124878). All participants consented to use of left-over foreskin tissue for research.
Histopathology
Foreskins were fixed in 100% ethanol and stored at −80°C [9]. Samples of foreskins were systematically obtained by unrolling the tissue and exposing the epithelial surface. Foreskins with visible scars or abnormal tissue, such as discoloration or nodules, were excluded. Cross-sections from the epithelial to subepithelial connective tissue, spaced roughly equally across the tissue surface, were cut with a razor blade into ∼1-cm thick sections. This technique was used to obtain tissue from a variety of areas of the foreskin, including the internal and external surface. A minimum of three sections per subject specimen were taken and embedded in paraffin blocks. Five-micrometer–thick paraffin sections were cut using a microtome and mounted without pretreatment.
An ordinal scoring system of inflammatory infiltrates was used to determine inflammation status, as described elsewhere [9]. Scores were assigned for epidermal and dermal compartments separately, and the observers were blinded to all clinical data, including HIV and HSV-2 serostatus.
Immunohistochemistry
Standard immunohistochemistry protocol was applied to the unstained 5-μm sections of tissue. In brief, primary antibody incubation was performed at 37oC for 15 min with optimized antibody dilutions. Primary monoclonal antibodies to CD1a (clone 220; NovaCastra), CD4 (clone IF6; NovaCastra), and CD8 (clone C8-144B; Ventana) were used for identification of CD1a+ dendritic cells and CD4+ and CD8+ T cells in the foreskin tissue. Horseradish-peroxidase–labeled secondary antibodies were optimized using positive and negative controls. The primary-secondary antibody complex was revealed with the chromogens napthol and fast red and counterstained with hematoxylin. This process produced a bright red precipitate, allowing for visualization of positively stained cellular populations with use of light microscopy. Slides were washed to remove any unbound reagent. All staining was performed on an automated Leica Bond-maX staining system (Mount Waverly). Immunostains were included in the analysis only if there was positive staining of control lymph node sections per staining round.
QUANTIFICATION OF CELLULAR POPULATIONS
All slides were digitally scanned at 20× magnification with use of a ScanScope CS slide scanner (Aperio Technologies). With use of custom software, 10 fields of uniform size (450 μm2) were selected using a low power view of the entire slide image, which precluded visualization of any gross cell formations, such as inflammatory foci when selecting fields (Custom software; image_selection tool, and Count_Macro for ImageJ; see supplementary materials). The areas of tissue were selected at intact epidermal-dermal junctions with use of the four compass points as a guide. Images of these fields were then evaluated by two independent technicians masked to all subject data. Technicians first estimated percent coverage of epidermis and dermis across each captured field in 5% increments and then counted positively staining cells at approximately full 20× magnification of the field as captured from scanscope for the epidermis or dermis separately (Figure 1). The counting of cell types was based on localization in the tissue (epidermis vs dermis) and cell morphology. Positively staining cells were identified by the presence of a clearly visualized nucleus and distinct cytoplasmic staining with red chromogen for CD4+ and CD8+ cells. Because of the larger size and arborized structure of the CD1a+ dendritic cells, cells without visible nuclei but displaying a distinct dendritic morphology were also counted. To obtain a total cell density for each tissue compartment, the intra-compartmental cell counts for all 10 images were added and divided by the total area of the epidermis or dermis estimated from the proportion of epidermis or dermis in the image. This was done for all 10 images, and the results were multiplied by 450 μm2 (the fixed area for each image), yielding the cell density estimate.
Figure 1.
Representative images used for cell density quantification are shown for immunohistochemical stains CD1a (A, B), CD4 (C, D), and CD8 (E, F) with positively staining cells in red (20× magnification, as captured by Scanscope). The images are from different men who were HIV-uninfected, HSV-2–uninfected (A), HIV-infected, HSV-2–uninfected (B), HIV-uninfected, HSV-2–infected (D), or HIV–HSV-2–coinfected (C, E, F). The surface area percentages of epidermal and dermal compartments were estimated for each image in increments of 5%. The numbers of cells were then counted according to the presence of a stained cell with a clearly defined nucleus. The CD1A stained cells with distinctive dendritic morphology were also counted if no nucleus was present. The top row (A, C, E) demonstrates a moderate level of cell density for each cell type. Higher CD1A density is also shown (B). The presence of a focus (arrow) of positively staining CD4 (D) or CD8 (F) cells in the image was also recorded.
In addition, a CD4+ and CD8+ cell focus was defined as a group of ≥20 positively staining cells in close proximity (Figure 1D and 1F). The number of focus-containing images was divided by the total examined tissue surface area to estimate the focal density for each subject.
HIV and HSV-2 Detection
HIV status at enrollment was determined using two separate enzyme-linked immunosorbent assays (Vironostika HIV-1 [Organon Teknika] and Welcozyme HIV 1 + 2 [Murex Diagnostics]). Discordant results and HIV seroconversion was confirmed by Western blot, as described elsewhere (Cambridge Biotech HIV-1 Western blot; Caltype Biomedical) [3]. HSV-2 serostatus was determined using an IgG glycoprotein enzyme immunoassay (Kalon Biological), as described elsewhere [4].
Statistical Analysis
Population characteristics in the HIV-infected and uninfected groups were compared using χ2 and Fisher exact tests. The distributions of cell density and inter-subject variability were first explored by histograms and normal quantile plots. A linear regression was then used to model the relationship between cell density and predictor variables, including HIV and HSV-2 infection status, presence of inflammation, and other potential confounders (eg, age, education level, occupation, number of sex partners during the previous year, and condom use). All exposure groups were compared using an unadjusted pairwise comparison model that incorporated the repeated measurements of the two observers. The repeated measurements from the two independent counters were modeled by multivariate linear regression assuming a compound symmetry structure on the variance-covariance matrix in each infection group. For illustrative purpose, the figures show only one observer's counts, and all group differences and P values presented are estimated from the model using repeated measurements of the two observers. Models were built for each cell type in each tissue compartment, and 2-sided P values were reported. All the regression analyses were performed using SAS PROC glimmix (SAS Institute). Correlation analyses were performed using the Spearman rank order test, because the focal inflammation data were not continuous. Differences between cell densities according to the presence of inflammation in each infection group were analyzed using the Mann-Whitney rank sum test.
RESULTS
Characteristics of HIV-Infected and Uninfected Participants
Foreskin samples from a random sample of 40 HIV-infected and 51 HIV-uninfected men were used for this study (Table 1) [3, 9, 18]. Compared with HIV-uninfected men, HIV-infected men were more likely to have HSV-2 coinfection (P = .003) and to have higher rates of dermal inflammation in the foreskin (P = .009) [9]. HIV-infected men were also older (P < .001) and had higher rates of condom use (P = .014) (Table 1). There were no differences between HIV-infected and uninfected men with regard to symptoms of urethral discharge, dysuria, and genital ulcers at the time of circumcision (Table 1).
Table 1.
Demographic characterisitics of the study population.
| Characteristic | HIV-uninfected |
HIV-infected |
|||
| n=51 | Percentage of total | n=40 | Percentage of total | P | |
| HSV-2 infection status | |||||
| Positive | 22 | 43.1 | 35 | 87.5 | .003 |
| Presence of inflammation | |||||
| Epidermis | 2 | 3.9 | 7 | 17.5 | .040 |
| Dermis | 8 | 15.7 | 17 | 42.5 | .009 |
| Currently married | 30 | 58.8 | 24 | 60 | .919 |
| Age, years | |||||
| 15–24 | 25 | 49 | 1 | 2.5 | <.001 |
| 25–34 | 14 | 27.5 | 23 | 57.5 | |
| >35 | 12 | 23.5 | 16 | 40 | |
| No. of sex partners in past year | |||||
| 0 | 8 | 15.7 | 6 | 15 | .167 |
| 1 | 27 | 52.9 | 14 | 35 | |
| ≥2 | 16 | 31.4 | 20 | 50 | |
| Condom use in past year | |||||
| No | 26 | 56.5 | 9 | 26.5 | .014 |
| Yes | 20 | 43.5 | 25 | 73.5 | |
| Highest education level | |||||
| Primary | 44 | 86.3 | 34 | 85 | .897 |
| Secondary | 7 | 13.7 | 6 | 15 | |
| Occupation | |||||
| Agriculture | 37 | 72.5 | 17 | 42.5 | .014 |
| Waged and student | 7 | 13.7 | 10 | 25.0 | |
| Other | 7 | 13.7 | 13 | 32.5 | |
| Sexual hygiene | |||||
| Wash after sex | 41 | 80.4 | 36 | 90 | .333 |
| Self-reported STI | |||||
| Urethral discharge | 0 | 0 | 0 | 0 | |
| Dysuria | 0 | 0 | 0 | 0 | |
| Genital ulcer | 0 | 0 | 1 | 2.5 | .440 |
NOTE. aTotals are for men who answered question with yes including any condom use over the past year.
CD1a+, CD4+, and CD8+ T Cell Densities in HIV- and HSV-2–Infected Foreskins
CD1a+ cells were found almost exclusively in the epidermis, and CD4+ cells were found predominantly in the dermis. CD8+ cells were present in both tissue compartments (Figure 1). Only tissue compartments containing each cell population of interest were examined and included in the subsequent analyses. The inter-observer cell density estimates for each cell type were highly correlated (Spearman correlation coefficient; r = .927–.973, P < .0001). Dermal CD4+ cell density was significantly correlated with dermal CD8+ cell density (Spearman correlation; r = .24, P < .05).
CD1A+ epidermal cell density did not differ by HIV or HSV-2 status (Figure 2A). However, CD4+ dermal cell density was significantly lower in the HIV-infected, HSV-2–uninfected population (mean ± standard deviation [SD], 11.2 ± 14.0 cells/mm2) than in HIV-uninfected, HSV-2–uninfected men (26.8 ± 9.08 cells/mm2; P = .029) and HIV-uninfected, HSV-2–infected men (68.7 ± 39.4 cells/mm2; P = .005) (Figure 2B). The CD4+ cell density was also significantly higher in the HIV-uninfected, HSV-2–infected group than in the HIV-uninfected, HSV-2–uninfected group (P = .037) and the HIV–HSV-2–coinfected group, with borderline statistical significance (28.7 ± 17.3 cells/mm2; P = .055) (Figure 2B).
Figure 2.
Cell densities stratified by HIV and HSV-2 infection status. Foreskin epidermal CD1A (A), dermal CD4 (B), epidermal and dermal CD8 (C, D) cell densities are shown according to neither infection (none), HIV-1 infection alone, HSV-2 infection alone, or HIV–HSV-2–coinfection. One counter's readings are shown. Mean cell density is shown with 95% confidence intervals. All pairwise comparisons between groups were performed using model that incorporated the repeated measurements of two technician counters, with significant differences indicated (P < .05).
The epidermal and dermal CD8+ cell densities were highest in the HIV–HSV-2–coinfected group (epidermal mean ± SD , 61.2 ± 29.0 cells/mm2; dermal mean ± SD, 102.9 ± 39.4 cells/mm2), which were significantly higher than in the other 3 groups (Figure 2C and 2D). In addition, the HIV-uninfected, HSV-2–infected group had significantly higher epidermal CD8 cell density (mean ± SD, 28.9 ± 24.2 cells/mm2) than did the HIV-infected, HSV-2–uninfected group (7.9 ± 20.9 cells/mm2; P = .036) and higher dermal density (25.3 ± 13.6 cells/mm2) than did the HIV-uninfected, HSV-2–uninfected group (10.0 ± 5.6 cells/mm2; P = .028) (Figure 2C and 2D).
Multivariate linear regression was used to estimate adjusted differentials in dermal CD4+ and CD8+ cell densities associated with HIV and HSV-2 infection status (Table 2). After adjusting for age and inflammation status, HIV-infected, HSV-2–uninfected individuals had significantly lower CD4+ cell densities than did the HIV-uninfected, HSV-2–uninfected population (P = .009) and HIV-uninfected, HSV-2–infected individuals (P = .005). The HIV–HSV-2–coinfected group also had lower CD4+ density than did the HIV-uninfected, HSV-2–infected group (P = .050) (Table 2). The presence of dermal inflammation was associated with an increase in CD4+ cell density. CD8+ cell densities were significantly increased in the coinfected group, compared with the other three groups (Table 2). The presence of histological inflammation was not significantly associated with increased dermal CD8+ cell density (Table 2). The dermal cell densities of CD4+ and CD8+ were not associated with other parameters that associated with differences in HIV infection prevalence, such as age, marital status, education, occupation, sexual hygiene, condom use, or number of sex partners during the past year.
Table 2.
Multivariate fixed effects model results for differences in CD4 and CD8 dermal cell densitya
| Variable | CD4 Dermal Cell Density |
CD8 Dermal Cell Density |
||||
| Infection Status | Mean | 95% Confidence interval | P | Mean | 95% Confidence interval | P |
| None | Mean, 43.6 | --- | Ref | 5.13 | --- | Ref |
| HIV | 26.9 | (-29.1 to 4.2) | .009 | 25.6 | (-13.0 to 53.9) | .228 |
| HSV-2 | 82.9 | (-0.3 to 78.7) | .052 | 21.0 | (-15.6 to 47.5) | .318 |
| HIV–HSV-2 | 40.5 | (-25.3 to 19.1) | .785 | 103.0 | (50.2–145.6) | <.001 |
| Age, years | ||||||
| ≥35 | Ref | --- | Ref | --- | ||
| 25–34 | -2.7 | (-11.5 to 6.1) | .543 | 0.31 | (-16.5 to 17.1) | .971 |
| 15–24 | 1.9 | (-15.0 to 18.8) | .824 | 10.3 | (-6.2 to 26.8) | .216 |
| Dermal inflammation | ||||||
| None | Ref | --- | Ref | --- | ||
| Present | 18.8 | (2.1–35.1) | .027 | 0.67 | (-17.6 to 19.0) | .942 |
| Difference in mean | Difference in Mean | |||||
| HSV-2 vs HIV | 55.9 | (17.3–94.5) | .005 | -4.5 | (-46.4 to 40.4) | .842 |
| HIV–HSV-2 vs HSV-2 | -42.3 | (-84.6 to .01) | .050 | 82 | (25.9–138.1) | .005 |
| HIV–HSV-2 vs HIV | 13.6 | (-3.3 to 30.5) | .113 | 77.5 | (20.6–134.3) | .008 |
NOTE. aMean is adjusted cells/mm2 in dermal tissue samples from uninfected patients. Epidermal cell densities were not included, because inflammation was found primarily in the dermis. The multivariate model controls for age and presence of inflammation.
CD8 Cellular Density is Significantly Associated with Local Inflammation
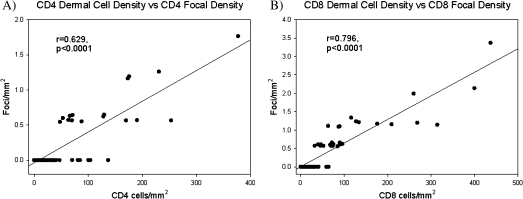
Dermal CD8+ cell density was significantly higher in men with inflammation than in those without it (mean ± SD, 31.3 ± 14.7 cells/mm2 vs 12.5 ± 15.9 cells/mm2; P = .020). CD4+ and CD8+ cell densities were examined for each infection group by presence of inflammation to determine which infections might be associated with this observation. There were no statistically significant differences in CD4+ cell densities among infection groups when stratified by inflammation status (Figure 3A). However, the HIV–HSV-2–coinfected men with inflammation had higher dermal CD8+ cell density than did HIV–HSV-2–coinfected men without inflammation (P = .02). CD8+ cell densities did not vary by inflammation status among the other infection groups (Figure 3B). In addition, the correlation between the number of inflammatory foci per mm2, and CD4+ and CD8+ cell densities were examined for the population as a whole (Figure 4A and 4B). Higher numbers of cellular foci were strongly correlated with higher CD4+ and CD8+ cell densities in the dermis (Spearman correlation r = .629 and .769, respectively; P < .0001) (Figure 4A, 4B).
Figure 3.
CD4 (A) and CD8 (B) dermal cell densities stratified by HIV and HSV-2 status and the presence of inflammation. The differences according to inflammation status were compared within each infection group. One counter's readings are shown. Mean cell density is shown with 95% confidence intervals. Significance was determined at a P < .05 by a Mann-Whitney rank sum analysis.
Figure 4.
Focal inflammation is significantly associated with higher cell densities. Dermal CD4 (A) and CD8 (B) cell densities were found to be significantly correlated with the focal density (Spearman correlation coefficient (r) = .629 and .796 respectively, P < .0001). Results shown are the readings of a single counter for the entire population.
DISCUSSION
To our knowledge, the present study was the first systematic study of immune cell densities in the foreskin and HIV and HSV-2 infection status. CD1a+ cell densities did not differ by HIV and HSV-2 infection status, similar to findings from a previous foreskin study [7]. CD1a+ cell densities also did not vary with the presence of inflammation, suggesting that the Langerhans cell population remains relatively stable in the foreskin epidermis despite underlying infection and local inflammation.
In contrast, individuals with HIV infection alone or HIV–HSV-2 coinfection had decreased CD4+ cell count in the foreskin, compared with HIV-uninfected individuals with or without HSV-2 infection. This finding was most likely attributable to direct or bystander cell killing of CD4+ cells by HIV [19–22]. Men with HSV-2 infection alone had substantially increased CD4+ cell densities, possibly a result of residual CD4 inflammatory response at previous sites of HSV-2 lytic infection [8].
Conversely, CD8+ cell densities were significantly higher in the coinfected and HIV-uninfected, HSV-2-infected groups than in the uninfected group, suggesting that HIV and HSV-2 may synergistically activate the CD8+ cellular immune response. HSV-2–specific CD8+ T cells have been shown to persist for weeks to months at sites of HSV-2 reactivation, and localized physiologic stress can trigger HSV-2 reactivation [16]. Interestingly, the increased CD8+ cell densities observed in the tissue samples from HIV–HSV-2–coinfected men was observed in the absence of evidence of active visible or healed ulcers. The persistent immunologic activation observed in HIV infection, particularly at the mucosal barrier, may stimulate more frequent subclinical HSV-2 reactivation, as has been reported in clinical studies, which subsequently increases the CD8+ cell density [8, 16, 23–25]. However, the cross-sectional design of this study precludes assessments of such causal relationships. Because of this cross-sectional design, the HIV–HSV-2–coinfected men were analyzed as a distinct exposure group, to more clearly observe possible associations between the two viruses. This was especially important in the CD4+ cell analysis because of direct cell killing of these cells by HIV.
The significant association between higher cell densities and the CD4+ and CD8+ focal densities suggest that the differences in cell densities associated with HIV and HSV-2 infection are, at least in part, a consequence of increased focal inflammation. This agrees with the previous report that HSV-2 lesions are associated with persistently elevated CD4+ and CD8+ cell densities at the site of the lesion even after it heals [8].
Male circumcision significantly reduces the rates of acquisition of HIV-1 and HSV-2 and has been shown to lower the amount of genital ulceration [1–4]. The data reported here suggest that these effects may be mediated by the removal of vulnerable CD4+ lymphocytic target cells found in the foreskin, particularly in HSV-2–infected individuals.
Funding
Bristol-Myers Squibb virology fellowship and support from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K23AI083100) to KEJ; and Divisions of Intramural Research, National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institutes of Health.
Acknowledgments
We thank all the participants of the circumcision trials and the staff of the Rakai Health Science Program; Alexander P. Rabkin, for assistance in tissue processing; and Maire Duggan, for assistance in the inflammation analysis.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, Lissouba P, Puren A, Auvert B. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect. 2009;85:116–20. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 7.Donoval BA, Landay AL, Moses S, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–91. [PubMed] [Google Scholar]
- 8.Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–92. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KE, Sherman ME, Ssempiija V, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS. 2009;23:1807–15. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwanzura L, McFarland W, Alexander D, Burke RL, Katzenstein D. Association between human immunodeficiency virus and herpes simplex virus type 2 seropositivity among male factory workers in Zimbabwe. J Infect Dis. 1998;177:481–4. doi: 10.1086/517381. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SJ, Risbud AR, Shepherd ME, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–21. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 12.Todd J, Grosskurth H, Changalucha J, et al. Risk factors influencing HIV infection incidence in a rural African population: a nested case-control study. J Infect Dis. 2006;193:458–66. doi: 10.1086/499313. [DOI] [PubMed] [Google Scholar]
- 13.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celum C, Wald A, Lingappa J, et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Koelle DM, Cao J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacic MB, Katki HA, Kreimer AR, Sherman ME. Epidemiologic analysis of histologic cervical inflammation: relationship to human papillomavirus infections. Hum Pathol. 2008;39:1088–95. doi: 10.1016/j.humpath.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374:229–37. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 22.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–9. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 23.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–9. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 25.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]